Abstract
Background. Human immunodeficiency virus (HIV)–tuberculosis coinfection is associated with heightened immune activation, viral replication, and T cell dysfunction. We compared changes in T cell activation and function between patients receiving concurrent treatment for HIV-tuberculosis coinfection and those receiving treatment for tuberculosis alone.
Methods. HIV-infected adults with tuberculosis and CD4+ T cell counts >350 cells/mm3 were randomized to receive tuberculosis treatment alone (control arm; n = 36) or 6 months of antiretroviral therapy (ART) concurrent with tuberculosis treatment (intervention arm; n = 38). HIV viral load, T cell subsets, T cell activation, and cytokine production were measured at enrollment and every 3 months for 12 months.
Results. Differences in absolute CD4+ and CD8+ T cell counts were not observed between arms. Viral load was reduced while participants received ART; control patients maintained viral load at baseline levels. Both arms had significant reductions in T cell expression of CD38 and HLA-DR. Interferon-γ production in response to mitogen increased significantly in the intervention arm.
Conclusions. In HIV-infected adults with tuberculosis and CD4+ T cell counts >350 cells/mm3, both tuberculosis treatment and concurrent HIV-tuberculosis treatment reduce T cell activation and stabilize T cell counts. Concurrent ART with tuberculosis treatment does not provide additional, sustained reductions in T cell activation among individuals with preserved immunologic function.
Coinfection with Mycobacterium tuberculosis remains a leading cause of morbidity and mortality among human immunodeficiency virus (HIV)–infected individuals in the developing world. HIV-infected individuals are more likely to develop tuberculosis disease following M. tuberculosis exposure, are at risk for severe, disseminated forms of tuberculosis, and have higher case fatality rates and rates of recurrent tuberculosis than individuals without HIV infection [1–5]. Tuberculosis accelerates the progression of HIV infection, especially among individuals who develop tuberculosis with preserved CD4+ T cell counts [6–10]. Uganda, where at least 39% of incident tuberculosis cases are complicated by HIV infection, is particularly affected by the overlapping epidemics of HIV and tuberculosis [11].
Tuberculosis has multiple deleterious effects on host immunity, including increased immune activation, apoptosis of CD4+ T cells, loss of both M. tuberculosis–specific and naive CD4+ T cells, and defective cytokine production [12–15]. Immune activation and erosion of the naive T cell pool are strongly associated with HIV infection. T cell activation, specifically CD38 expression on CD8+ T cells, is a marker for AIDS progression and death among untreated HIV-infected individuals and is predictive of poor CD4+ T cell recovery among patients receiving antiretroviral therapy (ART) [16–21]. Although the pathogenesis of HIV-tuberculosis coinfection is not well understood, the immunologic environment during M. tuberculosis infection is characterized by cytokine and chemokine irregularities that are believed to increase T cell activation, enhance HIV replication, and result in a dysfunctional immune response [22–28]. Thus, heightened immune activation as a result of both HIV and tuberculosis leads to multiple perturbations of the immune system that likely contribute to disease severity and progression.
The immunologic benefits of ART for HIV-infected patients, including reduction in viral load, immune activation, and restoration of CD4+ T cells, are well recognized [29, 30]. The effect of ART on T cell activation and function among HIV-infected adults with tuberculosis, however, is poorly characterized. In our earlier study, we reported that tuberculosis treatment alone significantly reduced T cell activation among HIV-infected adults with tuberculosis [31]. Therefore, we hypothesized that combination HIV-tuberculosis treatment would permit greater reduction in HIV replication than tuberculosis therapy alone, leading to decreased T cell activation and restoration of CD4+ and CD8+ T cell subsets and function. To test this hypothesis, we examined HIV-infected Ugandan adults with pulmonary tuberculosis and baseline CD4+ counts >350 cells/mm3 who were participants in a randomized clinical trial of ART during tuberculosis treatment. During this trial, which was initiated prior to recognition of the risks of both episodic and delayed ART, patients were treated with either a 6-month course of tuberculosis therapy (n = 36) or tuberculosis treatment combined with a punctuated 6-month course of ART (n = 38) [32, 33]. Using flow cytometry and a whole-blood enzyme-linked immunosorbent assay (ELISA), we compared markers of T cell activation, changes in T cell subsets, and production of interferon-γ (IFN-γ) in response to mitogen and M. tuberculosis– and HIV-specific antigens between study cohorts during the 6-month period of assigned therapy and for an additional 6-month period of observation.
