Abstract
Background. A key missing element in the development of a successful human immunodeficiency virus (HIV) vaccine is an immunogen that can generate broadly cross-neutralizing antibodies against primary isolates of the virus.
Methods. This phase 1 clinical trial employed a DNA prime and subunit envelope protein boost in an attempt to generate cellular and humoral immune responses that might be desirable in a protective HIV vaccine. Priming was performed via intramuscular injection with gag and env DNA adsorbed to polylactide coglycolide microspheres, followed by boosting with a recombinant trimeric envelope (Env) glycoprotein delivered in MF59 adjuvant.
Results. The DNA prime and protein boost were generally safe and well-tolerated. Env-specific CD4+ cellular responses were generated that were predominantly detected after Env protein boosting. Neutralizing antibody responses against the homologous SF162 viral isolate were remarkably strong and were present in the majority of vaccine recipients, including a strong response against CD4-induced epitopes on gp120. Despite the promising potency of this vaccine approach, neutralization breadth against heterologous tier 2 strains of HIV-1 was minimal.
Conclusions. Potent neutralization against neutralization-sensitive strains of HIV is achievable in humans through a DNA prime, recombinant oligomeric Env protein boost regimen. Eliciting substantial breadth of neutralization remains an elusive goal.
Clinical Trials Registration. NCT00073216.
A successful preventative human immunodeficiency virus (HIV) vaccine will likely need to generate a combination of broad cellular immunity and broadly cross-neutralizing antibody responses. Twenty-five years of research and development of HIV vaccines have not yet succeeded in the generation of a candidate vaccine that achieves this goal. Two phase 3 trials of recombinant subunit gp120 envelope protein vaccines demonstrated that the limited humoral responses generated by this approach had no protective efficacy [1, 2]. The Merck-HIV Vaccine Trials Network (HVTN) phase 2b trial of the MRK Ad5 HIV-1 gag/pol/nef vaccine, commonly known as the STEP trial, demonstrated that this prototypical cytotoxic T-lymphocyte (CTL)–based vaccine did not protect volunteers from infection and failed to reduce the viral load setpoint [3]. Recently, a phase 3 trial of a recombinant canarypox prime followed by a gp120 boost provided the first evidence that some protective efficacy can be achieved by a preventive HIV vaccine. Results from the RV144 trial revealed that, in the modified intent-to-treat analysis, the vaccine efficacy rate was 31.2%, while showing no effect on subsequent viremia or T cell count in individuals who became infected [4]. Further understanding of the mechanism underlying the modest and transient protection seen in this trial is warranted.
The DNA and protein vaccines used in the present trial represented an attempt to elicit both cellular and humoral responses against HIV and, in particular, to generate neutralizing antibodies that would neutralize not just highly neutralization-sensitive (tier 1) viruses, but also heterologous isolates that exhibit a more typical neutralization phenotype (tier 2) of primary isolates [34]. The envelope immunogen was a recombinant, purified gp140 protein that was trimeric and derived from the SF162 primary isolate of HIV [5]. To enhance exposure of the coreceptor binding site, a deletion was introduced in the V2 loop. This deletion enhanced binding of CD4-induced antibodies 17b and 48d, indicating that the coreceptor binding site was indeed more accessible [5]. Gag and Env DNA priming was performed in a formulation with polylactide coglycolide (PLG) microparticles and followed by trimeric Env protein boosting. This approach appeared promising in small animals and generated an enhanced breadth of neutralization in nonhuman primates [5, 6]. Here, we report results from the first human trial of this vaccination approach. CD4+ T cell responses against Env were generated, whereas CD8+ CTL responses were not successfully elicited by this approach. Remarkably high titers of neutralizing antibodies were generated against the homologous SF162 virus. Nevertheless, the approach failed to elicit antibodies capable of neutralizing heterologous, tier 2 clade B viruses.
METHODS
Construction of Codon-optimized Gag and Env DNA Vectors
Priming vaccinations were performed with a combination of plasmid DNA encoding the gag gene from HIV-1 SF2 and a V2-deleted, gp140 env gene from HIV-1 SF162. The details of the design of the codon-optimized, V2-deleted env gene were reported elsewhere [5, 6]. The optimized SF2 gag gene was placed under the control of the CMV immediate-early promoter and the resulting plasmid termed “pCMVgagB.” The optimized SF162 env gene was inserted downstream from the viral subgenomic promoter in the Sindbis virus–based DNA vector system, which was described elsewhere [7] and is termed “pSINenvB.”
