Abstract
Objective. We investigated coinfection patterns for 25 human papillomavirus (HPV) types and assessed the risk conferred by multiple HPV types toward cervical disease.
Methods. Sexually active women (n=5,871) in the NCI-sponsored Costa Rica HPV Vaccine Trial's prevaccination enrollment visit were analyzed. Genotyping for 25 HPVs was performed using SPF10/LiPA25. We calculated odds ratios (ORs) to assess coinfection patterns for each genotype with 24 other genotypes. These ORs were pooled and compared with pair-specific ORs to identify genotype combinations that deviated from the pooled OR. We compared risk of CIN2+/HSIL+between multiple and single infections and assessed additive statistical interactions.
Results. Of the 2478 HPV-positive women, 1070 (43.2%) were infected with multiple types. Multiple infections occurred significantly more frequently than predicted by chance. However, this affinity to be involved in a coinfection (pooled OR for 300 type-type combinations=2.2; 95% confidence interval [CI]=2.1-2.4) was not different across HPV type-type combinations. Compared with single infections, coinfection with multiple α9 species was associated with significantly increased risk of CIN2+(OR=2.2; 95% CI=1.1–4.6) and HSIL+(OR=1.6; 95% CI=1.1–2.4). However, disease risk was similar to the sum of estimated risk from individual types, with little evidence for synergistic interactions.
Conclusions. Coinfecting HPV genotypes occur at random and lead to cervical disease independently.
Cervical coinfection with more than 1 human papillomavirus (HPV) genotype is common, especially among young women [1–6]. Given the common sexual mode of transmission of genital HPV infections, women infected with 1 HPV type are more likely to harbor additional genotypes [2–7]. Nonetheless, it is unclear whether any 2 HPV types are more or less likely to be involved in a coinfection than would be expected by the sexual transmission. This question is particularly important given the ability to prevent infections with certain HPV types through prophylactic HPV vaccination [8–10]. Theoretically, vaccination could indirectly either increase or decrease the prevalence of HPV types not targeted by the vaccine [5, 6, 11]. Current evidence from vaccine trials, however, indicates no type-replacement and some evidence of cross-protection for phylogenetically related HPV types [12–15]. Additionally, few studies have formally evaluated synergistic interactions across coinfecting HPV types on cervical disease risk [16–18].
Given the large number of genital HPV types [19], addressing the epidemiology of HPV coinfections requires large studies. In the current study within the National Cancer Institute-sponsored Costa Rica Vaccine trial's (CVT) prevaccination enrollment visit, we cross-sectionally evaluated the epidemiology of multiple cervical HPV infections among 5871 women aged 18–25 years [20]. We specifically investigated coinfection patterns for 25 HPV genotypes and evaluated the risk conferred by multiple HPV types toward cervical disease.
MATERIALS AND METHODS
The design and methods of CVT have been described elsewhere [20]. Briefly, CVT is a community-based double-blind randomized phase III trial aimed at evaluating the efficacy of HPV 16/18 bivalent vaccine in preventing cervical cancer precursors. The trial recruited 7466 women aged 18– 25 years from the Guanacaste and Puntarenas provinces, Costa Rica. Women were randomized into either the HPV vaccine arm or the hepatitis A vaccine control arm using a 1:1 ratio [20]. The current report uses cross-sectional data; because all specimens were collected at the enrollment visit before vaccination, we included women randomized to both the HPV vaccine arm and the control arm into this analysis.
Women who were sexually active at enrolment (n = 5871) provided demographic, medical and reproductive history, and risk factor information and underwent a pelvic examination. The 1595 women who reported no sexual activity did not have cervical exams, as specified in the protocol, and were excluded from the current analysis. Exfoliated cervical cells were collected in Preservcyt liquid medium (Hologic, Inc.) for ThinPrep Pap test and HPV DNA testing. Liquid-based cytology testing was performed in Costa Rica and results were classified using the Bethesda system. For quality control purposes, all abnormal results as well as 10% of randomly selected normal results were reinterpreted in the United States. Abnormal Pap test results indicative of high-grade disease were further evaluated through colposcopically-directed biopsies. HPV DNA testing was performed at Delft Diagnostic Laboratory (Voorburg, The Netherlands) using the broad-spectrum polymerase chain reaction (PCR)-based HPV SPF10/DEIA/LiPA25 system (version 1; Labo Bio-Medical Products, Rijswijk, The Netherlands). All samples were run through an HPV DNA enzyme immunoassay (DEIA), and DEIA positive samples were genotyped by the linear probe assay (LiPA25) for 25 HPV types (6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68–73, 70, and 74) [20, 21]. Additionally, SPF10/DEIA positive but HPV16/18 LiPA25 negative samples were subjected to HPV16/18 type-specific PCR.