METHODS
Participants
We present analysis of a subset of subjects enrolled in a larger phase 3 open-label randomized clinical trial entitled “Randomized clinical trial of a 6-month punctuated course of antiretroviral therapy in Ugandan HIV+ adults with pulmonary tuberculosis and CD4+ >350 cells/mm3 (#NCT00078247/PART).” PART's objective was to compare HIV disease progression and clinical outcomes of HIV-infected adults with tuberculosis and high CD4+ T cell counts treated with standard tuberculosis therapy (control arm) and those treated with tuberculosis therapy plus a limited 6-month course of ART (intervention arm). Participants aged between 13 and 60 were recruited from the Uganda–Case Western Reserve University Research Collaboration within the Mulago Hospital Complex in Kampala, Uganda. All participants had HIV type 1 (HIV-1) infection and smear- and/or culture-confirmed acute pulmonary tuberculosis. HIV-1 infection was diagnosed by means of ELISA and Western blot test and confirmed with HIV RNA copy levels. Only HIV-1–infected participants who were ART naive and had CD4+ counts >350 cells/mm3 were eligible for study participation. Data for this substudy were accrued during the period March 2006 through February 2009. Participants were selected for substudy enrollment if results of immunologic assessments (flow cytometry and whole-blood ELISA) were available from study enrollment and at least 2 additional time points during a 12-month period. Written informed consent was obtained from participants prior to enrollment, and the study protocol was approved by the institutional review boards of Case Western Reserve University/University Hospitals, University of California at San Francisco, the Joint Clinical Research Center in Kampala, Uganda, and the Ugandan National Council for Science and Technology.
Participants initially received a standard regimen of directly observed tuberculosis therapy: 2 months of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 4 months of daily isoniazid and rifampin. Two weeks after study enrollment, participants were randomized to receive either 6 months of standard tuberculosis therapy (control arm) or tuberculosis therapy plus a punctuated 6-month course of ART (trizivir, a combination of abacavir, lamivudine, and zidovudine) (intervention arm). Daily cotrimoxazole was provided to participants in both arms after they had completed 2 months of tuberculosis or HIV-tuberculosis therapy.
Measurements
Data regarding demographic characteristics and symptoms of pulmonary tuberculosis were collected on standardized forms. Participants underwent a physical exam, baseline chest radiograph, and collection of sputum samples for acid-fast bacilli (AFB) smear and culture at enrollment. Additional sputum specimens for AFB smear and culture were obtained at 2 and 5 months after initiation of tuberculosis treatment.
Median viral load was measured using Amplicor quantitative restriction transcriptase-polymerase chain reaction assay (Roche Amplicor 1.5) at enrollment (baseline) and every 3 months during 12 months of study observation. The lower limit of detection of the assay was 400 copies/mm3.
Immunophenotyping was performed at enrollment and every 3 months for 12 months. Whole blood was freshly collected in sodium heparin tubes, and 200 μL was aliquotted to each of the 12 × 75 mm test tubes. For flow cytometry, the following antibodies were used: anti-CD4 allophycocyananin (APC), anti-CD8 APC, anti–HLA-DR phycoerythrin (PE), anti-CD38 or anti-CD62L phycoerythrin Cy5 (PECy5), and anti-CD45RO fluorescein isothiocyanate (FITC). Mouse monoclonal isotypic control conjugated with PE, PECy5, FITC, and APC was used to determine nonspecific binding and to set gating boundaries. Antibodies were obtained from BD Pharmingen.
Cells were acquired using a FACS Calibur flow cytometer (BD Bioscience) using Cellquest software (BD Bioscience) with additional analysis performed using FlowJo Software (Tree Star). A total of 25,000–50,000 cells were analyzed for each condition. Lymphocytes were selected on the basis of forward and side scatter characteristics, and CD4+ or CD8+ T cell populations were then determined. T cells were then evaluated for proportion of cells expressing HLA-DR and CD38 or CD45RO and CD62L, with gating strategies identical to those we previously reported [31]. All immunologic studies were performed at the Joint Clinical Research Center in Kampala, Uganda.
Whole-blood ELISAs were performed at enrollment and every 3 months for 12 months to measure IFN-γ in response to phytohemagglutinin (PHA), as well as M. tuberculosis– and HIV-specific antigens. Whole blood was diluted 1:5 with RPMI 1640 and cultured in 48-well tissue culture plates (1 mL/well) with medium alone, PHA (5 μg/mL; Sigma), M. tuberculosis culture filtrate (10 μg/mL; Colorado State University), M. tuberculosis antigen 85B (20 μg/mL; Colorado State University), and purified p24 protein from HIV clade A or HIV clade D, the 2 dominant HIV clades in Uganda (10 μg/mL; Case Western Reserve University, Molecular Core). After 7 days of incubation, cell-free supernatants were collected and cryopreserved for batch testing with ELISA. IFN-γ concentration (pg/mL) was determined in duplicate wells by using sandwich ELISA with the anti–IFN-γ antibody pairs ENM-700A and biotinylated ENM-701B (Pierce). Background IFN-γ production (response to medium alone) was subtracted from IFN-γ production in response to each individual stimulus, and values were log transformed prior to statistical analysis.
Statistical Analysis
Nonparametric rank tests were performed to compare median changes in viral load, CD4+ and CD8+ T cell counts and subsets, markers of immune activation, and IFN-γ production from baseline to months 3, 6, 9, and 12, within and between study arms. Analysis was performed using Statistical Analysis Software (SAS) version 9.2 (SAS Institute). A P value of <.05 was considered to reveal a significant difference.
