Abstract
Background. Some human immunodeficiency virus (HIV)–infected individuals are not able to achieve a normal CD4+ T cell count despite prolonged, treatment-mediated viral suppression. We conducted an intensification study to assess whether residual viral replication contributes to replenishment of the latent reservoir and whether mucosal HIV-specific T cell responses limit the reservoir size.
Methods. Thirty treated subjects with CD4+ T cell counts of <350 cells/mm3 despite viral suppression for ≥1 year were randomized to add raltegravir (400 mg twice daily) or matching placebo for 24 weeks. The primary end points were the proportion of subjects with undetectable plasma viremia (determined using an ultrasensitive assay with a lower limit of detection of <.3 copy/mL) and a change in the percentage of CD38+HLA-DR+CD8+ T cells in peripheral blood mononuclear cells (PBMCs).
Results. The proportion of subjects with undetectable plasma viremia did not differ between the 2 groups (P = .42). Raltegravir intensification did not have a significant effect on immune activation or HIV-specific responses in PBMCs or gut-associated lymphoid tissue.
Conclusions. Low-level viremia is not likely to be a significant cause of suboptimal CD4+ T cell gains during HIV treatment.
Clinical Trials Registration. NCT00631449.
Highly active antiretroviral therapy (HAART) has been effective in decreasing morbidity and mortality associated with human immunodeficiency virus (HIV) infection [1]. However, a significant proportion of individuals are unable to achieve a normal CD4+ T cell count despite prolonged viral suppression with effective HAART. In one study, ∼25% of patients who started HAART at a CD4+ T cell count of <200 cells/mm3 were unable to achieve a CD4+ T cell count of >500 cells/mm3 even after more than 7–10 years of HAART [2]. Moreover, having a suboptimal CD4+ T cell response has been associated with significant clinical consequences, including increased AIDS-related and non–AIDS-related morbidity and mortality [3–6].
The mechanisms of suboptimal immune recovery are not completely understood. Persistent T cell activation may be causally related to the inability to reconstitute normal CD4+ T cell counts, perhaps owing to its effect on lymphoid tissue architecture [7–11]. Moreover, ongoing low-level viral replication may be the proximal cause of persistent activation during HAART [12–15]. Several studies have attempted to address this issue [15–18], although none have focused on the host responses to HIV in both blood and gut-associated lymphoid tissue (GALT).
We conducted a randomized, double-blinded, placebo-controlled study of raltegravir intensification to assess whether ongoing viral replication contributes to immune activation and suboptimal immune recovery during HAART. Our secondary objective was to explore the host determinants of viral persistence in both blood and GALT.
METHODS
Subjects
Thirty HIV-infected, HAART-treated subjects with CD4+ T cell counts of <350 cells/mm3 for ≥1 year despite viral suppression (<40 to 75 copies/mL) for ≥1 year were enrolled in this randomized, placebo-controlled study. In 15 subjects raltegravir (400 mg twice daily) was added to their current suppressive HAART regimens for 24 weeks, and in 15 subjects matching placebo was added for 24 weeks; subjects were randomized to treatment in a double-blinded fashion. Twenty-one of 30 subjects also consented to undergo 3 serial colorectal biopsies. All subjects provided written informed consent. This study was approved by the University of California San Francisco (UCSF) Committee on Human Research. Subjects were seen every 4 weeks. Plasma and peripheral blood mononuclear cells (PBMCs) were stored, and detailed interviews were conducted at most visits. Adherence to study drug was measured at every study visit by self-report and by pill count.
An independent data monitoring committee including 3 individuals from the scientific community met 12, 24, 48, and 60 weeks after the enrollment of the first subject and 60 weeks after the enrollment of the last subject. No significant adverse events occurred during the study. The 2 primary end points were (1) the proportion of subjects with undetectable plasma RNA (<.3 copy/mL assay [19]) at week 12 and (2) a change in the percentage of CD38+HLA-DR+CD8+ T cells in PBMCs, given that immune activation levels have been shown to be a strong and independent predictor of HIV disease progression [7, 8, 20].
The sample size was based on data from prior studies [12, 21]. To achieve 80% statistical power to detect a 35% difference in proportion of subjects with undetectable plasma viremia, 14 subjects would be needed in each group (assuming a type I error of 5%) [22]. Similarly, to achieve 80% statistical power to detect a 5.5% difference in activated CD8+ T cells, 13 subjects would be needed in each group (assuming a type I error of 5%). Our prior work has indicated that a 5.5% difference in activated CD8+ T cells is clinically meaningful; among treated patients with virologic suppression for a median of 2 years, we observed that for every 5% increase in the percentage of activated CD8+ T cells, there was a mean of 35 fewer CD4+ T cells/mm3 gained [7]. We therefore enrolled 15 subjects in each group to account for potential losses to follow-up and early treatment discontinuations.