Statistical Analyses
A woman infected with more than 1 of the 25 HPV types was considered to have multiple infections. We assessed predictors of multiple infections versus single HPV infections using binary logistic regression. We initially evaluated whether the number of coinfecting HPV types in a woman represented independent infections by comparing the observed frequency of number of infections per woman with the null frequency expected by chance. Under independence, expected frequencies for the number of coinfecting HPV types in a woman would arise from a Poisson distribution (ie, having 1 HPV infection would neither increase nor decrease the probability of another infection). We then calculated observed/expected ratios and exact 95% Poisson confidence intervals (CIs). Because a Poisson distribution prescribes an equal mean and variance, we quantified the degree of departure from independence for the number of coinfecting types (unadjusted and adjusted for number of lifetime sex partners) by calculating a variance inflation factor (VIF, calculated as the Pearson χ2 divided by the degrees of freedom). VIF values >1.0 would indicate that multiple infections occurred more than expected by chance, whereas values <1.0 would indicate less than expected multiple infections. Given the probability of concurrent transmission of more than 1 HPV type between partners and the common mode of transmission, we anticipated that occurrence of multiple infections would not follow a Poisson distribution and would be more than expected by chance.
We conducted analyses to investigate whether any 2 genotypes were more or less likely to occur in multiple infections when compared with all other genotype combinations. Using each of the genotypes targeted by currently available vaccines—16, 18, 6, and 11—as the predictor variable, we calculated odds ratios (OR) for coinfection with 24 other HPV types after adjustment for age, number of lifetime sex partners, and smoking. Because occurrence of multiple infections was significantly higher than expected by chance, the OR for any pair of HPV types would be >1.0 [2]. Therefore, separately for HPV types 6, 11, 16, and 18, we calculated a fixed-effects common underlying (pooled) OR by averaging the 24 pair-specific ORs weighted by the inverse of the variance of each OR. This pooled OR represents the underlying affinity of 16, 18, 6, or 11 to be involved in a coinfection with another HPV type. To assess whether any particular pair of genotypes deviated from the pooled OR, we calculated the difference (on a log scale) between the pair-specific OR and the pooled OR. Because each woman could contribute more than 1 infection, we used 200 bootstrap replications to correct the variance estimates for the pooled OR as well as for the differences between pair-specific ORs and the pooled OR. We repeated the above analyses for all 300 possible pair combinations over 25 HPV types (coinfection of each type with 24 other HPV types), except that these ORs were unadjusted for risk factors.
We evaluated risk of cervical disease conferred by multiple HPV infections when compared with single infections (reference group) using binary logistic regression, with histology (cervical intraepithelial lesion grade 2 or worse [CIN2+] vs. < CIN2) or cytology (high-grade squamous intraepithelial lesions or worse [HSIL+] vs. <HSIL) as the dependent variable. The CIN2+ analyses used consensus results from Costa Rica and U.S. reviews, whereas the HSIL+ analyses used results from the cytology review conducted in the United States. In addition to high-grade lesions, carcinoma in situ, and invasive cervical cancers, atypical squamous cells suggestive of high-grade disease as well as rare glandular abnormalities were considered as HSIL+. The main predictor variables were: coinfection status (multiple vs. single types); coinfection with oncogenic types (multiple oncogenic vs. single oncogenic; oncogenic HPV types defined as HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68–73); coinfection with oncogenic types excluding HPV16; coinfection with more than 1 type belonging to α9 species (multiple α9 species with or without other types vs. single α9 species with or without other types); coinfection with more than 1 type belonging to α7 species (as defined above); coinfection of HPV16 with other types (HPV16 coinfections with other types vs. single HPV16); and coinfection of HPV18 with other types (HPV18 coinfection with other types vs. single HPV18). All models incorporated adjustment for age; number of lifetime sex partners; number of pregnancies; and smoking, which were selected for adjustment a priori as well as based on significant univariate associations with CIN2+ and HSIL+. Finally, for α9 genotypes, we assessed evidence for type-type additive statistical interactions on risk of CIN2+ or HSIL+ by computing synergy indices and 95% confidence intervals [22]. For example, for HPV16 and HPV31, the synergy index was calculated as [exp(b1+b2+b3)−1]/[(exp(b1)+exp(b2)− 2], where b1 = main effect of HPV16, b2 = main effect of HPV31, and b3 = coefficient for cross-product term between HPV16 and HPV31 in a logistic regression model.
Because of the large number of statistical comparisons, we used Bonferroni-corrected P-value thresholds to assess statistical significance of coinfection patterns: P < .002 (.05/24) for coinfection of HPV types 16, 18, 6, and 11 and P < .0001 (.05/300) for all possible coinfection patterns. Analyses of CIN2+ and HSIL+ used a P-value of .05. All analyses were 2-sided.
RESULTS
The study included 5871 of 7466 women who were sexually active at enrollment. HPV prevalence at enrollment was 50.0% (2938/5871) and at least 1 of the 25 genotypes was detected among 42.3% (2478/5871) of women. Prevalence of oncogenic HPV types was 33.8% (1983/5871). Multiple HPV infections were observed among 18.2% of women (1070/5871).
Women with increased number of lifetime sex partners (eg, OR for >5 partners vs. 1 partner = 2.05; 95% CI = 1.41–2.97) and current smokers (OR vs. never smokers = 1.41; 95% CI = 1.10–1.82) were more likely to harbor multiple HPV infections (Table 1). Within this group of young women, age, marital status, number of pregnancies, oral contraceptive use, age at sexual debut, and condom use were unrelated to multiple HPV infections.
Table 1.
