Abstract
Background. Oseltamivir, a widely used anti-influenza drug, is hydrolytically activated by carboxylesterase 1 (CES1). The expression of this carboxylesterase is developmentally regulated. This study was performed to determine when after birth infants acquire competence of activating this prodrug.
Methods. Liver tissue samples were collected and divided into 5 age groups: group 1 (1–31 d old), group 2 (35–70 d old), group 3 (89–119 d old), group 4 (123–198 d old), and group 5 (>18 years of age). These samples were analyzed for oseltamivir hydrolysis and CES1 expression.
Results. Liver samples in group 1 expressed the lowest level of CES1 with the lowest hydrolytic activity toward oseltamivir. A 4–7-fold increase between groups 1 and 2 (1–31 vs 35–70 d of age) was detected in the hydrolysis and expression analyses, respectively. Liver samples in the other 3 pediatric groups (35–198 d of age) exhibited similar expression and hydrolysis levels. Overall, liver samples in group 1 had CES1 expression and hydrolysis levels that were 10% of those of adults, whereas liver samples in the other 3 pediatric groups had levels that were ∼50% of adult levels.
Conclusions. The post-neonatal surge in CES1 expression ensures the hydrolytic capacity to be gained rapidly after birth in infants, but the larger variability during this period suggests that caution should be exercised on the extrapolated dosing regimens of ester drugs from other age groups.
Carboxylesterases constitute a large class of hydrolytic enzymes [1, 2]. These enzymes are major pharmacokinetic determinants of ester/amide dugs and detoxify against many insecticides [1–3]. In addition, these enzymes hydrolyze endogenous lipids and are involved in the assembling of lipoproteins [4, 5]. Carboxylesterases exhibit overlapping substrate specificity; however, many drugs are hydrolyzed predominately by a single carboxylesterase [1, 2, 6]. Although there are exceptions, the relative sizes between the alcohol and acyl (acid) moieties of an ester contribute significantly to the isoform-specific hydrolysis. For example, the anticancer prodrug irinotecan has a larger alcohol moiety and is hydrolyzed primarily by carboxylesterase 2 (CES2) [7]. In contrast, the anti-influenza prodrug oseltamivir has a larger acid group and is hydrolyzed by carboxylesterase 1 (CES1) [8].
The recent influenza pandemic led to the wide use of oseltamivir among patients of all age groups [9, 10]. Initial reports showed that infants and children, even those only months old, produced sufficient levels of the hydrolytic metabolite [11, 12]. A previous study from this laboratory has shown that fetal livers had only ∼5% of the adult CES1 capacity, depending on the experimental parameters (messenger RNA [mRNA], protein, or hydrolysis levels) [13]. The present study was performed to test the hypothesis that a surge in expression of CES1 during the early developmental period enables a rapid gain of oseltamivir activation capacity. To test this hypothesis, liver tissues from donors at birth to age ∼6 months were collected and analyzed for the activation of oseltamivir and the expression of CES1.
MATERIALS AND METHODS
Liver RNA and S9 Fractions
A total of 59 tissue samples were used in this study (Table 1). Liver tissues were acquired primarily from the University of Maryland Brain and Tissue Bank for Developmental Disorders (Baltimore, MD). The isolation of total RNA from the liver tissues is described elsewhere [14], and the quality was determined by electrophoresis. S9 fractions were prepared by differential centrifugation, as described elsewhere [14]. The use of the human tissue samples was approved by the Institutional review board.
Table 1.