Virologic Measurements
Plasma RNA was measured at baseline and week 12 with an ultrasensitive assay, with a lower limit of detection of <.3 copy/mL (median, 6.5 mL of plasma) [19]. Cell-associated RNA and proviral DNA was measured from PBMCs at baseline and weeks 4 and 24. The transcription-mediated amplification assay (Aptima; Gen-Probe) was used to measure cell-associated RNA. This assay is a nucleic acid-amplification test that has been approved by the Food and Drug Administration for the early detection of HIV infection in plasma (in the screening of blood donors) and has also been validated for clinical diagnostic use [23]. A modified approach of published methods for PBMC extraction and transcription-mediated amplification of cell-associated hepatitis C virus was used [24, 25]. The output is a signal-cutoff (S/Co) ratio (range, 0–30), with S/Co <1.0 considered HIV RNA “negative” and S/Co ≥1.0 considered “positive.” All S/Co ratios were normalized to the input number of PBMCs.
Total proviral DNA was extracted from PBMCs using modifications of methods described elsewhere [25, 26]. This assay has an overall sensitivity of 1 copy/3 μg of input DNA, equivalent to ∼450,000 PBMCs [27, 28]. All proviral DNA levels were normalized to the input number of PBMCs.
T Cell Immunophenotyping and Cytokine Flow Cytometry
The percentages of CD38+HLA-DR+CD8+ and CD38+HLA-DR+CD4+ T cells and the percentages of HIV Gag-specific CD8+ and CD4+ T cells expressing interferon (IFN) γ and interleukin (IL) 2 were measured in PBMCs at baseline and at weeks 4 and 24. We focused on Gag-specific IFN-γ+IL-2+ T cell responses because these have been associated with control of HIV replication in elite controllers [29–31].
PBMCs were isolated from whole blood, cryopreserved, and stored at the UCSF AIDS Specimen Bank. Markers of T cell activation and antigen-specific T cell responses were measured using flow cytometry at the UCSF Core Immunology Laboratory, according to methods described elsewhere that have been optimized and validated for frozen PBMCs [32]. Frozen PBMCs were rapidly thawed in warm medium, counted on a Guava PCA system with the ViaCount assay (Millipore), and rested overnight (T cell response assays) or washed and stained the same day (T cell immunophenotyping). The average viability of thawed cells was 93% (range, 61%– - 98%; 80% of samples had a viability of >90%).
For T cell immunophenotyping, thawed cells were stained with Aqua Amine Reactive Dye (Invitrogen) to discriminate dead cells, washed, and then stained with the following fluorescently conjugated monoclonal antibodies: CD3–Pacific Blue, CD38–phycoerythrin (PE), HLA-DR–fluorescein isothiocyanate (FITC), CD4–PE–Texas Red, and CD8–Qdot 605 (Invitrogen). In each experiment a fluorescence-minus-one control was included for CD38 and HLA-DR to help determine the cutoff for positive staining. Stained cells were washed, fixed in .5% formaldehyde (Polyscience), and held at 4°C until analysis.
For cytokine flow cytometry (CFC), rested PBMCs were stimulated for 18–22 h at 37°C with overlapping peptide pools corresponding to HIV-1 Con B Gag peptides (NIH 8117) in the presence of .5 μg/mL brefeldin A and .5 μg/mL monensin (Sigma-Aldrich). A control well with no stimulation was tested in parallel for each sample. After stimulation, cells were washed and stained with Aqua Amine Reactive Dye, fixed, and then permeabilized for intracellular staining with antibodies against CD3–Pacific Blue, IFN-γ–FITC, IL-2–PE (all BD BioScience), CD4–PE–Texas Red, and CD8-Qdot 605 (both Invitrogen). Cells were then washed and stored at 4°C until analysis.
Stained cells were analyzed on a customized BD LSR II flow cytometer; 100,000 and 500,000 lymphocytes were collected for immunophenotyping and CFC samples, respectively. Data were compensated and analyzed using FlowJo software version 9.0.2 (Tree Star) to determine the proportions of CD4+ and CD8+ T cells expressing each of the T cell or cytokine markers. Combinations of markers were calculated with FlowJo software using the Boolean gate function. For CFC data, results from control wells with no stimulation were subtracted from results obtained with stimulation.
Gut-Associated Lymphoid Tissue
Colorectal biopsies were performed in 21 of 30 subjects at 2 weeks before baseline, and weeks 6 and 22. At each of the 3 visits, 30 biopsy specimens were obtained with 3-mm jumbo forceps, approximately 10–15 cm from the anal verge. Eighteen of 30 biopsy specimens were processed on the same day for measurement of T cell activation (percentages of CD38+HLA-DR+CD8+ and CD38+HLA-DR+CD4+ T cells) or rested overnight and assayed for total Gag-specific CD8+ and CD4+ T cells (all Gag-specific CD8+ and CD4+ T cells expressing IFN-γ, IL-2, tumor necrosis factor (TNF) α, or CD107a). We focused on mucosal T cell responses because they have been associated with control of HIV replication in elite controllers [33].