Characteristics of Sexually Active Participants at Enrollment in the Costa Rica Vaccine Trial, Stratified by HPV Status (n = 5871)
| Characteristic | No HPV infection n= 3392 n (%) | Single HPV infection n= 1409 n (%) | Multiple HPV infection n= 1070 n(%) | OR (95% CI) a Multiple HPV vs. single HPV (reference) |
| Age, years, mean (SD) | 21.5 (2.2) | 21.3 (2.2) | 21.1 (2.2) | 0.96 (.92–1.00) |
| Marital status | ||||
| Single | 1247 (36.8) | 722 (51.2) | 621 (58.0) | 1.00 |
| Married | 2055 (60.6) | 632 (44.9) | 389 (36.4) | 0.86 (.71–1.05) |
| Divorced/widowed | 87 (2.5) | 55 (3.9) | 57 (5.3) | 1.26 (.84–1.90) |
| Missing | 3 (.1) | 0 (.0) | 3 (.3) | - b |
| Number of pregnancies | ||||
| 0 | 1218 (35.9) | 600 (42.5) | 505 (47.2) | 1.00 |
| 1 | 1328 (39.1) | 502 (35.7) | 355 (33.2) | 0.93 (.75–1.15) |
| 2 | 601 (17.7) | 215 (15.3) | 133 (12.4) | 0.84 (.62–1.14) |
| ≥3 | 245 (7.3) | 92 (6.5) | 77 (7.2) | 1.21 (.81–1.82) |
| Oral contraceptive use | ||||
| Never | 641 (18.9) | 277 (19.6) | 255 (23.8) | 1.00 |
| Ever | 2626 (77.4) | 1087 (77.2) | 786 (73.5) | 0.85 (.68–1.05) |
| Missing | 125 (3.7) | 45 (3.2) | 29 (2.7) | - |
| Age at sexual debut | ||||
| ≤15 years | 1051 (31.0) | 463 (32.9) | 341 (31.9) | 1.00 |
| >15 years | 2336 (68.9) | 946 (67.1) | 725 (67.8) | 1.15 (.94–1.41) |
| Missing | 5 (.1) | 0 (.0) | 4 (.3) | - |
| Number of lifetime sex partners | ||||
| 1 | 1750 (51.6) | 468 (33.2) | 255 (23.8) | 1.00 |
| 2–5 | 1505 (44.4) | 854 (60.7) | 716 (66.9) | 1.53 (1.26–1.87) |
| >5 | 120 (3.5) | 73 (5.3) | 89 (8.3) | 2.05 (1.41–2.97) |
| Missing | 17 (.5) | 12 (.8) | 10 (1.0) | - |
| Condom use | 1287 (37.9) | 501 (35.6) | 350 (32.7) | 1.00 |
| Never | 242 (7.1) | 111 (7.9) | 69 (6.4) | 0.80 (.57–1.12) |
| Rarely | 426 (12.6) | 189 (13.4) | 175 (16.4) | 1.16 (.89–1.50) |
| Sometimes | 383 (11.3) | 206 (14.6) | 189 (17.7) | 1.08 (.83–1.39) |
| Most of the time | 901 (26.6) | 342 (24.2) | 252 (23.5) | 0.93 (.74–1.16) |
| Always | 153 (4.5) | 60 (4.3) | 35 (3.3) | - |
| Missing | ||||
| Cigarette smoking | ||||
| Never smoker | 2947 (86.9) | 1161 (82.4) | 813 (76.0) | 1.00 |
| Former smoker | 192 (5.7) | 104 (7.4) | 87 (8.1) | 1.03 (.75–1.40) |
| Current smoker | 250 (7.3) | 144 (10.2) | 167 (15.6) | 1.41 (1.10–1.82) |
| Missing | 3 (.1) | 0 (.0) | 3 (.3) | - |
NOTE. aOdds ratios were adjusted for all variables listed in the table. Age was modeled as a linear variable with 1 degree-of-freedom.
Subjects with missing values were excluded.
The number of coinfecting HPV genotypes in a woman ranged from 2 to 8. The frequency of number of coinfecting genotypes did not conform to a Poisson distribution (Table 2). Women were more likely than expected by chance to present with no infection or with 3 or more coinfections, whereas the occurrence of single and double infections was less likely than predicted by chance. Variance inflation factors, measuring the degree of departure from a Poisson assumption of independence, were 1.57 and 1.31 without and with adjustment for sexual behavior, respectively; again indicating that occurrence of multiple infections was higher than expected by chance.
Table 2.
Prevalence of Multiple HPV Infections at Enrollment among Sexually Active Women in the Costa Rica Vaccine Trial (n = 5412) a
| Number of coinfecting HPV types | Observed number of women (O) | Poisson Expected number of women (E) | O/E (95% CI) |
| 0 | 2933 | 2488.4 | 1.18 (1.14–1.22) |
| 1 | 1409 | 1933.5 | 0.73 (.69–.77) |
| 2 | 646 | 751.1 | 0.86 (.79–.93) |
| 3 | 267 | 194.6 | 1.37 (1.21–1.55) |
| 4 | 102 | 37.8 | 2.7 (2.2–3.3) |
| 5 | 39 | 5.9 | 6.6 (4.7–9.1) |
| 6 | 12 | 0.8 | 15.8 (8.2–27.6) |
| 7 | 2 | 0.1 | 23.7 (2.9–85.6) |
| 8 | 2 | 0.01 | 243.9 (29.5–881.2) |
NOTE. aAnalyses were restricted to 25 HPV types genotyped using SPF10/LiPA. Women with untyped HPV types (n = 459) were excluded.