Formulation of DNA-PLG Microparticles
The DNA/PLG vaccine consisted of env or gag DNA adsorbed onto biodegradable polymer microparticles (PLG), as has been described elsewhere [8, 9]. Plasmids were produced in Escherichia coli, precipitated, and purified by 2 chromatography steps and transferred by ultrafiltration into formulation buffer. Positively charged PLG microparticles were mixed with the negatively charged DNA, which bound tightly via ionic interaction to form the DNA/PLG immunogen. The formulation was then aseptically filled into single-dose vials and lyophilized to produce a more stable product. The final dosage form was reconstituted with sterile water when prepared for injection.
Design and Production of Recombinant Trimeric, V2-Deleted Env Protein
The production and purification of a codon-optimized, V2-deleted SF162 Env protein has been described previously [5, 10]. Stable Chinese hamster ovary cell lines secreting gp140SF162ΔV2 were derived as detailed elsewhere [10] and adapted to large-scale production and purification.
MF59 Adjuvant
MF59 adjuvant (MF59C.1) is an oil-in-water emulsion with a squalene internal oil phase and a citrate buffer external aqueous phase. Two nonionic surfactants, sorbitan trioleate and polysorbate 80, serve to stabilize the emulsion. This adjuvant is part of an influenza vaccine that is approved for marketing in Europe [11, 12].
Clinical Trial Design and Conduct
The clinical trial was conducted by the HIV Vaccine Clinical Trials Network at clinical sites in Nashville, Tennessee; Seattle, Washington; St Louis, Missouri; and Providence, Rhode Island. The primary objective was to determine the safety and tolerability of the vaccination regimen in HIV-uninfected volunteers, with secondary objectives of measuring the humoral and cellular immunogenicity of this regimen in humans. Part A of the study included a dose-escalating design for the DNA/PLG microparticle prime. Part B included additional volunteers at the highest DNA priming dose. An additional comparison group (group 5) was added during the performance of the trial and assessed oligomeric Env protein immunization in the absence of a DNA prime. The resulting (final) trial design is shown in Table 1. A Protocol Safety Review Team examined the safety data in part A for all participants in each group through day 42 of the study before the study proceeded with dose escalation. Healthy, HIV-1–uninfected adult subjects were recruited at 4 centers in the United States; enrollment began in December 2003 and was completed in May 2006 (www.clinicaltrials.gov identifier, NCT00073216).
Table 1.
Study Schema
| Study Agents | ||||||||
| DNA/PLG : |
Prime : Clade B Gag DNA/PLG and Clade B Env DNA/PLG microparticles, given as a single IM injection, doses of 250/250, 500/500,1000/1000 |
|||||||
| gp140 : |
Boost : Clade B recombinant oligomeric Env gp140 protein with MF59 adjuvant, given as a single IM injection, 100 mcg dose for all injections |
|||||||
| Control : |
placebo - 0.9% Sodium Chloride (NaCl) |
|||||||
| PART A : Dose escalation | ||||||||
| Group | DNA/PLG (mcg Gag/Env) | Number of subjects | Injection Schedule in Months (Days) | |||||
| 0 (0) | 1 (28) | 2 (56) | 6 (168) | 9 (273) | ||||
| 1 | 250/250 | 10 | DNA/PLG | DNA/PLG | DNA/PLG | gp140 | gp140 | |
| Control | 2 | NaCl | NaCl | NaCl | NaCl | NaCl | ||
| 2 | 500/500 | 10 | DNA/PLG | DNA/PLG | DNA/PLG | gp140 | gp140 | |
| Control | 2 | NaCl | NaCl | NaCl | NaCl | NaCl | ||
| 3 | 1000/1000 | 10 | DNA/PLG | DNA/PLG | DNA/PLG | gp140 | gp140 | |
| Control | 2 | NaCl | NaCl | NaCl | NaCl | NaCl | ||
| PART B : | ||||||||
| Group | DNA/PLG (mcg gag/env) | Number | Injection Schedule in Months (Days) |
|||||
| 0 (0) | 1 (28) | 2 (56) | 6 (168) | 9 (273) | ||||
| 4 | 1000/1000 | 20 | DNA/PLG | DNA/PLG | DNA/PLG | gp140 | gp140 | |
| Control | 4 | NaCl | NaCl | NaCl | NaCl | NaCl | ||
| 0 (0) | 3 (84) | 9 (273) | ||||||
| 5 | N/A | 30 | gp140 | gp140 | gp140 | |||
| Control | 6 | NaCl | NaCl | NaCl | ||||
| Total (Parts A & B) | 80 16 (control) | |||||||
Assays of Cellular Immune Function
T cell responses were assessed by interferon (IFN)–γ enzyme-linked immunosorbent spot (ELISPOT) assay using cryopreserved peripheral blood mononuclear cells (PBMCs), which had been stimulated overnight with synthetic peptide pools that span the clade B Env or Gag proteins. Assays were performed using validated assay procedures [13] that included the use of cryopreserved PBMCs stimulated ex vivo with pools of peptides that were 15 amino acids in length and overlapped by 11 amino acids. Peptides matched the gp120 sequence of the SF-162 construct used for vaccination. Responses were measured as the number of spot-forming cells per 1 million PBMCs and expressed as the geometric means. Intracellular cytokine staining (ICS) for IFN-γ or interleukin (IL)–2 were performed using PBMCs collected 2 weeks after receipt of the last vaccine dose for all groups. The 8-color ICS assay has been previously validated [14] and was used in this trial to identify IFN-γ– and IL-2–secreting CD3+/CD8+ and CD3+/CD4+ HIV-specific T cells.