Samples were placed directly into 10 mL of Roswell Park Memorial Institute 1640 medium supplemented with fetal calf serum (15%), penicillin (100 U/mL), streptomycin (100 μg/mL), glutamine (2 mmol/L), and piperacillin-tazobactam (.5 mg/mL) (Zosyn; Wyeth) (R15 medium). Rectal mononuclear cells (RMCs) were isolated from biopsy specimens according to a published protocol that was optimized for high yield and viability of mucosal lymphocytes without compromising the detection of most surface antigens [34]. Briefly, biopsy specimens underwent 3 rounds of digestion in .5 mg/mL collagenase type II (Sigma-Aldrich). Each round was followed by disruption of the tissue with a syringe bearing a 16-gauge blunt-end needle and subsequent passage through a 70-μm cell strainer. Yields ranged from 4 to 21 × 106 RMC from 18 biopsy specimens.
For cell surface immunophenotyping of freshly isolated RMCs, methods were similar to those used for PBMCs [34]. For CFC, freshly isolated RMCs were rested overnight at 37°C and 5% CO2 in R15 medium containing .5 mg/mL piperacillin-tazobactam to discourage bacterial growth, and then similar methods were used as for PBMCs [34]. The expression of IFN-γ, IL-2, TNF-α, and/or CD107a was measured using a modification of methods described elsewhere [35, 36]. To account for fewer events and elevated baseline cytokine staining in mucosal samples, response data from peptide-stimulated wells were first compared against data from unstimulated controls using an algorithm to determine statistical significance, described elsewhere, before background subtraction [33, 37].
Statistical Methods
Baseline characteristics were compared using the Wilcoxon rank sum test or Fisher's exact test. Generalized estimating equations were calculated to estimate the mean change in plasma RNA, cell-associated RNA, proviral DNA, immune activation (PBMCs and GALT), and HIV-specific responses (PBMCs and GALT) over time. Interaction terms were created to assess whether these changes over time differed significantly between the raltegravir and placebo groups. All statistical analyses were conducted with Stata software (version 9.2; Stata Corp).
RESULTS
Forty-one subjects were screened to enroll 30 subjects; 11 subjects were excluded from the study because the screening CD4+ T cell count was > 350 cells/mm3, plasma HIV RNA was > 75 copies/mL, concurrent corticosteroid or growth hormone use, or patient preference. All 30 completed the study. At baseline, the median age for all 30 subjects was 49 years, the median duration of HIV infection was 17.5 years, and the median duration of HAART suppression was 2.6 years (Table 1). The median baseline CD4+ T cell count was 232 cells/mm, and the nadir count was 53 cells/mm3. The median adherence to study drug was 100% by self-report and 98% by pill count; there were no statistically significant differences in adherence between the 2 groups.
Table 1.
Baseline Characteristics for Subjects in Raltegravir and Placebo Treatment Groupsa
| Baseline Characteristic | Raltegravir (n = 15) | Placebo (n = 15) | P |
| Age, median, years | 48 | 49 | .72 |
| Sex | 15 male | 14 male, 1 MTF | |
| CD4+ T cell count, median, cells/mm3 | 233 | 231 | .56 |
| Nadir CD4+ T cell count, median, cells/mm3 | 17 | 90 | .13 |
| Plasma HIV-1 RNA, median, copies/mLb | 2.6 | 5.5 | .52 |
| Time since HIV diagnosis, median, years | 15 | 18 | .23 |
| Duration of HAART suppression, median, years | 3.1 | 2.1 | .76 |
| Treatment with PI-containing HAART, no. of subjects | 10/15 | 8/15 | .71 |
| HCV coinfection, no. of subjects | 4/15c | 4/15 | 1.00 |
NOTE. aHAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type 1; MTF, male-to-female transgender subject; PI, protease inhibitor.
Plasma HIV-1 RNA levels as detected by an ultrasensitive assay (lower limit of detection, <.3 copy/mL).
c Includes 1 subject who was HCV Ab positive but had undetectable HCV RNA levels after completion of treatment with pegylated interferon and ribavarin, >5 years before study entry.