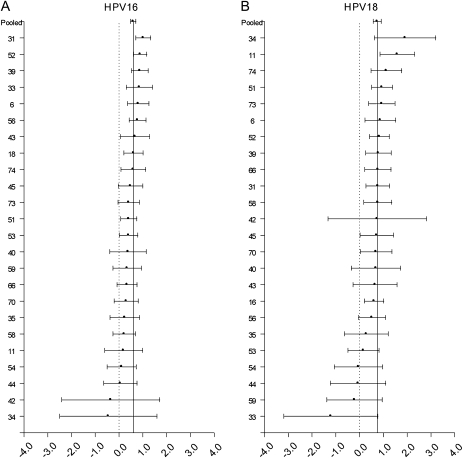
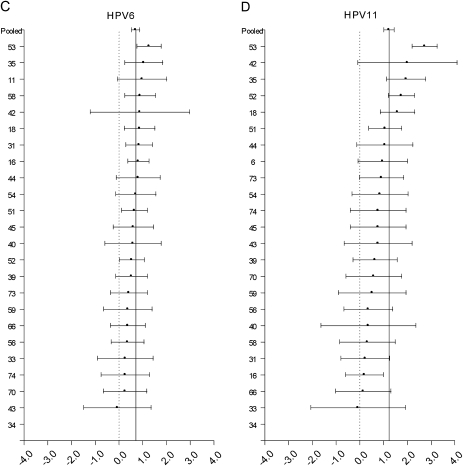
A high proportion of each HPV type was involved in multiple infections, ranging from 48.6% for HPV54 to 86.7% for HPV34. For HPV types 16, 18, 6, and 11, no overall heterogeneity was observed for coinfection with 24 other types (all χ2 P >.05). For HPV16 (pooled OR = 1.82), HPV18 (pooled OR = 2.11), and HPV6 (pooled OR = 2.02) we did not find statistically significant differences (Bonferroni-corrected P < .002) between the pooled OR and the OR for any particular coinfection pattern (Figure 1A, 1B, and 1C). HPV11 was significantly more likely to be involved in a coinfection with HPV53 when compared with other types (OR = 15.5 vs. pooled OR = 3.42, P < .002).
Figure 1.
Log odds ratios and 95% confidence intervals for HPV16 (A), HPV18 (B), HPV6 (C), and HPV11 (D) for coinfection with 24 other HPV types are shown. The vertical solid line represents the odds ratio pooled across the 24 individual pair-specific odds ratios. The dashed vertical line represents the null log odds ratio of .0. Although odds ratios for some type-type combinations were significantly different from the pooled odds ratio at the .05 level, these associations did not retain statistical significance at a Bonferroni-corrected P-value threshold of P < .002, with the exception of coinfection of HPV11 with HPV53.
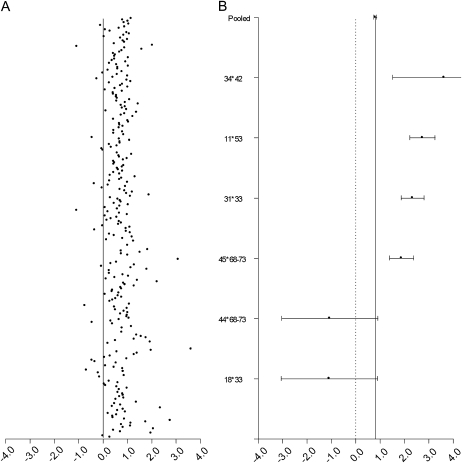
When all possible pair combinations across 25 HPV types were considered, the pooled underlying OR for all 300 pair combinations was 2.25; 95% CI = 2.12–2.38 (Figure 2A). Of these, ORs for 6 genotype combinations were significantly different from the pooled OR at a Bonferroni-corrected P-value threshold of .0001 (Figure 2B). HPV11–HPV53; HPV31–HPV33; HPV34–HPV42; and HPV45 with HPV68–73 were significantly more likely to be involved in a coinfection when compared with all other type-type combinations, whereas HPV44 with HPV68-73 and HPV18-HPV33 were significantly less likely to be involved in a coinfection.
Figure 2.
(A) Log odds ratios for 300 type-type combinations across 25 HPV genotypes. The vertical line represents the null log odds ratio of .0. (B) Pooled odds ratios across 300 individual odds ratios as well as pair-specific odds ratios that differ significantly from the pooled odds ratio at a Bonferroni-corrected P-value threshold of P < .0001.
At the phylogenetic clade level, for α9 types (HPV16, 31, 33, 35, 52, and 58), no difference was observed for involvement in coinfection with α9 types (OR = 2.32; 95% CI = 1.83–2.77) vs. non- α9 types (OR = 1.86; 95% CI = .97–2.72). Likewise, α7 types (HPV18, 39, 45, 59, and 70) were involved in multiple infections to a similar extent with other α7 types (1.94; 95% CI = 1.33–2.47) or non- α7 types (OR = 1.80; 95% CI = 1.12–2.39).