Demographic Distribution of Liver Donors
| Group (age) | No. of donors | No. male/no. female | No. white | No. African American | No. Hispanic |
| 1 (1–31 d) | 12 | 6/6 | 6 | 4 | 2 |
| 2 (35–70 d) | 13 | 5/8 | 5 | 8 | 0 |
| 3 (89–119 d) | 10 | 7/3 | 4 | 6 | 0 |
| 4 (123–198 d) | 10 | 8/2 | 4 | 5 | 1 |
| 5 (>18 years) | 14 | 7/7 | 10 | 4 | 0 |
Reverse-transcription Quantitative Polymerase Chain Reaction and Western Blot Analysis
The mRNA levels were determined by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) with a TaqMan gene expression assay (Applied Biosystems) [11]. The TaqMan assay identification numbers were as follows: CES1, Hs00275607_m1 (NM_001266); and polymerase (RNA) II, Hs01108291_m1 (NM_000937). The CES1 probe could detect both carboxylesterase 1A1 (CES1A1) and CES1A2 transcripts [15]. The CES1A1- and CES1A2-specific TaqMan probes were custom-prepared by Applied Biosystems according to reported sequences [15]. All samples were analyzed in triplicate, and the signals were normalized to those of polymerase (RNA) II [20] and then expressed as relative levels of mRNA. For Western blot analysis, S9 fractions (.25 μg) were resolved by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis - in a mini gel apparatus and transferred electrophoretically to nitrocellulose membranes. After nonspecific binding sites were blocked with 5% nonfat milk, the blots were incubated with an antibody against CES1 and glyceraldehyde-3-phosphate dehydrogenase, respectively. The preparation of the antibody against CES1 is described elsewhere [16]. The primary antibodies were subsequently localized with goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase, and horseradish peroxidase activity was detected with a chemiluminescent kit (SuperSignal West Pico, ThermoFisher Scientific). The chemiluminescent signals were captured by a Kodak Image Station 2000, and the relative intensities were quantified by means of Kodak 1D Image Analysis Software 3.6.5. K2.
Enzymatic Assays and Monitoring of Hydrolysis by Liquid Chromatography Mass Spectrometry
The enzyme incubations were conducted as described elsewhere, with minor modifications [8]. All enzymatic assays were performed at 37°C in a total volume of 100 μL. Pilot studies were performed to determine conditions (eg, protein concentrations) to maintain the metabolism in the linear range. Generally, S9 fractions (5 μg of protein) were prepared in 50 μL of incubation buffer (Tris-HCl; concentration, 50 mmol/L; pH, 7.4) and then mixed with an equal volume of oseltamivir solution at a concentration of 2 μmol/L (in the same buffer). The incubations usually lasted for 15 min, and the reactions were terminated with 150 μL of acetonitrile containing the internal standard deuterated oseltamivir carboxylate. The reaction mixtures were subjected to centrifugation for 15 min at 4°C (15,000 g). Oseltamivir and oseltamivir carboxylate were obtained from Toronto Research Chemicals. The deuterated oseltamivir carboxylate was provided by Hoffmann–La Roche. Various controls were performed such as 0 min of incubation and incubation without microsomes. The hydrolysis of oseltamivir was monitored by a liquid chromatography mass spectrometry system (API 3200), essentially as described elsewhere [15]. The analytes were detected in positive ion mode using the following mass transitions: m/z for oseltamivir, 313 → 166; m/z for oseltamivir carboxylate, 285→138; and m/z for internal standard (IS), 288 → 139. Flow injection analysis was performed at a flow rate of 20 μL/min to obtain optimum source parameters. The calibration curve was 1–250 ng/mL with good linearity for oseltamivir and 4–1000 ng/mL for oseltamivir carboxylate.
Statistical Analysis
Statistical analyses were performed with PASW Statistics software (version 18; SPSS). Significant differences were tested according to the Spearman test for correlation or 1-way analysis of variance followed by a Duncan test for comparison of means. In all cases, significant differences were observed when P values were <.05.
RESULTS
Surge in the Level of CES1 mRNA During the Post-neonatal Period
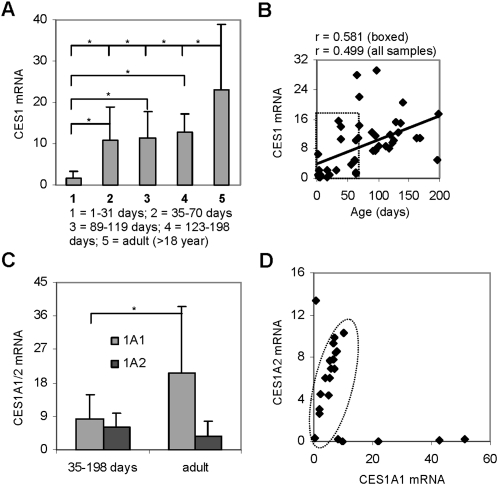
To ascertain the activation capacity of the anti-influenza agent oseltamivir during the early developmental period, we collected a large number of individual liver tissue samples from donors of age 1–198 d. The samples were divided into several groups: group 1 (1–31 d old), group 2 (35–70 d old), group 3 (89–119 d old), group 4 (123–198 d old) and group 5 (>18 years old). We have shown elsewhere that oseltamivir is hydrolytically activated by CES1 [10]. The expression of CES1 in these samples was first determined by RT-qPCR. The results are summarized in Figure 1A and Table 2. Samples in group 1 (1–31 d old) expressed the lowest level, whereas those in group 5, the adult group, expressed the highest level of CES1 mRNA. Samples in group 1 expressed only ∼7% of the adult level (Table 2). Samples in groups 2–4, the other 3 pediatric groups, expressed much higher levels of CES1 mRNA, 43%– - 55% of the adult CES1 mRNA level (Table 2). More importantly, a 7-fold increase in CES1 mRNA level was detected between groups 1 and 2, supporting our hypothesis that there is a surge in CES1 expression during the early developmental period. There were large interindividual variations in the level of CES1 mRNA, ranging from 4-fold to 24-fold, particularly in groups 1 and 2 (Table 2).