Ultrasensitive Plasma RNA Assay
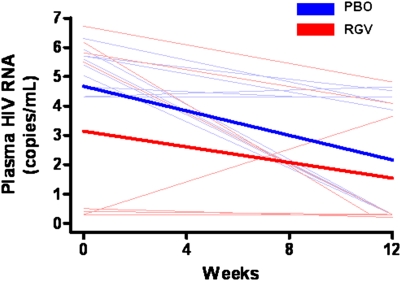
At baseline, 19 of 28 subjects had plasma RNA detectable with an ultrasensitive assay (lower limit of detection, <.3 copy/mL) (7 subjects receiving raltegravir, 12 receiving placebo; P = .103); for 2 subjects the internal standard failed. The median baseline plasma RNA levels were 2.6 and 5.5 copies/mL for the raltegravir and placebo groups, respectively (P = .52). The proportions of subjects with undetectable plasma RNA at week 12 did not differ significantly between the raltegravir and placebo groups (73% vs 54%, respectively, P = .42). Both groups had a significant decrease in plasma RNA at week 12 (raltegravir, −1.6 copies/mL [P < .05]; placebo, −2.5 copies/mL [P < .01]) (Figure 1), but raltegravir intensification did not decrease plasma RNA more than placebo (P = .39).
Figure 1.
Plasma human immunodeficiency virus (HIV) RNA (as determined by ultrasensitive assay with a lower limit of detection of <.3 copy/mL [19]). Both raltegravir (RGV) (red) and placebo (PBO) (blue) groups had significant decreases in plasma RNA at week 12 (raltegravir, −1.6 copies/mL [P < .05]; placebo, −2.5 copies/mL [P < .01]). However, raltegravir intensification did not decrease plasma RNA more than placebo (P = .39). Thin lines represent raw data for each subject; thick lines, estimated mean values over time from generalized estimating equations for each group. Eight time points at which the internal standard for the assay failed were excluded from analyses and from the figure. “Undetectable” outcomes were assigned the lower limit of the assay (.3 copy/mL).
Cell-Associated RNA and Proviral DNA
Baseline proviral DNA levels in PBMCs for the raltegravir and placebo groups were 15 and 23 copies/1 million PBMC, respectively. Compared with placebo, raltegravir intensification did not have a significant effect on cell-associated RNA (P = .90) or proviral DNA (P = .60) levels.
CD4+ T Cell Counts and T cell Activation
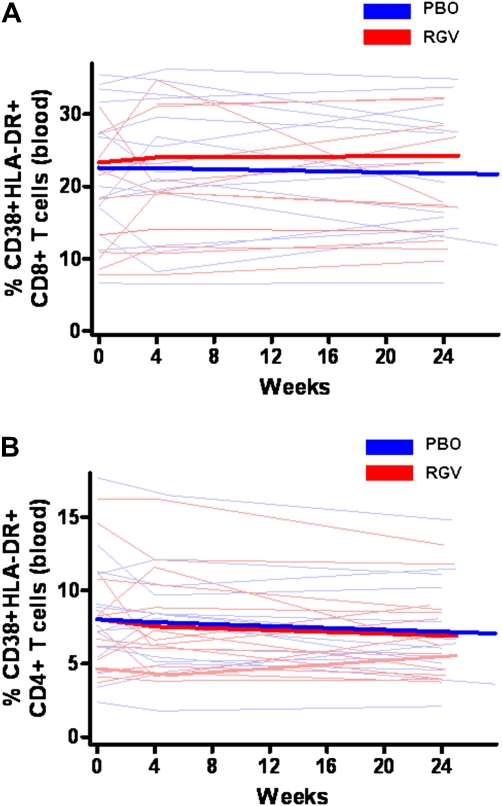
After 24 weeks of intensification, peripheral CD4+ T cell count increased by 19 cells/mm3 in the raltegravir group and 36 cells/mm3 in the placebo group (P = .28). The baseline percentage of CD38+HLA-DR+CD8+ T cells in PBMCs was elevated in both groups (22.3% for raltegravir and 20% for placebo, compared with a median percentage of 1% among HIV-uninfected persons found in another study from our group [7]). Raltegravir intensification had no effect on CD8+ T cell activation (P = .33) (Figure 2A). The baseline percentage of CD38+HLA-DR+CD4+ T cells was also elevated in both groups (7.6% for raltegravir, 7.2% for placebo), compared with a median of 1% among HIV-uninfected persons [7]. Both groups had a significant decrease in CD4+ T cell activation in PBMCs (raltegravir, −1.1% [P = .02]; placebo, −.9% [P = .02]) (Figure 2B). However, raltegravir intensification did not decrease CD4+ T cell activation more than placebo (P = .67).
Figure 2.
Percentages of CD38+HLA-DR+CD8+ and CD38+HLA-DR+CD4+ T cells in peripheral blood mononuclear cells (PBMCs). RGV, raltegravir (red); PBO, placebo (blue). Thin lines represent raw data for each subject; thick lines, estimated mean values over time from generalized estimating equations for each group. A, Raltegravir intensification had no effect on CD8+ T cell activation in PBMCs (P = .33). B, Both groups had significant decreases in CD4+ T cell activation in PBMCs (raltegravir, −1.1%, [P = .02]; placebo, −.9% [P = .02]), but raltegravir intensification did not decrease CD4+ T cell activation in PBMCs more than placebo (P = .67).