Associations of multiple HPV infections with risk of CIN2+ and HSIL+ are shown in Table 3. For HPV types 16 and 18, coinfection with additional HPV types was not associated with risk of CIN2+. In contrast, coinfection with more than 1 HPV type belonging to clade α9 was associated with significantly increased risk of CIN2+ (OR = 2.28; 95% CI = 1.13–4.62). Coinfection with multiple oncogenic HPV types was associated with marginally increased CIN2+ risk (OR = 1.74; 95% CI = .95–3.19; P = .072). However, this association was largely driven by coinfection with HPV16 (OR for multiple oncogenic types excluding HPV16 = .49). For HSIL+, presence of multiple infections (OR = 1.34; 95% CI = 1.02–1.78), multiple oncogenic infections (OR = 1.48; 95% CI = 1.10–1.99), and coinfection with more than 1 type belonging to clade α9 (OR = 1.64; 95% CI = 1.11–2.42) were associated with significantly increased risk.
Table 3.
Relationship of Multiple HPV Infections with Risk of CIN2+ or HSIL+
| CIN2+ |
HSIL+ |
||||
| No. of women | No. (%) | OR (95% CI) a | No. (%) | OR (95% CI) a | |
| Single HPV | 1409 | 25 (1.8) | 1.00 | 113 (8.0) | 1.00 |
| Multiple HPV | 1070 | 21 (2.0) | 1.12 (.62–2.04) | 112 (10.5) | 1.34 (1.02–1.78) |
| HPV 16 | |||||
| Single | 170 | 7 (4.1) | 1.00 | 29 (17.2) | 1.00 |
| Multiple | 318 | 15 (4.7) | 1.15 (.46–2.88) b | 46 (14.6) | 0.84 (.49–1.42) |
| Non HPV-16 | |||||
| Single | 1239 | 18 (1.5) | 1.00 | 84 (6.8) | 1.00 |
| Multiple | 752 | 6 (.8) | 0.50 (.19–1.31) | 66 (8.8) | 1.32 (.84–1.96) |
| HPV 18 | |||||
| Single | 55 | 1 (1.8) | 1.00 | 8 (14.5) | 1.00 |
| Multiple | 133 | 2 (1.5) | 0.82 (.07–9.28) b | 21 (15.8) | 1.26 (.49–3.21) |
| Oncogenic types | |||||
| Single | 1310 | 24 (1.8) | 1.00 | 123 (9.4) | 1.00 |
| Multiple | 673 | 21 (3.1) | 1.74 (.95–3.19) | 90 (13.4) | 1.48 (1.10–1.99) |
| Oncogenic types excluding | |||||
| HPV16 | 1082 | 17 (1.6) | 1.00 | 91 (8.4) | 1.00 |
| Single | 316 | 3 (.9) | 0.49 (.13–1.80) | 35 (11.1) | 1.37 (.90–2.08) |
| Multiple | |||||
| α9 species | |||||
| Single | 1004 | 25 (2.5) | 1.00 | 117 (11.7) | 1.00 |
| Multiple | 241 | 13 (5.4) | 2.28 (1.13–4.62) | 43 (18.1) | 1.64 (1.11–2.42) |
| α7 species | |||||
| Single | 650 | 13 (2.0) | N/A | 64 (9.9) | 1.00 |
| Multiple | 71 | 0 (.0) | 8 (11.3) | 1.02 (.46–2.62) | |
NOTE. aOdds ratios were adjusted for age, lifetime number of sex partners, number of pregnancies, and smoking
These odds ratios were not adjusted for any factors owing to small sample sizes
For α9 genotypes (16, 31, 33, 35, 52, and 58), we evaluated evidence for type-type interactions on cervical disease risk (Table 4). For CIN2+, coinfection with any 2 α9 genotypes was generally associated with significantly increased risk. Nonetheless, there was no significant evidence for type-type interaction in increasing CIN2+ risk, and none of the synergy indices was statistically significant. Likewise, for HSIL+, none of the synergy indices was statistically significant, indicating that effect measures were consistent with risk additivity (ie, risk for joint infection was similar to the sum of risk from individual infections).
Table 4.