Figure 1.
Levels of carboxylesterase 1 (CES1) messenger RNA (mRNA; isoforms (CES1A1 and CES1A2) in various age groups and correlation analysis. A, The mRNA expression level of CES1. Total RNA was subjected to reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysis for the level of CES1 mRNA by a TaqMan probe recognizing both CES1A1 and CES1A2. The signals from each target were normalized based on the signal from polymerase II and expressed as relative levels among all samples. The data are presented as mean (± SD). *P < .05. B, Correlation analysis between age and the level of CES1 mRNA. C, The mRNA levels of CES1A1 and CES1A2. Total RNA was subjected to RT-qPCR with a CES1A1 and CES1A2 isoform-specific TaqMan probe. The signals from each target were normalized based on the signal from Pol II and expressed as relative levels among all samples. The data are presented as mean (± SD). *P < .05. D,Correlation analysis between CES1A1 and CES1A2 in samples that expressed both transcripts.
Table 2.
Carboxylesterase 1 Messenger RNA Levels and Oseltamivir Hydrolysis
| Messenger RNA level/arbitrary units |
Oseltamivir hydrolysis |
|||
| Group (age) | Mean | Variability, fold increasea | Mean, pmol products formed/mg protein/min | Variability, fold increasea |
| 1 (1–31 d) | 1.54 | 14 | 5.98 | 62 |
| 2 (35–70 d) | 10.87 | 24 | 23.38 | 1689b |
| 3 (89–119 d) | 11.29 | 4 | 23.00 | 4 |
| 4 (123–198 d) | 12.74 | 4 | 24.87 | 15 |
| 5 (>18 years) | 23.04 | 12 | 47.00 | 5 |
NOTE. aVariability was calculated as the maximum value divided by the minimum value in each group.
The fold increase of the variability was 25 after a single sample with extremely low activity was not considered.
The larger individual variation in groups 1 and 2 suggests that developmental regulation plays a greater role in the expression of CES1 during the period from 1 to 70 d of age. To test this possibility, we performed correlation analyses with the entire set of data points (groups 1–4) or the data points of groups 1 and 2. As shown in Figure 1B, when pediatric groups were considered together, the levels of CES1 mRNA were correlated with age with a coefficient of correlation of .499. However, groups 1 and 2 exhibited much better correlation, with a coefficient of correlation as high as .581 (Figure 1B). The adult group (group 5) consisted of donors 18–39 years of age and exhibited no age-related increase in CES1 mRNA level.
Differential Effect of Age on the Expression of CES1A1 and CES1A2
The CES1 activity is contributed by 2 isoforms: CES1A1 and CES1A2. Although CES1A1 and CES1A2 are distinct genes, they produce the same mature carboxylesterase [15]. To specify the relative expression of CES1A1 and CES1A2, isoform-specific TaqMan probes were used to detect the respective transcripts. The CES1A1 transcript was detected in all samples, but the CES1A2 transcript was detected only in 25% of the samples in group 1 and ∼40% of the samples in other groups. The pediatric samples expressed CES1A1 mRNA at one-half of the adult level, and surprisingly, samples in the pediatric groups expressed higher levels of CES1A2 mRNA than those in the adult group (Figure 1C). CES1A1 and CES1A2 mRNA levels correlated relatively well in the majority of the samples (Figure 1D, inside the circle); however, one-third of the samples showed predominant expression of either CES1A1 or CES1A2 (Figure 1D, outside the circle).