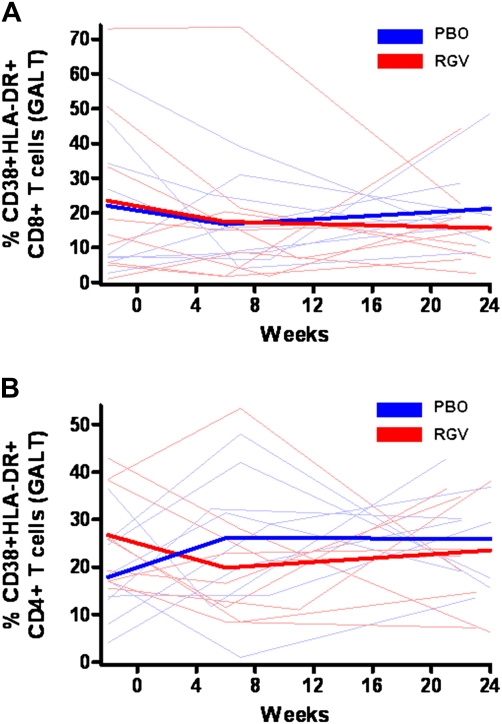
The baseline percentages of CD38+ HLA-DR+CD8+ T cells in GALT were 14% and 8% in the raltegravir and placebo groups, respectively. Raltegravir intensification had no effect on CD8+ T cell activation in GALT (P = .53) (Figure 3A). The baseline percentages of CD38+HLA-DR+CD4+ T cells in GALT were 26% and 17% in the raltegravir and placebo groups, respectively. Initially, CD4+ T cell activation levels in GALT seemed to decrease in the raltegravir group, but this finding did not reach statistical significance at week 6 (P = .08) or week 22 (P = .14) (Figure 3B).
Figure 3.
Percentages of CD38+HLA-DR+CD8+ and CD38+HLA-DR+CD4+ T cells in gut-associated lymphoid tissue (GALT). RGV, raltegravir (red); PBO, placebo (blue). Thin lines represent raw data for each subject; thick lines, estimated mean value over time from generalized estimating equation for each group. Raltegravir intensification had no effect on CD8+ (A) (P = .53) or CD4+ (B) (P = .14) T cell activation in GALT.
HIV-Specific Responses
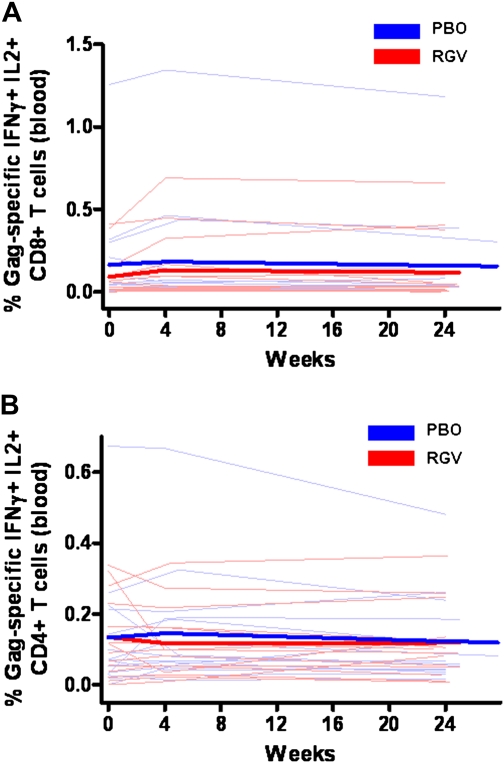
We hypothesized that ongoing viral replication may stimulate higher HIV-specific T cell responses and that raltegravir intensification might decrease this antigen-specific response. The baseline percentages of Gag-specific IFN-γ+IL2+CD8+ T cells in PBMCs were .03% and .05% in the raltegravir and placebo groups, respectively, and the corresponding baseline percentages for Gag-specific IFN-γ+IL2+CD4+ T cells were .2% and .07%, respectively. Raltegravir intensification had no significant effect on Gag-specific IFN-γ+IL2+CD8+ (P = .19) (Figure 4A) or IFN-γ+IL2+CD4+ (P = .80) (Figure 4B) T cell responses.
Figure 4.
Percentages of Gag-specific CD8+ and CD4+ T cells expressing interferon (IFN) γ and interleukin (IL) 2 in peripheral blood mononuclear cells (PBMCs). RGV, raltegravir (red); PBO, placebo (blue). Thin lines represent raw data for each subject; thick lines, estimated mean values over time from generalized estimating equations for each group. Raltegravir intensification had no effect on Gag-specific IFN-γ+IL2+CD8+ (A) (P = .19) or IFN-γ+IL2+CD4+ (B) (P = .80) T cell responses in PBMCs.