Interaction between Coinfecting α9 Genotypes and Risk of Cervical Disease
| CIN2+OR (95% CI) a | HSIL+OR (95% CI) a | |
| 16-31 | 1.00 | 1.00 |
| Negative | 10.42 (5.31–20.41) | 5.20 (3.82–7.09) |
| 16 only | 7.74 (3.06–19.58) | 3.79 (2.41–5.95) |
| 31 only | 32.51 (12.46–84.84) | 8.23 (4.26–15.87) |
| 16 and 31 | 1.94 (.71–5.35) | 1.03 (.46–2.27) |
| Synergy index b | ||
| 16-33 | 1.00 | 1.00 |
| Negative | 10.19 (5.55–18.70) | 5.26 (3.92–13.92) |
| 16 only | 6.56 (1.51–28.4) | 7.73 (4.29–13.92) |
| 33 only | 30.65 (6.63–141.74) | 9.04 (2.91–28.01) |
| 16 and 33 | 2.00 (.37–10.74) | 0.73 (.19–2.75) |
| Synergy index | ||
| 16-35 | 1.00 | 1.00 |
| Negative | 11.10 (6.14–20.08) | 5.92 (3.97–7.06) |
| 16 only | 5.26 (1.22–22.69) | 2.77 (1.32–5.82) |
| 35 only | NE | NE |
| 16 and 35 | NE | NE |
| Synergy index | ||
| 16-52 | 1.00 | 1.00 |
| Negative | 13.47 (7.13–25.45) | 5.29 (3.86–7.26) |
| 16 only | 5.05 (2.00–12.74) | 3.32 (2.22–4.97) |
| 52 only | 7.37 (1.68–32.23) | 7.85 (4.36–14.15) |
| 16 and 52 | 0.38 (.07–2.07) | 1.03 (.50–2.10) |
| Synergy index | ||
| 16-58 | 1.00 | 1.00 |
| Negative | 11.63 (6.21–21.78) | 5.49 (4.08–7.39) |
| 16 only | 8.28 (3.06–22.35) | 5.30 (3.34–8.40) |
| 58 only | 27.37 (5.99–125.37) | 8.13 (2.67–24.77) |
| 16 and 58 | 1.47 (.29–7.25) | 0.81 (.22–2.95) |
| Synergy index | ||
| 31-33 | 1.00 | 1.00 |
| Negative | 6.09 (2.87–12.89) | 3.03 (1.98–4.63) |
| 31 only | 2.65 (.35–19.73) | 4.76 (2.38–9.50) |
| 33 only | 19.20 (5.51–66.88) | 11.50 (5.14–25.71) |
| 31 and 33 | 2.67 (.53–13.21) | 1.81 (.63–5.19) |
| Synergy index | ||
| 31-35 | 1.00 | 1.00 |
| Negative | 7.93 (4.04–15.54) | 3.67 (2.50–5.36) |
| 31 only | 3.68 (.87–15.59) | 1.98 (.90–4.33) |
| 35 only | NE | 2.97 (.37–23.89) |
| 31 and 35 | NE | 0.36 (.01–16.13) |
| Synergy index | ||
| 31-52 | 1.00 | 1.00 |
| Negative | 6.91 (3.24–14.74) | 3.57 (2.35–5.44) |
| 31 only | 2.46 (.94–6.38) | 3.15 (2.18–4.54) |
| 52 only | 15.33 (4.46–52.65) | 7.56 (3.40–16.81) |
| 31 and 52 | 1.94 (.45–8.20) | 1.38 (.52–3.69) |
| Synergy index | ||
| 31-58 | 1.00 | 1.00 |
| Negative | 8.51 (4.20–17.23) | 4.04 (2.74–5.95) |
| 31 only | 7.12 (2.91–17.40) | 4.96 (3.19–7.73) |
| 58 only | 9.81 (1.27–75.75) | 2.86 (.66–12.43) |
| 31 and 58 | 0.64 (.06–6.51) | 0.26 (.02–2.58) |
| Synergy index | ||
| 33-35 | 1.00 | 1.00 |
| Negative | 6.47 (2.26–18.47) | 6.20 (3.67–10.47) |
| 33 only | 2.77 (.66–11.64) | 1.98 (.95–4.14) |
| 35 only | NE | NE |
| 33 and 35 | NE | NE |
| Synergy index | ||
| 33-52 | 1.00 | 1.00 |
| Negative | 5.80 (1.74–19.24) | 6.93 (3.98–12.07) |
| 33 only | 2.75 (1.21–6.22) | 3.32 (2.35–4.69) |
| 52 only | 18.61 (2.26–152.67) | 7.34 (1.51–35.59) |
| 33 and 52 | 2.68 (.23–30.97) | 0.76 (.11–5.02) |
| Synergy index | ||
| 33-58 | 1.00 | 1.00 |
| Negative | 5.37 (1.62–17.76) | 6.41 (3.75–10.95) |
| 33 only | 5.16 (2.14–12.37) | 4.36 (2.83–6.71) |
| 58 only | 150.37 (9.23- >999) | 24.57 (1.53–394.16) |
| 33 and 58 | 17.51 (.93–326.72) | 2.68 (.14–49.87) |
| Synergy index | ||
| 35-52 | 1.00 | 1.00 |
| Negative | 3.53 (.83–14.89) | 2.61 (1.24–5.50) |
| 35 only | 3.12 (1.44–6.76) | 3.42 (2.44–4.79) |
| 52 only | NE | NE |
| 35 and 52 | NE | NE |
| Synergy index | ||
| 35-58 | 1.00 | 1.00 |
| Negative | 3.21 (.76–13.52) | 2.20 (1.05–4.60) |
| 35 only | 6.09 (2.68–13.84) | 4.49 (2.93–6.87) |
| 58 only | NE | NE |
| 35 and 58 | NE | NE |
| Synergy index | ||
| 52-58 | 1.00 | 1.00 |
| Negative | 3.58 (1.64–7.84) | 3.42 (2.42–4.86) |
| 52 only | 7.35 (3.19–16.92) | 4.81 (3.07–7.55) |
| 58 only | NE | 4.95 (1.43–17.13) |
| 52 and 58 | NE | 0.63 (.12–3.09) |
| Synergy index |
NOTE. NE= Not estimable
Unadjusted odds ratios
Synergy index was calculated as (exp(b1+b2+b3)−1)/((exp(b1)+exp(b2))−2), where b1 = main effect of HPV type1, b2 = main effect of HPV type2, and b3 = coefficient for cross-product term between HPV type 1 and HPV type 2.