Measurement of HIV-Specific Binding Antibody
Anti-Gag and anti-Env binding antibody responses were determined by validated enzyme-linked immunosorbent assay, as described elsewhere [15, 16]. Sera from cryopreserved samples were tested in duplicate in microtiter plates (NUNC) coated with purified p55 Gag (Protein Sciences), o-gp140 deltaV2 (Novartis), and gp41 (Immunodiagnostics).
HIV Neutralization Assays
Neutralization assays used the TZM-bl (JC53bl-13) reporter cell line. This assay employs a luciferase gene responsive to the HIV Tat protein and has been optimized as the end point neutralization assay for the HVTN, as described elsewhere [17]. Samples from the indicated time points were tested for neutralization of the homologous SF162 viral isolate and against the NIH clade B panel of pseudoviruses to assess neutralization breadth [18]. A response to an isolate was considered positive if the neutralization titer was ≥25 or if the neutralization potency at serum dilution level 1:10 was ≥50%.
Statistical Analysis
The statistical analysis employed an intent-to-treat principle (ie, all data from enrolled participants were used according to the initial randomization assignment, regardless of how many injections they received). Two-sided 95% confidence intervals (CIs) for the response rates were calculated using the score test method of Agresti and Coull [19]. Differences in immunogenicity response rates and magnitudes were tested with 2-sided Fisher exact tests and Wilcoxon rank sum tests, respectively. Reported P values were not adjusted for multiple comparisons. For comparison of the response rates between treatment groups, a significant difference was declared if the 2-sided 95% CI for the difference in response rates between the 2 groups excluded 0. The magnitude of the T cell immune response was calculated by summing across the maximum responses for each PTE peptide pool for each protein. All analyses were performed using SAS (SAS Institute), S-Plus (TIBCO Software Inc.), and/or R statistical software. Pie charts were prepared using Spice, version 4.3 (provided by Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Disease, National Institutes of Health).
RESULTS
Safety and Reactogenicity
The DNA prime-protein boost vaccination regimen was generally well tolerated. Mild or moderate pain and/or tenderness at the injection site were the most commonly reported side effects, with somewhat higher percentages of vaccine than placebo recipients in the DNA/gp140 groups (P = .03) but not the gp140 alone groups reporting symptoms. No severe local or systemic reactogenicities were reported. Four serious adverse events were reported during the trial. A death attributed to cocaine overdose occurred in 1 placebo participant. Two vaccine recipients (in groups 4 and 5) had elevated creatinine phosphokinase levels of significant magnitude to require reporting as an serious adverse event, but these serious adverse events were judged to be unrelated to study vaccine. Supplementary Table S1 presents the vaccine-related adverse events in tabular form.