Correlation Between CES1 mRNA and Protein
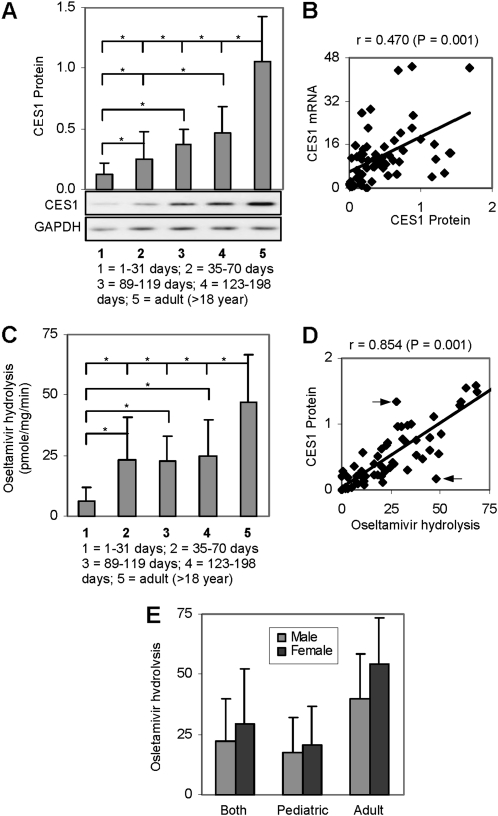
We next performed Western blot analyses and examined whether the levels of CES1 mRNA (CES1A1 and CES1A2 combined) are translated into the levels of protein. As shown in Figure 2A, the patterns of the levels of CES1 protein among various age groups were similar to the patterns of the levels of CES1 mRNA (Figures 1A and 2A). However, there was a difference in the statistical significance. The mRNA levels among groups 2, 3, and 4, although increased with age, showed no statistical significance (Figure 1A). In contrast, the difference in the protein levels of CES1 reached statistical significance between groups 2 and 4 (Figure 2A). Such a discrepancy was likely due to the intrinsic difference between these 2 approaches. RT-qPCR generally has better quantitative power than Western blot analysis. Overall, the protein levels correlated well with the levels of mRNA, and the correlation was statistically significant (Figure 2B).
Figure 2.
Levels of carboxylesterase 1 (CES1) protein and hydrolysis of oseltamivir. A, Western blot analysis S9 fractions (.25 μg) resolved by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis - and transferred electrophoretically to nitrocellulose membranes. The blots were incubated with an antibody against CES1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and developed with chemiluminescent substrate. The signal was captured by a Kodak Image Station 2000, and the relative intensities were quantified by Kodak 1D Image Analysis software. *P < .05. B, Correlation analysis of CES1 protein and messenger RNA (mRNA). C, Hydrolysis of oseltamivir S9 fraction (5 μg) from various age groups incubated with oseltamivir (1 μmol/L) at 37°C for 15 min; the formation of oseltamivir carboxylate was detected by liquid chromatography mass spectrometry. *P < .05. D, Correlation between CES1 protein and hydrolysis of oseltamivir. E, Difference in relative activity toward oseltamivir between samples from male (n = 33) and female (n = 26) donors.
Hydrolysis of Oseltamivir as a Function of Age
Next we examined whether the levels of CES1 protein among various age groups represent the relative activity toward oseltamivir. Consistent with the lowest level of CES1 protein and mRNA in group 1, samples in this group showed the lowest hydrolysis of oseltamivir (Table 2 and Figure 2C). The hydrolysis by this group was 12% of that by the adult group and ∼25% of that by other pediatric groups (Table 2 and Figure 2C). Samples in the other 3 pediatric groups showed comparable hydrolysis of oseltamivir and had one-half of the hydrolytic activity of samples in the adult group (Table 2 and Figure 2C). Among all groups, the largest interindividual variation (1689-fold) was detected in group 2. The second largest interindividual variation was 62-fold, which was detected in group 1 (Table 2). Such a large variation in group 2 was attributed to a single sample with extremely low activity toward oseltamivir, and variability was decreased to 25-fold when this data point was not considered. RT-qPCR and Western blot analysis detected evident expression of CES1 in this sample. Importantly, this sample effectively hydrolyzed p-nitrophenylacetate, a standard substrate for carboxylesterases [17].