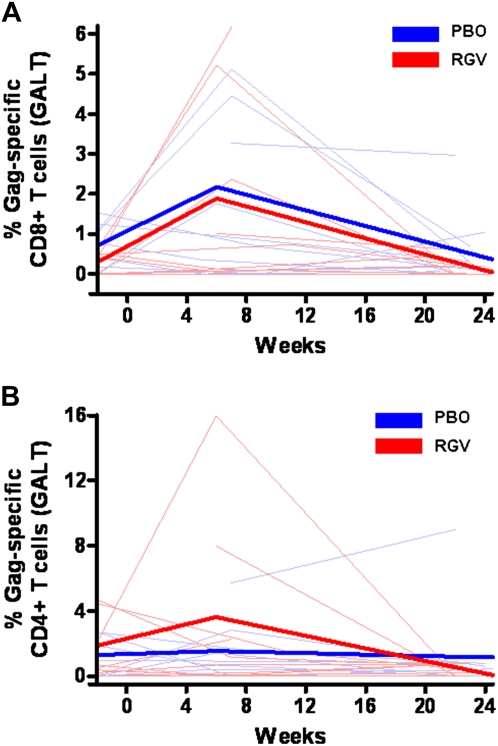
The baseline percentages for CD8+ total Gag-specific responses (IFN-γ, IL-2, TNF-α, or CD107a) in GALT were .41% and .77% in the raltegravir and placebo groups, respectively, and the corresponding baseline percentages for CD4+ total Gag-specific responses were .59% and 0%. Raltegravir intensification had no effect on total Gag-specific CD8+ (P = .91) (Figure 5A) or CD4+ (P = .30) (Figure 5B) T cell responses in GALT.
Figure 5.
Percentages of Gag-specific interferon (IFN) γ, interleukin (IL) 2, tumor necrosis factor (TNF) α, or CD107a CD8+ and CD4+ T cell responses in gut-associated lymphoid tissue (GALT). RGV, raltegravir (red); PBO, placebo (blue). Thin lines represent raw data for each subject; thick lines, estimated mean values over time from generalized estimating equations for each group. Raltegravir intensification had no effect on Gag-specific IFN-γ, IL-2, TNF-α, or CD107a CD8+ (A) (P = .91) or CD4+ (B) (P = .30) T cell responses in GALT.
Mucosal HIV-Specific Responses and Viral Persistence
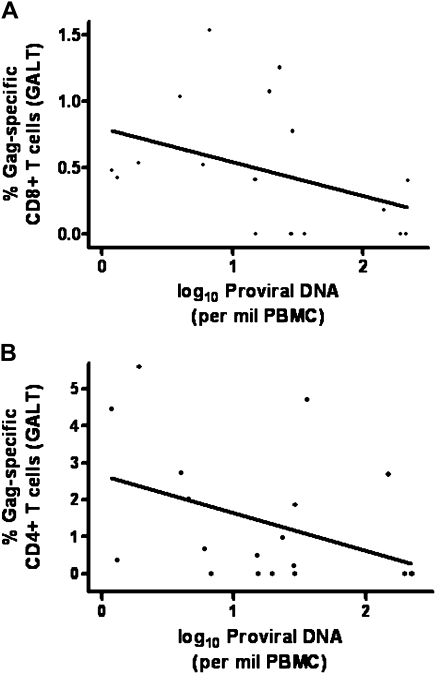
Our secondary objective was to explore the host determinants of viral persistence. We hypothesized that HIV-specific T cell responses in GALT may be a critical determinant of the size of the latent reservoir, and we conducted cross-sectional analyses between HIV-specific T cell responses in GALT and measures of viral persistence in PBMCs. We found that higher baseline CD8+ total Gag-specific responses (IFN-γ, IL-2, TNF-α, or CD107a) in GALT were associated with lower levels of baseline proviral DNA in PBMCs (Spearman's ρ = −.50; P = .03) (Figure 6). Similar findings were observed with baseline CD4+ total Gag-specific responses (IFN-γ, IL-2, TNF-α, or CD107a) in GALT and baseline proviral DNA levels in PBMCs, although these associations did not reach statistical significance (Spearman's ρ= −.42; P = .07). There were no significant associations between baseline HIV-specific T cell responses in PBMCs and measures of viral persistence in PBMCs.
Figure 6.