Cellular Immunogenicity
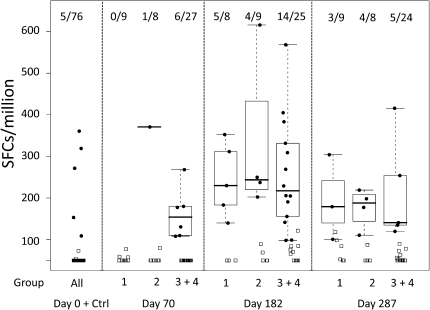
Gag-specific IFN-γ ELISPOT response rates did not increase substantially, compared with baseline, after the 3 vaccinations with DNA/PLG or after protein vaccination (data not shown). Responses against Env were very low 2 weeks after 3 DNA vaccinations, although positive responses were detectable in a few individual subjects (Figure 1). In contrast, substantial Env-specific IFN-γ ELISPOT responses were detected in samples taken 2 weeks after the first protein boost (Figure 1). Response rates ranged from 0% (95% CI, 0.0%–32.4%) for the placebo group to 62.5% (95% CI, 30.6%–86.3%), 44.4% (95% CI, 18.9%–73.3%) and 56.0% (95% CI, 37.1%–73.3%) after treatment with 250, 500, and 1000 μg of DNA/PLG plus 140 of μg gp140, respectively. Responses were generally lower following the second dose of protein (day 287) (Figure 1). These results indicate that Env DNA/PLG responses were quite limited after 3 doses but may have primed for the responses seen after protein boosting (described further in the paragraph below).
Figure 1.
Interferon-γ enzyme-linked immunosorbent spot assay Responses to Env Following DNA/ polylactide coglycolide (PLG) Priming and Oligomeric, V2-deleted Glycoprotein Boosting. Responses are expressed as the number of spot-forming units (SFUs) per 106 cells. Env responses are shown at 2 weeks following the completion of DNA/PLG priming (day 70) and 2 weeks after receipt of the first and second protein boosts (days 182 and 287, respectively). The numbers of positive responders in each group are indicated at the top of the figure.
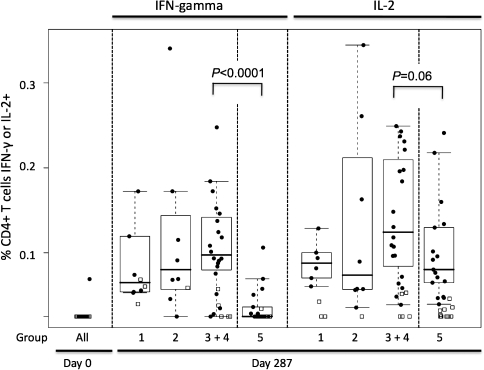
ICS assays were performed for production of IFN-γ and IL-2, to provide additional quantification of the cellular responses and to determine whether the observed responses were predominantly due to CD4+ or CD8+ T cells. The majority of responses by ICS were found in the CD4+ subset of T lymphocytes. Of these responses, >90% were directed against Env, whereas the responses to Gag peptides were minimal (as had been seen in the ELISPOT analysis). Responses to Env peptides measured 2 weeks following the final immunization in each group of vaccine recipients were significantly greater than those of placebo recipients and were modest in magnitude (Figure 2). The highest median responses for the CD4+ T cell population were seen in the combined groups 3 and 4, representing the recipients of the 1000 μg priming dose of Env DNA/PLG. Three doses of protein alone (group 5) resulted in mean CD4+ T cell responses equivalent to those in the groups receiving 250 or 500 μg of DNA/PLG priming (Figure 2). Responses in volunteers primed with 1000 μg of Env DNA/PLG demonstrated significantly higher responses than those receiving protein alone. Thus, DNA/PLG administration did prime for CD4+ T cell responses to Env that were boosted by recombinant protein, an effect detectable only at the highest DNA priming dose.
Figure 2.
CD4+ T cell Responses Measured by intracellular cytokine staining for interferon (IFN)–γ and interleukin (IL)–2. The percentage of the CD3+/CD4+ population expressing either cytokine following exposure to Env peptides is depicted. IFN-γ– and IL-2–specific responses are shown for each vaccination group. Note that for group 5, day 70 represents 2 weeks after receipt of the second protein dose, whereas day 182 for the DNA-primed groups represents 2 weeks after receipt of the first protein boost.