We next performed the correlation analysis between CES1 protein and oseltamivir hydrolysis. As expected, the protein level was correlated well with the hydrolytic activity with a coefficient of correlation of .854 (Figure 2D). Indeed, all data points were closely scattered along the correlation line with only 2 exceptions (Figure 2D, arrows). In addition, we tested whether the overall hydrolysis of oseltamivir differs between male and female donors. Interestingly, samples from female donors appeared to show a higher hydrolytic activity in both pediatric and adult groups (Figure 2E). The female-related higher hydrolysis in the adult group was more profound than that in the pediatric groups. However, apparent sex differences were not statistically significant in any age groups.
DISCUSSION
We and other investigators have recently reported that the expression of human carboxylesterases is developmentally regulated [13, 18]. In this study, we analyzed a large number of individual liver samples from pediatric donors (at birth to 198 d of age) and demonstrated a surge in the expression of CES1 during the post-neonatal stage. Samples from donors at 1–31 d of age had the lowest capacity of CES1 on both expression and oseltamivir hydrolysis. In contrast, samples from donors at 35–70 d of age (next age group) showed a 4–7-fold increase in CES1 capacity (Table 2). Three other pediatric groups (groups 2, 3, and 4), consisting of samples from donors of age 35-198 d, exhibited slight increases with age in the expression and hydrolysis. However, the increases were minimal (∼3%) and statistically insignificant (Figures 1A and 2C).
The age-related increases without statistical significance in groups 2, 3, and 4 suggest that the developmental regulation of CES1 occurs in 2 phases. The post-neonatal phase occurs quickly and dramatically (large magnitude in a short period). Such a surged expression ensures the hydrolytic capacity of CES1 to be gained rapidly after birth. The second phase, probably starting at the end of the surged expression, is slow and incremental. In support of this notion, we have previously reported that pediatric donors, largely consisting of those up to 10 years of age, expressed ∼70% of the adult level of CES1 mRNA [15]. Likewise, Zhu et al [18] reported that the level of CES1 protein in donors aged 12–18 years remained slightly lower than that in adult donors. In this study, we have shown that donors at ≥2 months of age had approximately one-half of the adult CES1 capacity. This finding, along with those of previous studies, suggests that the second phase lasts for almost the entire development period (up to 18 years of age) but only accounts for a 50% gain of total CES1 capacity compared with adults.
The precise mechanisms on the post-neonatal surge remain to be determined. There are many changes immediately after birth and in the early days and weeks of life, notably in hormones and food intake. Interestingly, many genes are expressed with a neonatal or post-neonatal surge as is CES1. For example, fibroblast growth factor 21 (FGF21) is induced rapidly and drastically after birth [19]. It was recently reported that the induction was initiated by suckling and required lipid intake. Importantly, the neonatal induction of FGF21 was diminished in mice lacking functional peroxisome proliferator-activated receptor alpha [19]. It happens that this receptor is also implicated in the regulation of the expression of carboxylesterases in rodents [20–22]. Another interesting observation was that the composition of dietary lipids appeared to alter the surged expression of FGF21 [19], pointing to a potential difference between formula milk and breastfeeding.
CES1 has been shown to play critical roles in the metabolism of lipids in CES1 transgenic animals [5]. Therefore, it is conceivable that the post-neonatal surge of CES1 is a functional adaptation to deal with lipid intake. However, CES2 also shows a potential post-neonatal surge in expression [13], but CES2 primarily hydrolyzes drugs and other xenobiotics [1, 2]. In addition to nutrient stimuli, hormonal factors are likely involved in the post-neonatal surge of CES1 and other carboxylesterases [23]. However, some hormones may act independently of age. In this study, we have shown that samples from female donors had higher expression of CES1 and higher hydrolysis of oseltamivir in both pediatric and adult groups, although samples in the adult group showed much greater sex difference than samples in the pediatric group (26% vs 14%, respectively) (Figure 2E).