Possible associations between baseline human immunodeficiency virus (HIV)–specific responses in gut-associated lymphoid tissue (GALT) and baseline measures of viral persistence. A, Higher percentages of Gag-specific interferon (IFN) γ, interleukin (IL) 2, tumor necrosis factor (TNF) α, or CD107a CD8+ T cells in GALT were associated with lower levels of proviral DNA in peripheral blood mononuclear cells (PBMCs) (Spearman's ρ = −.50; P = .03). Gag-specific IFN-γ, IL-2, TNF-α, or CD107a CD8+ T cell responses and proviral DNA levels were measured at baseline (figure includes subjects in both raltegravir and placebo groups). B, There appeared to be a possible association between Gag-specific IFN-γ, IL-2, TNF-α, or CD107a CD4+ T cell responses in GALT and proviral DNA in PBMCs, but this association did not reach statistical significance (Spearman's ρ = −.42; P = .07). Gag-specific IFN-γ, IL-2, TNF-α, or CD107a CD4+ T cell responses and proviral DNA levels were measured at baseline (figure includes subjects in both raltegravir and placebo groups).
DISCUSSION
There remains an ongoing debate as to whether viral replication persists in the context of suppressive HAART [12, 15,38–40]. This question is critical in the continuing efforts toward viral eradication, and multiple studies, including raltegravir intensification studies, have attempted to address the issue [15–18].
In this study, raltegravir intensification for 24 weeks did not decrease plasma RNA more than placebo. This finding is consistent with those in 2 other recent studies of raltegravir intensification [16, 18]. However, several important differences deserve comment. Our study focused on immunologic nonresponders, and the duration of intensification was longer (24 weeks) than in the other studies. We found that both groups had a significant decrease in plasma RNA, probably because subjects in each group were in a relatively early phase of viral suppression (2.6 years) [41]. More importantly, and in contrast to other studies, we focused on the host responses to HIV in both the blood and the gut, assuming that these responses might be a more sensitive measure of low-level viral replication [20]. We hypothesized that higher levels of viral replication would result in higher levels of T cell activation and perhaps higher levels of HIV-specific T cell responses, with these effects being most evident in the GALT, which is thought to be the major reservoir of HIV during HAART [42, 43]. Our data consistently failed to reveal any effect of raltegravir intensification on any of these measurements.
However, we acknowledge that our study did not fully rule out the presence of ongoing viral replication in the context of effective HAART. Subjects in our study were not required to have detectable plasma RNA at baseline; indeed, only 19 of 28 subjects had plasma RNA that was detectable at baseline with an ultrasensitive assay (lower limit of detection, <.3 copy/mL). Thus, it is possible that had we selected only subjects with plasma RNA detectable at baseline with an ultrasensitive assay, we may have observed a decrease in plasma viremia with raltegravir intensification. However, results from such a study were recently published and did not show an effect with intensification [16]. Moreover, additional studies, including measurement of 2–long terminal repeat circles [15] and measurement of viral persistence in other tissues, such as GALT, are necessary to completely rule out the presence of ongoing viral replication [43]. In a recent randomized clinical trial of raltegravir intensification, Buzón and colleagues found that raltegravir may have an effect on both virus and T cell dynamics in individuals who are taking a protease inhibitor as part of their HAART regimen [15]. Of note, we observed similar findings in our study. When we limited our analysis of residual viremia to subjects taking a protease inhibitor as part of their HAART regimen, the proportion of subjects with undetectable plasma RNA at week 12 was higher in the raltegravir group than in the placebo group (100% vs 44%, respectively; P = .04). These data suggest that residual viral replication may occur in an anatomic compartment that is less accessible to protease inhibitors. Further studies will be necessary to confirm these findings.
Another limitation of this study, and of this particular area of research, is the lack of uniformity in the definition of “immunologic nonresponders.” Although we examined subjects with CD4+ T cell counts of <350 cells/mm3 despite viral load suppression for ≥1 year, it is possible and indeed likely that the biology associated with having a low CD4+ T cell count during relatively early HAART may prove to be different from that in long-term, immunologic nonresponders. This is another area that requires further study.
Immunologic nonresponders may have a larger latent reservoir than responders, and most of this reservoir—a disproportionate amount—resides in the gut [42, 43]. Moreover, immunologic nonresponders may have insufficient or ineffective HIV-specific responses in this critical location where most of the residual virus exists. The degree of immune reconstitution after HAART has been shown to be related to the degree of local fibrosis in lymph nodes and the gut [10, 11]. Immunologic nonresponders may be caught in a continuous, self-sustaining cycle of increased immune activation, greater damage and fibrosis to the gut, and increased microbial translocation [44, 45]. To elucidate further the determinants of immunologic nonresponse, cross-sectional studies comparing the size of the latent reservoir and HIV-specific mucosal responses in immunologic responders and nonresponders will be necessary.