Assessment of Polyfunctional CD4+ T cell Responses in DNA Primed versus Unprimed Individuals
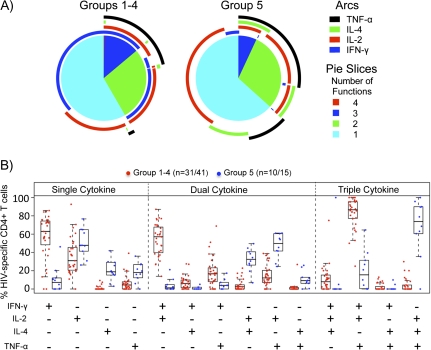
To determine whether the qualitative nature of the CD4+ T cell responses differed in those who had been primed with DNA (groups 1-4) versus those receiving protein alone (group 5), we assessed IFN-γ, IL-2, tumor necrosis factor–α, and IL-4 production in response to Env peptide pools by ICS 2 weeks after receipt of the final protein boost. Figures 3A and 3B show the relative distribution of the number of cytokines expressed or the degree of polyfunctional response in those receiving DNA priming (groups 1–4) or protein alone (group 5). Although the total numbers of polyfunctional responses did not differ significantly between the DNA primed and unprimed groups (Figure 3A, number of functions), analysis of individual cytokines secreted revealed important differences. Figure 3B illustrates graphically the individual cytokine secretion patterns, grouped by single cytokine responses, secretion of 2 cytokines, or secretion of 3 cytokines. In this analysis, marked differences were observed in the DNA primed versus unprimed groups. Responses in individuals primed with DNA were dominated by IFN-γ, whereas IL-4 secretion was much more prominent in cells from those receiving protein alone. Thus, although polyfunctional responses were elicited equally in both groups, DNA priming had an important effect on the quality of the CD4+ T cell response.
Figure 3.
Analysis of Polyfunctional CD4+ T cell Responses. A, interferon (IFN)–γ, interleukin (IL)–2, tumor necrosis factor (TNF)–α, and IL-4 responses to Env peptides were assessed 2 weeks after receipt of the final protein boost by intracellular cytokine staining. Pie charts show the percentage of Env-specific cells responding with secretion of 1, 2, 3, or 4 cytokines averaged for individuals receiving DNA priming (groups 1–4; left) or for individuals receiving protein alone (group 5; right). Arcs show the percentage of cells producing each of the 4 cytokines. The color keys for the pie slices and arcs are shown. B, Specific cytokine secretion patterns are shown for those cells expressing 1 cytokine (left), 2 cytokines (middle), or 3 cytokines (right) following in vitro peptide stimulation. Red dots indicate responses from individuals primed with DNA (groups 1–4); blue dots indicate responses from individuals receiving protein alone (group 5).
HIV-Specific Binding and Neutralizing Antibody Responses
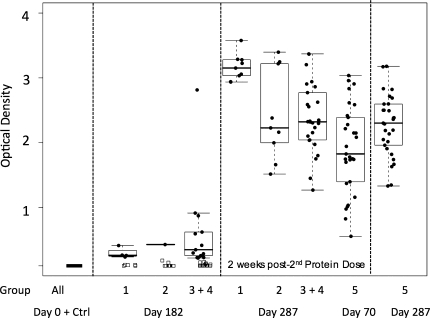
Binding antibody responses were minimal following DNA/PLG priming (day 182) (Figure 4), wheras substantial levels of binding antibodies against Env developed in 100% of vaccinees following the protein boost (day 287) (Figure 4). Those individuals who received protein alone demonstrated similar, high levels of Env-specific binding antibodies 2 weeks after the third vaccination (day 287) (Figure 4, group 5).
Figure 4.
Binding Antibody Responses Measured by enzyme-linked immunosorbent assay, Shown as Optical Density Units. The heavy bar indicates the median, with a box around the 25th and 75th percentiles, with whiskers on the plot representing the 5th and 95th percentiles. Responses to Env are shown for serum samples 2 weeks after receipt of the first and second protein boosts for groups 1–4 (days 182 and 287, respectively) and 2 weeks after receipt of the second and third protein doses for group 5 (days 70 and 287, respectively). For comparison of responses following the second protein boost in primed (groups 1–4) versus unprimed (group 5) individuals, we present these side by side, as indicated. Ctrl, results from volunteers receiving saline.