DISCUSSION
In a large sample of young women in Costa Rica, the occurrence of multiple infections was significantly more common than expected by chance. However, there was no strong evidence that particular HPV genotypes were more or less likely to be involved in a coinfection when compared with other type-type combinations. Risk of cervical disease in women coinfected with more than 1 HPV type was similar to the sum of the risks from the coinfecting types.
Prevalence of multiple infections was significantly higher among women with more lifetime sex partners, consistent with sexual transmission of genital HPV infections [11]. Multiple infections were more common than would be expected by chance alone [5, 6], as evidenced by the deviation of the number of coinfecting genotypes from a Poisson distribution. This lack of conformity to a Poisson distribution was expected given the common mode of transmission and the possibility of concurrent acquisition of more than 1 type [5, 6]. Although these results indicate that prevalence of multiple infections is largely driven by sexual behaviors, we observed overdispersion in the distribution of coinfecting genotypes even after adjustment for lifetime number of sex partners, indicating that female sexual behaviors alone do not explain increased prevalence of multiple infections. For example, multiple infections were common even among women with few lifetime sex partners. Although behaviors of the male partners and cotransmission of HPV types would no doubt have an influence, it is possible that prevalence of multiple infections is also determined by immunologic mechanisms. Indeed, prevalence of multiple infections is high among immunosuppressed HIV-infected women [5]. Similarly, multiple infections were more common among current smokers in our study, which could also reflect immunologic mechanisms because current smoking has been shown to increase incidence, persistence, and viral load of HPV infections [23–25]. Additional studies are needed to characterize the immunologic determinants of multiple infections.
Women infected with 1 type were significantly more likely to harbor additional HPV types, but this increased affinity to be involved in a coinfection was not heterogeneous across HPV type-type combinations. We did, however, find some exceptions to this general observation even at conservative Bonferroni-corrected P-value thresholds. Certain HPV type combinations were more likely to be involved in a coinfection when compared with all other combinations (HPV31–33, HPV45–HPV68–73, and HPV34–HPV42), while some HPV types were less likely to be involved in a coinfection (HPV18–33 and HPV44–HPV68–73). The reasons for these observations are unclear. While PCR cross-reactivity could explain some of these results (eg, 31–33), we found that for α7 and α9 genotypes, there was no difference for involvement in a coinfection based on phylogenetic relatedness. Likewise, the majority of significant associations were for genotypes belonging to different phylogenetic clades. Our results indicate that HPV genotypes involved in coinfections occur at random, with little evidence for enhancement of susceptibility or competitive exclusion/cross-protection with any specific HPV type.
Although consistent with previous analyses [1–3, 5, 7, 26], the lack of decreased frequency in a coinfection for phylogenetically related HPV types in unvaccinated women contrasts with results of partial cross-protection for HPV31 (with HPV16) and HPV45 (with HPV18) among vaccinated women [12–14]. Similarly, a recent study reported significantly lower HPV16 and HPV18 viral loads among women coinfected with phylogenetically-related HPV types [27], suggesting some evidence for immunologic cross-protection among unvaccinated women. It is possible that the weak immune responses in natural HPV infections [28], as opposed to the strong responses following vaccination [29], do not confer protection against phylogenetically-related HPV types.
Women with multiple infections were at significantly increased risk of CIN2+ and HSIL+ when compared with those with single infections, particularly those with coinfections involving multiple oncogenic types and multiple α9 genotypes. Nonetheless, risk of CIN2+/HSIL+ for those with multiple α9 types was similar to the sum of estimated risk from individual types. However, we note that, particularly for CIN2+, a majority of synergy indices were nonsignificantly higher than 1.0. Therefore, it is possible that the low number of CIN2+ cases in our study (n = 47) may have afforded limited statistical power to detect significant interactions. Our results indicate that women with multiple infections are at significantly increased risk of cervical disease than those with single infections by virtue of each additional type conferring incremental risk, but coinfecting HPV types follow an independent course toward causing disease. Therefore, our observations suggest that HPV vaccination will not have a profound impact on the carcinogenicity of non-vaccine targeted HPV genotypes.
Our study has several strengths, including the large sample size and the use of state-of-the-art HPV detection and disease diagnosis methods. We also note the limitations of our study. Importantly, we evaluated prevalent and not incident HPV infections, which precluded an assessment of the natural history of coinfecting HPV types. We may have missed some type-type associations if a majority of prevalent coinfections were recently acquired as opposed to being established infections. Additionally, we could not assess concurrently versus sequentially acquired infections, and immunologic responses following concurrent acquisition of multiple HPV types could be different from responses for sequentially acquired infections. Given the narrow age range of women included in our study (18–25 years), our results are not generalizable to older women. Because HPV detection among older women may represent persistent infections, HPV coinfection patterns could differ among older women. Finally, despite the large number of women included in our study, assessments of certain coinfection patterns as well as synergy indices may have been affected by low statistical power.