Although multiple studies have defined the immunologic correlates of virus control in untreated persons, to our knowledge there has not been an extensive study examining the immunologic correlates of viral persistence in the context of long-term antiretroviral therapy. In our study, we considered the level of viremia and cell-associated virus in the peripheral blood as well as other possible determinants, including the level of immune activation and the magnitude of HIV specific T cell responses. In a secondary analysis, we observed negative correlations between mucosal HIV-specific T cell responses and measurements of the cellular reservoir; however, these findings are preliminary and require confirmation by other, larger studies. Although the mechanisms accounting for these associations cannot be fully addressed in this cross-sectional analysis, strong mucosal T cell responses might contribute to rapid clearance of virus during HAART, and presumably this is testable. Moreover, this hypothesis could coexist with a scenario in which HIV-infected cells continue to produce virus but are susceptible to immune clearance. Approaches aimed at expanding HIV-specific CD4+ and CD8+ T cell responses in the gut mucosa may accelerate clearance of the viral reservoir. The next logical step would be to pursue therapeutic vaccine studies using HIV vaccines that elicit strong mucosal T cell responses in HAART-treated patients.
In summary, we found that suboptimal CD4+ T cell gains during long-term HAART are not likely to be due to low-level ongoing viral replication, at least as detected in peripheral blood. Other therapeutic options for this challenging patient population will be needed. In the context of complete or near complete viral suppression, mucosal HIV-specific T cell responses may be a determinant of the size of the latent reservoir. This latter observation provides support for future studies aimed at using therapeutic vaccines to affect the size of the reservoir [46].
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers K23AI075985, K24AI069994, AI052745, AI055273, RR16482, R01 AI087145, R01 AI057020), the American Foundation for AIDS Research (grant number 106710-40-RGRL), the Universitywide AIDS Research Program (grant number CC99-SF-001), the UCSF/Gladstone Institute of Virology and Immunology Center for AIDS Research (grant number P30 AI027763), the Clinical Research Center at San Francisco General Hospital, supported by the UCSF Clinical and Translational Research Institute Clinical Research Center (grant number UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246), and the Center for HIV/AIDS Vaccine Immunology (grant number U01-AI067854). Mucosal samples were analyzed in a facility constructed with support from NCRR (grant number C06 RR - 12088). S.P. was funded, in part, by the Swedish Research Council and by the American Foundation for AIDS Research (grant number 107170-44-RGRL). J.M.M. is a recipient of the National Institutes of Health (NIH) Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research (grant number DPI OD00329).
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DM, Hogg RS, Chan K, Tyndall M, Yip B, Montaner JS. Disease progression in patients with virological suppression in response to HAART is associated with the degree of immunological response. AIDS. 2006;20:371–7. doi: 10.1097/01.aids.0000196180.11293.9a. [DOI] [PubMed] [Google Scholar]
- 4.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Martin JN, Ssewanyana I, et al. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections (San Francisco, CA) 2010. Impact of CD8+ T cell activation on CD4+ T cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy [abstract 306] [Google Scholar]
- 9.Anthony KB, Yoder C, Metcalf JA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–33. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–71. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 11.Estes J, Baker JV, Brenchley JM, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–64. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havlir DV, Strain MC, Clerici M, et al. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J Virol. 2003;77:11212–9. doi: 10.1128/JVI.77.20.11212-11219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2005;191:348–57. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 14.Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4:e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzón MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:373–4. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–60. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 21.Deeks SG, Hoh R, Neilands TB, et al. Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J Infect Dis. 2005;192:1537–44. doi: 10.1086/496892. [DOI] [PubMed] [Google Scholar]
- 22.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd ed. Oxford University Press; 2002. [Google Scholar]
- 23.Nugent CT, Dockter J, Bernardin F, et al. Detection of HIV-1 in alternative specimen types using the APTIMA HIV-1 RNA Qualitative Assay. J Virol Methods. 2009;159:10–4. doi: 10.1016/j.jviromet.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernardin F, Tobler L, Walsh I, Williams JD, Busch M, Delwart E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology. 2008;47:1446–52. doi: 10.1002/hep.22184. [DOI] [PubMed] [Google Scholar]
- 25.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee TH, el-Amad Z, Reis M, et al. Absence of HIV-1 DNA in high-risk seronegative individuals using high-input polymerase chain reaction. AIDS. 1991;5:1201–7. doi: 10.1097/00002030-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Chafets DM, Reed W, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006;46:1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 29.Emu B, Sinclair E, Favre D, et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005;79:14169–78. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–46. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 33.Ferre AL, Hunt PW, Critchfield JW, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shacklett BL, Yang O, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 35.Critchfield JW, Lemongello D, Walker DH, et al. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–71. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 37.Critchfield JW, Young DH, Hayes TL, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 39.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–57. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton PA, Mitsuyasu RT, Deeks SG, et al. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS. 2003;17:53–63. doi: 10.1097/00002030-200301030-00008. [DOI] [PubMed] [Google Scholar]
- 43.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 44.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 46.Autran B, Debre P, Walker B, Katlama C. Therapeutic vaccines against HIV need international partnerships. Nat Rev Immunol. 2003;3:503–8. doi: 10.1038/nri1107. [DOI] [PubMed] [Google Scholar]