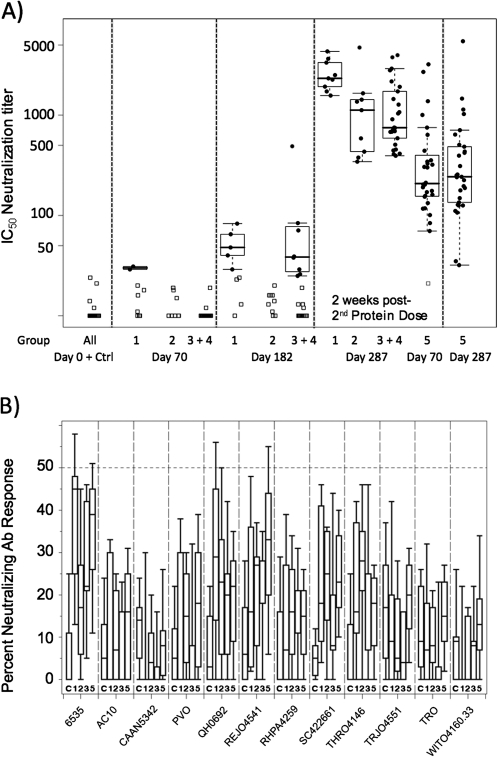
Next, we assessed neutralizing antibodies directed against the SF162 viral isolate. Responses were negative in all groups at the completion of the DNA/PLG priming regimen with the exception of 2 individuals in group 1 (day 70) (Figure 5A). In contrast, responses elicited by 2 doses of recombinant, oligomeric protein in the absence of priming were seen in 97% of volunteers, with a median 50% inhibitory concentration (IC50) of 208 (day 70) (Figure 5A, group 5). Measurable neutralizing antibody responses were detected in a minority of primed vaccine recipients following the first protein boost (day 182) (Figure 5A). Remarkably, 100% of vaccinees receiving 2 (groups 1–4) or 3 doses (group 5) of the oligomeric gp140 protein developed neutralizing antibody responses against SF162 (day 287) (Figure 5A). IC50 titers against SF162 were remarkably high in the DNA/PLG-primed groups, ranging from a median of 2328 in group 1 to 750 in group 3 + 4. The contribution of DNA/PLG priming to enhancing the neutralizing antibody titer against SF162, compared with that seen after 2 or 3 doses of protein alone, is suggested by these data (Figure 5A; compare groups 1, 2, and 3 +4 with group 5). Also notable was the apparent enhanced priming effect of lower doses of DNA (group 1), compared with the other groups. We conclude that DNA/PLG priming followed by recombinant oligomeric gp140 protein boosting elicited high titers of neutralizing antibodies against the homologous SF162 isolate in human volunteers and that the responses were superior to those generated by the administration of recombinant protein alone.
Figure 5.
Neutralizing Antibody Titers Measured Using the TZM-bl Luciferase Reporter Assay. A, Neutralization of SF162, shown as the reciprocal dilution providing 50% reduction in reporter signal. Group assignments are indicated below the plot and the time points in the trial are indicated above the plot. For comparison of responses following receipt of the second protein boost in primed (groups 1–4) versus unprimed (group 5) individuals, we present these side by side, as indicated. B, Neutralization of a panel of clade B primary isolate pseudoviruses at a single dilution (1:10). Isolate designation is provided below the plot. The dashed line indicates 50% neutralization cutoff. Group designation indicated below each box plot. Ab, antibody; Ctrl, results from subjects receiving saline; IC50, half maximal inhibitory concentration.
To assess breadth of neutralization, serum specimens from day 287 were tested for neutralization of a panel of clade B isolates derived from early transmission events. The panel of isolates and low neutralizing titers attained at a 1:10 dilution are represented in Figure 5B. Although responses above the level achieved in placebo recipients (C) can be appreciated, the overall conclusion from these data is that neutralization potency against tier 2 strains of clade B isolates was very weak. Thus, there was a lack of correlation between the magnitude of the homologous neutralizing antibody titers achieved and the ability to generate cross-neutralization of tier 2 clade B primary isolates.
Epitope Mapping of Neutralizing Antibodies in Serum Samples from Vaccines
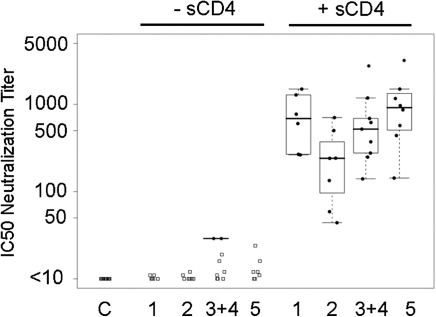
Peptide competition studies revealed that neutralizing antibody titers were diminished >50% in serum from 4 vaccinees following incubation with V1 peptide, whereas no serum samples demonstrated significant decrease in activity following incubation with V3 peptide (data not shown). Next, we reasoned that the V2 deletion in the Env glycoprotein vaccine may have facilitated the development of responses against CD4i epitopes. To assess vaccinee serum for the presence of neutralizing activity against CD4-induced (CD4i) epitopes, we used an HIV-2 molecular clone that is highly susceptible to CD4i antibody neutralization in the presence of subinhibitory concentrations of soluble CD4 [20]. Remarkably, neutralization of this HIV-2 isolate in the presence of subinhibitory concentrations of CD4 was robust, indicating that a substantial component of the neutralizing antibody activity found in trial volunteers indeed appeared to be directed against CD4i epitopes (Figure 6).
Figure 6.