In conclusion, in a large sample of young women, our key observations were that despite the significantly higher frequency of multiple HPV types, coinfecting HPV genotypes come together at random and, although associated with increased risk of cervical disease, coinfecting genotypes lead to cervical disease independently. Our results indicate that HPV16/18 vaccination would not result in either type-replacement of infection or modulation of the carcinogenic potential of untargeted HPV types.
Funding
The Costa Rican Vaccine Trial (CVT) is a longstanding collaboration between investigators in Costa Rica and Intramural Research Program (IRP) of the National Cancer Institute (NCI). The CVT trial is sponsored and funded by NCI (N01-CP-11005) with support from the NIH Office for Research on Women's Health and conducted in agreement with the Ministry of Health of Costa Rica.Vaccine was provided for CVT by GSK Biologicals, under a Clinical Trials Agreement with NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. Douglas Lowy and John Schiller from NCI are named inventors on U.S.-government owned HPV vaccine patents that are licensed to GSK and Merck, and so are entitled to limited royalties as specified by federal law.
Appendix 1.
Affiliations of the Costa Rica Vaccine Trial (CVT) group:
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytologist)
Manuel Barrantes (Field Supervisor)
M. Concepcion Bratti (co-Investigator)
Fernando Cárdenas (General Field Supervisor)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Yenory Estrada (Pharmacist)
Paula Gonzalez (co-Investigator)
Diego Guillén (Pathologist)
Rolando Herrero (co-Principal Investigator)
Silvia E. Jimenez (Trial Coordinator)
Jorge Morales (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Elmer Pérez (Field Supervisor)
Carolina Porras (co-Investigator)
Ana Cecilia Rodriguez (co-Investigator)
Maricela Villegas (Clinic M.D.)
University of Costa Rica, San José, Costa Rica
Enrique Freer (Director, HPV Diagnostics Laboratory)
Jose Bonilla (Head, HPV Immunology Laboratory)
Sandra Silva (Head Technician, HPV Diagnostics Laboratory)
Ivannia Atmella (Immunology Technician)
Margarita Ramírez (Immunology Technician)
United States National Cancer Institute, Bethesda, MD, USA
Allan Hildesheim (co-Principal Investigator)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Co-Project Officer & Medical Monitor)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor & QC Pathologist)
Sholom Wacholder (Statistician)
SAIC, NCI-Frederick, Frederick, MD, USA
Ligia Pinto (Head, HPV Immunology Laboratory)
Alfonso Garcia-Pineres (Scientist, HPV Immunology Laboratory)
Women's and Infants’ Hospital, Providence, RI, USA
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
DDL Diagnostic Laboratory, Voorburg, The Netherlands
Wim Quint (HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
References
- 1.Vaccarella S, Franceschi S, Snijders PJ, Herrero R, Meijer CJ, Plummer M. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol Biomarkers Prev. 2010;19:503–10. doi: 10.1158/1055-9965.EPI-09-0983. [DOI] [PubMed] [Google Scholar]
- 2.Thomas KK, Hughes JP, Kuypers JM, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182:1097–102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- 3.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Myers L, Hammons AF, et al. Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol Biomarkers Prev. 2005;14:2439–45. doi: 10.1158/1055-9965.EPI-05-0465. [DOI] [PubMed] [Google Scholar]
- 6.Mendez F, Munoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 7.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 8.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau MC, Abrahamowicz M, Villa LL, Costa MC, Rohan TE, Franco EL. Predictors of cervical coinfection with multiple human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2003;12:1029–37. [PubMed] [Google Scholar]
- 12.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–44. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 14.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 15.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199:919–22. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 16.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 18.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 19.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–8. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology. 1996;7:286–90. doi: 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 24.Koshiol J, Schroeder J, Jamieson DJ, et al. Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am J Epidemiol. 2006;164:176–83. doi: 10.1093/aje/kwj165. [DOI] [PubMed] [Google Scholar]
- 25.Xi LF, Koutsky LA, Castle PE, et al. Relationship between cigarette smoking and human papilloma virus types 16 and 18 DNA load. Cancer Epidemiol Biomarkers Prev. 2009;18:3490–6. doi: 10.1158/1055-9965.EPI-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rousseau MC, Villa LL, Costa MC, Abrahamowicz M, Rohan TE, Franco E. Occurrence of cervical infection with multiple human papillomavirus types is associated with age and cytologic abnormalities. Sex Transm Dis. 2003;30:581–7. doi: 10.1097/00007435-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Xi LF, Edelstein ZR, Meyers C, Ho J, Cherne SL, Schiffman M. Human papillomavirus types 16 and 18 DNA load in relation to coexistence of other types, particularly those in the same species. Cancer Epidemiol Biomarkers Prev. 2009;18:2507–12. doi: 10.1158/1055-9965.EPI-09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(Suppl 2) doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10) doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]