Like those of many other drug-metabolizing enzymes, the expression and the hydrolytic activity of CES1 exhibited large interindividual variation. Based on the high degree of correlation between the protein level and hydrolysis (r = .854) (Figure 2D), the observed variation in the hydrolysis of oseltamivir likely resulted from differences in the expression. On the other hand, the scattering patterns of the correlation in Figure 2D point to the existence of outliers with potentially large alteration in the catalytic activity toward oseltamivir. It is likely that these represent polymorphic variants of CES1. It should be noted that a single sample in group 2 showed extremely low activity toward oseltamivir, although this individual had evident expression of CES1 (Table 2). Zhu and Markowitz [24] recently reported that 2 CES1 mutations, namely, Gly143Glu and p.Asp260fs, showed impairment in the hydrolysis of oseltamivir. We also reported several CES1 variants with altered hydrolysis of oseltamivir [8]. For example, the variant with an Ser58Asn substitution, compared with the wild-type CES1, had a higher specific rate toward oseltamivir [8]. These findings collectively suggest that certain CES1 variants may differ from the wild-type CES1 in the hydrolysis of oseltamivir, leading to altered hydrolytic activation of this prodrug.
All the pediatric samples except those in group 1 had approximately one-half of the hydrolytic activity of the adult samples, suggesting that these infants can efficiently activate this prodrug. In support of this notion, a retrospective analysis has shown that the outcome of oseltamivir treatment was similar between infants (<1 year old) and older children [12]. As for individuals from birth to 31 d of age (group 1), the clinical effectiveness in the hydrolytic activation remains to be established. Based on the reported time to reach the maximum plasma concentration values of oseltamivir and its hydrolytic metabolite, it is clear that the activation of oseltamivir is achieved primarily during the first pass in the liver [25]. Such a high efficient conversion is due to the high hepatic concentration of oseltamivir right after oral administration. In neonates, the level of CES1 likely becomes a limiting factor. On the other hand, the plasma concentrations of the active metabolite also depend on the rate of elimination, particularly the renal clearance [26].
In summary, our work points to several important conclusions. First, the expression of CES1 surges during the post-neonatal period and such a surge ensures the hydrolytic capacity of this enzyme to be gained rapidly in infants. Second, there exists a large interindividual variability in the expression and hydrolysis of CES1 and the variability is greater in groups 1 and 2 (1–70 d of age). Such large variability during this period suggests that caution should be exercised in the extrapolation of the dose of ester drugs from other age groups. Third, the surge in expression, although it is significant, confers only one-half of the hydrolytic activity of the adult level, pointing to major differences between infants and adults in the overall hydrolytic biotransformation by CES1.
Funding
This work was supported by the National Institutes of Health (grant numbers R01ES07965 and R01GM61988); and Hoffmann–La Roche.
References
- 1.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Sanghani SP, Sanghani PC, Schiel MA, Bosron WF. Human carboxylesterases: an update on CES1, CES2 and CES3. Protein Pept Lett. 2009;16:1207–14. doi: 10.2174/092986609789071324. [DOI] [PubMed] [Google Scholar]
- 3.Yang D, Wang X, Yang D, Yan B. Pyrethroid insecticides: isoform-dependent hydrolysis, induction of cytochrome P450 3A4 and evidence on the involvement of the pregnane X receptor. Toxicol Appl Pharmacol. 2009;237:49–58. doi: 10.1016/j.taap.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow JA, Middleton BL, Borazjani A, Hatfield MJ, Potter PM, Ross MK. Inhibition of carboxylesterase 1 is associated with cholesteryl ester retention in human THP-1 monocyte/macrophages. Biochim Biophys Acta. 2008;1781:643–54. doi: 10.1016/j.bbalip.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei E, Alam M, Sun F, Agellon LB, Vance DE, Lehner R. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J Lipid Res. 2007;48:2597–606. doi: 10.1194/jlr.M700320-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Tang M, Mukundan M, Yang J, et al. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J Pharmacol Exp Ther. 2006;319:1467–76. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- 7.Sanghani SP, Quinney SK, Fredenburg TB, Davis WI, Murry DJ, Bosron WF. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 2004;32:505–11. doi: 10.1124/dmd.32.5.505. [DOI] [PubMed] [Google Scholar]