Measurement of Neutralizing Antibodies Against CD4i Epitopes. Human immunodeficiency virus (HIV)–2 7312A/V434M is a molecularly-cloned HIV-2 isolate that is susceptible to neutralization by CD4i antibodies in the presence of subinhibitory concentrations of sCD4. In this assay, SF162 or HIV-2 were incubated in the absence (left) or presence (right) of 0.5 μg/mL sCD4, and neutralization of virus assayed using TZM-bl luciferase reporter cells.
DISCUSSION
Modifications of the envelope glycoproteins of HIV and simian immunodeficiency virus have the potential to increase the exposure of neutralization epitopes and are being investigated as a possible means to enhance neutralization potency and breadth when employed in a vaccine. SF162 is a CCR5-using primary isolate that is classified as a tier 1 virus for being highly sensitive to neutralization by serum from HIV-1–infected individuals. Deletion of 30 amino acids from the central region of the V2 loop (SF162ΔV2) does not inhibit viral replication but renders the virus even more susceptible to neutralization by serum from HIV-infected individuals [21]. V2 deletion makes recognition by CD4-induced antibodies, such as 48d and 17b, more effective, suggesting that conserved regions of the Env complex involved in coreceptor binding are uncovered by this strategy [22, 23]. In rabbits, the SF162ΔV2 construct was injected as a DNA vaccine and elicited similar binding antibody titers but higher titers of neutralizing antibodies, compared with the unmodified Env immunogen [24]. When applied in a DNA prime/recombinant protein boost vaccination in rhesus macaques, neutralization titers against the homologous parental isolate were enhanced, and extended activity against some heterologous viruses was elicited [24]. Altogether, these preclinical data suggested that the DNA prime/recombinant oligomeric protein boost was a promising regimen worthy of study in human trials. We note, however, that some broadly neutralizing antibodies against HIV require contributions from the V2 loop [25], so the potential benefits of deletion of V2 in Env immunogen design must be entertained in this context.
The results of this clinical trial in humans provide a dramatic illustration of one of the central problems in the field of HIV vaccine development. The DNA/PLG prime/oligomeric protein boost regimen succeeded in eliciting very high homologous neutralizing antibody titers against the vaccine strain. Neutralizing antibodies were detected in 100% of volunteers after only 2 doses of the recombinant protein boost. These results were very encouraging and support the concept of generating neutralizing antibodies through immunization with oligomeric, modified Env glycoproteins. In addition, the magnitude of neutralization achieved was enhanced by priming with DNA encoding the same Env glycoprotein with a partial V2 deletion. However, despite eliciting high titers of neutralizing antibodies against SF162, neutralization breadth was not achieved in this trial. Antibody responses were potent and were elicited in all volunteers, but they were extremely specific for the vaccine strain. The basis for the narrow specificity of the response probably relates to the fact that antibodies targeting CD4-inducible epitopes in coreceptor binding domain of gp120 are rarely a target on primary patient isolates [20]. Such neutralization responses are unlikely to provide protection from infection or to impact the course of early events following transmission of HIV.
The nature of neutralization breadth remains an understudied and significant problem in the HIV vaccine field. In recent studies, serum samples from 10%–25% of chronically infected individuals have shown substantial neutralizing activity against many tier 2 viruses [26-29]. A portion of this broadly neutralizing activity appears to be directed against epitopes on gp120, primarily the CD4 binding site, with only rare activity directed against the membrane proximal external region of gp41 and with many other specificities that remain to be identified [26-28, 30, 31]. It remains to be determined how many envelope genes and proteins, as well as what form of the proteins, will be required in a vaccine construct to elicit the degree of breadth seen in this subset of infected individuals. We note that the V2-deleted, trimeric gp140 approach studied in this clinical trial was monovalent, but that a second Env component derived from a clade C primary isolate has been produced and characterized [32, 33]. Given the magnitude of the SF162-specific responses demonstrated in this trial, it would be of great interest to combine these antigens in a future trial and determine if cross-neutralizing antibodies can be generated that extend beyond the included isolates.
This trial failed to fully substantiate the enhanced potency of the use of PLG microparticles in humans as a means of delivering a DNA vaccine that had been established in preclinical studies [9, 34-36]. HIV DNA vaccines adsorbed onto PLG microparticles demonstrated 100- and 1000-fold increases in CD8+ T cell and antibody responses, respectively, compared with naked DNA when administered to mice [8] and were also enhanced in macaques [34]. Responses to DNA/PLG alone (prior to boosting) in this trial were very modest, with minimal stimulation of CD8+ T cell responses. Despite the modest effect of DNA priming on measurable HIV-specific immune responses prior to the boost, a marked difference in the cytokine profile of Env-specific CD4+ T cells was detected in those who had been primed with DNA. Although there was no difference in the percentage of polyfunctional Env-specific T cells induced, DNA priming led to a substantial increase in polyfunctional T cells secreting IFN-γ.
In summary, this phase 1 clinical trial of a DNA prime and oligomeric, V2-deleted gp140 protein boost was encouraging because it produced a strong neutralizing antibody response in 100% of volunteers, but it was also disappointing because the response was strain specific rather than broad. The ability to overcome this obstacle through combinations of Env DNA and protein immunogens will be an important subject of future trials.
Funding
National Institute of Allergy and Infectious Diseases, National Institutes of Health (U01 AI068614 and NO1-AI05396). The Emory Center for AIDS Research (P30 AI050409) provided some support for the preparation of this manuscript.
Acknowledgments
We thank the volunteers at four clinical sites who participated in this trial.
Potential conflicts of interest: J.D. and S.B. are employees and stakeholders of Novartis Vaccines and Diagnostics. All other authors: no conflicts.
References
- 1.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 2.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava IK, Stamatatos L, Kan E, et al. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol. 2003;77:11244–59. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett SW, Srivastava IK, Ulmer JB, Donnelly JJ, Rappuoli R. Development of V2-deleted trimeric envelope vaccine candidates from human immunodeficiency virus type 1 (HIV-1) subtypes B and C. Microbes Infect. 2005;7:1386–91. doi: 10.1016/j.micinf.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Dubensky TW, Jr., Driver DA, Polo JM, et al. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J Virol. 1996;70:508–19. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Hagan D, Singh M, Ugozzoli M, et al. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J Virol. 2001;75:9037–43. doi: 10.1128/JVI.75.19.9037-9043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000;97:811–6. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava IK, Stamatatos L, Legg H, et al. Purification and characterization of oligomeric envelope glycoprotein from a primary r5 subtype B human immunodeficiency virus. J Virol. 2002;76:2835–47. doi: 10.1128/JVI.76.6.2835-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansaldi F, Canepa P, Parodi V, et al. Adjuvanted seasonal influenza vaccines and perpetual viral metamorphosis: the importance of cross-protection. Vaccine. 2009;27:3345–8. doi: 10.1016/j.vaccine.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Siber GR. Adjuvants for human vaccines–current status, problems and future prospects. Vaccine. 1995;13:1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 13.Russell ND, Hudgens MG, Ha R, Havenar-Daughton C, McElrath MJ. Moving to human immunodeficiency virus type 1 vaccine efficacy trials: defining T cell responses as potential correlates of immunity. J Infect Dis. 2003;187:226–42. doi: 10.1086/367702. [DOI] [PubMed] [Google Scholar]
- 14.Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323:39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goepfert PA, Tomaras GD, Horton H, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25:510–8. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agresti A, Coull BA. Order-restricted tests for stratified comparisons of binomial proportions. Biometrics. 1996;52:1103–11. [PubMed] [Google Scholar]
- 20.Decker JM, Bibollet-Ruche F, Wei X, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201:1407–19. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–5. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–33. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt R, Kwong PD, Desjardins E, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–11. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 24.Barnett SW, Lu S, Srivastava I, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J Virol. 2001;75:5526–40. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhillon AK, Donners H, Pantophlet R, et al. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol. 2007;81:6548–62. doi: 10.1128/JVI.02749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Svehla K, Louder MK, et al. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83:1045–59. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sather DN, Armann J, Ching LK, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–69. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 30.Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–68. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Migueles SA, Welcher B, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke B, Gomez-Roman VR, Lian Y, et al. Neutralizing antibody responses to subtype B and C adjuvanted HIV envelope protein vaccination in rabbits. Virology. 2009;387:147–56. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava IK, Kan E, Sun Y, et al. Comparative evaluation of trimeric envelope glycoproteins derived from subtype C and B HIV-1 R5 isolates. Virology. 2008;372:273–90. doi: 10.1016/j.virol.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Otten GR, Schaefer M, Doe B, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J Virol. 2005;79:8189–200. doi: 10.1128/JVI.79.13.8189-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hagan D, Singh M, Ugozzoli M, et al. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J Virol. 2001;75:9037–43. doi: 10.1128/JVI.75.19.9037-9043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajdy M, Singh M, Kazzaz J, et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res Hum Retroviruses. 2004;20:1269–81. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]