- 8.Shi D, Yang J, Yang D, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase HCE1 and the activation is inhibited by anti-platelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319:1477–84. doi: 10.1124/jpet.106.111807. [DOI] [PubMed] [Google Scholar]
- 9.Adisasmito W, Chan PK, Lee N, et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a Global Patient Registry. J Infect Dis. 2010;202:1154–60. doi: 10.1086/656316. [DOI] [PubMed] [Google Scholar]
- 10.Lee VJ, Yap J, Cook AR, et al. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. N Engl J Med. 2010;362:2166–74. doi: 10.1056/NEJMoa0908482. [DOI] [PubMed] [Google Scholar]
- 11.Acosta EP, Jester P, Gal P, et al. Oseltamivir dosing for influenza infection in premature neonates. J Infect Dis. 2010;202:563–6. doi: 10.1086/654930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siedler K, Skopnik H. Oseltamivir for treatment of influenza in infants less than one year: a retrospective analysis. Pediatr Infect Dis J. 2010;29:495–8. doi: 10.1097/INF.0b013e3181cc4d01. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Pearce R, Wang X, Gaedigk R, Wan YJY, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–47. doi: 10.1016/j.bcp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Shi D, Yang D, Song X, Yan B. Interleukin-6 suppresses the expression of carboxylesterases HCE1 and HCE2 through transcriptional repression. Mol Pharmacol. 2007;72:686–94. doi: 10.1124/mol.107.036889. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa M, Furihata T, Yaginuma Y, et al. Structural organization and characterization of the regulatory element of the human carboxylesterase (CES1A1 and CES1A2) genes. Drug Metab Pharmacokinet. 2008;23:73–84. doi: 10.2133/dmpk.23.73. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, Song L, Zhang H, Matoney L, LeCluyse E, Yan B. Dexamethasone differentially regulates the expression of carboxylesterase genes in humans and rats. Drug Metab Dispos. 2000;28:186–91. [PubMed] [Google Scholar]
- 17.Shi D, Yang J, Yang D, You L, Yan B. Dexamethasone suppresses the expression of multiple rat carboxylesterases through transcriptional repression: evidence for an involvement of the glucocorticoid receptor. Toxicology. 2008;254:97–105. doi: 10.1016/j.tox.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu HJ, Appel DI, Jiang Y, Markowitz JS. Age- and sex-related expression and activity of carboxylesterase 1 and 2 in mouse and human liver. Drug Metab Dispos. 2009;37:1819–25. doi: 10.1124/dmd.109.028209. [DOI] [PubMed] [Google Scholar]
- 19.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–12. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan EW, Yan B, Greenway D, Parkinson A. Regulation of two rat liver microsomal carboxylesterase isozymes: species differences, tissue distribution and the effects of age, sex and xenobiotic treatment of rats. Arch Biochem Biophys. 1994;315:514–26. doi: 10.1006/abbi.1994.1532. [DOI] [PubMed] [Google Scholar]
- 21.Hosokawa M, Hirata K, Nakata F, Suga T, Satoh T. Species differences in the induction of hepatic microsomal carboxylesterases caused by dietary exposure to di(2-ethylhexyl)phthalate, a peroxisome proliferator. Drug Metab Dispos. 1994;22:889–94. [PubMed] [Google Scholar]
- 22.Poole M, Bridgers K, Alexson SE, Corton JC. Altered expression of the carboxylesterases ES-4 and ES-10 by peroxisome proliferator chemicals. Toxicology. 2001;165:109–19. doi: 10.1016/s0300-483x(01)00416-4. [DOI] [PubMed] [Google Scholar]
- 23.Hosokawa M, Satoh T. Effects of hypophysectomy and pituitary hormones on hepatic microsomal carboxylesterase isozymes in male rats. Res Commun Chem Pathol Pharmacol. 1988;62:279–88. [PubMed] [Google Scholar]
- 24.Zhu HJ, Markowitz JS. Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants. Drug Metab Dispos. 2009;37:264–7. doi: 10.1124/dmd.108.024943. [DOI] [PubMed] [Google Scholar]
- 25.Massarella JW, He GZ, Dorr A, Nieforth K, Ward P, Brown A. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol. 2000;40:836–43. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- 26.Holodniy M, Penzak SR, Straight TM, et al. Pharmacokinetics and tolerability of oseltamivir combined with probenecid. Antimicrob Agents Chemother. 2008;52:3013–21. doi: 10.1128/AAC.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]