RESULTS
Baseline Characteristics
A total of 74 HIV-infected participants with tuberculosis were included in the analysis, with 36 randomized to receive tuberculosis therapy alone (control arm) and 38 randomized to receive concurrent ART and tuberculosis treatment (intervention arm). Baseline demographic and clinical characteristics of the 2 groups were similar (Table 1). Demographic and baseline clinical characteristics of participants in this substudy were not significantly different from those of participants in the overall clinical trial (data not shown).
Table 1.
Demographic and Baseline Clinical Characteristics of Study Participants
| Characteristic | Tuberculosis treatment alone(n = 36) | HIV-tuberculosis treatment(n = 38) | P |
| Age, median years (range) | 32 (19–54) | 32 (23–47) | .50 |
| Female sex, % | 36 (n = 13) | 39 (n = 15) | .77 |
| BMI, median (range) | 9.1 (15.6–26.0) | 9.7 (15.2–29.2) | .56 |
| CD4 cell count, median (range) | 609.5 (374–1368) | 532.5 (283–1415) | .15 |
| Log viral load, median (range) | 4.4 (2.0–5.9) | 4.5 (2.0–5.9) | .84 |
| Extent of disease according to chest radiograph, % | .83 | ||
| Normal/minimal | 17 | 21 | |
| Moderately advanced | 36 | 32 | |
| Far advanced | 44 | 47 | |
| AFB smear, median grade (%) | 3+ (62) | 3+ (74) | .25 |
NOTE. AFB, acid-fast bacilli; BMI, body mass index; HIV, human immunodeficiency virus.
Tuberculosis Therapy Alone Has No Effect on HIV Viral Load
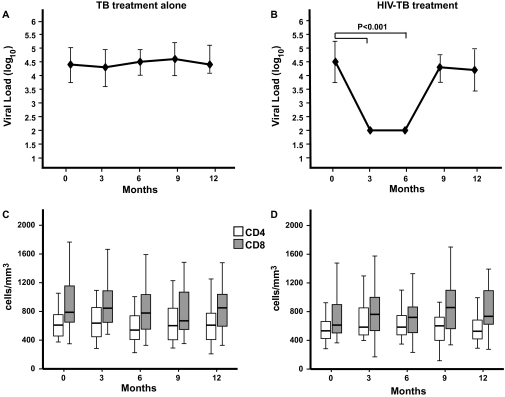
HIV viral load was measured at study enrollment and every 3 months for 12 months. There was no significant change in median HIV log10 viral load, compared with baseline, at 3 (4.3 log10 copies/mL, IQR 3.5--4.9), 6 (4.5 log10 copies/mL, IQR 3.9--4.9), 9 (4.6 log10 copies/mL, IQR 3.9--5.2), or 12 (4.4 log10 copies/mL, IQR 4.1--5.0) months in patients receiving tuberculosis therapy alone (Figure 1A). As expected, median HIV log10 viral load was significantly reduced, compared with baseline, in patients receiving combined HIV-tuberculosis treatment while they continued therapy, with a 2.5 log10 copies/mL reduction at both 3 and 6 months (P < .001 for both time points). 95% of subjects in the intervention arm achieved an undetectable viral load by 3 months (data not shown). However, HIV log10 viral load rebounded and returned to near-baseline levels at 9 (4.3 log10 copies/mL , IQR 3.6–4.6) and 12 (4.2 log10 copies/mL, IQR 3.4–4.9) months (Figure 1B).
Figure 1.
Changes in median human immunodeficiency virus (HIV) viral load, CD4+, and CD8+ T cell counts among participants receiving tuberculosis treatment alone (A,C) and combined HIV-tuberculosis treatment (B,D). Median values ± interquartile range are shown (A,B). Boxes indicate the interquartile ranges, the horizontal lines transecting the boxes indicate the medians, and the whiskers indicate the highest and lowest values (C,D). Nonparametric rank tests were performed to compare median changes in viral load, CD4+, and CD8+ T cell counts within each treatment arm from baseline to months 3, 6, 9, and 12. Significant P values are indicated. TB, tuberculosis.
CD8+ but Not CD4+ T Cell Subsets Are Altered by Combined HIV-Tuberculosis Therapy
Total CD4+ and CD8+ T cell counts, as well as the proportions of naive (CD45RO–/CD62L+), memory (CD45RO+/CD62L+), and effector (CD62L–) CD4+ and CD8+ T cells, were measured at enrollment and every 3 months during the 12-month study period. There was no significant change in absolute CD4+ or CD8+ T cell counts during the 12-month study period in either arm (Figure 1 C,D). At the 6-month time point, participants who received combined HIV-tuberculosis therapy had a significant increase from baseline in the proportion of naive CD8+ T cells, as well as a significant decrease from baseline in proportion of effector CD8+ T cells, when compared with participants who received tuberculosis treatment alone (P = .01 and P = .04, respectively, Table 2). By 12 months, however, these differences were no longer observed. Although there was a trend toward restoration of the naive CD4+ T cell pool in both arms during the 12-month study period, these changes were not significant (P = .84 and P = .92 at 6 and 12 months, respectively, Table 2).
Table 2.
Changes in CD4+ and CD8+ T Cell Subsets during 12 Months of Immunologic Follow-up
| Subset | Tuberculosis treatment alone |
HIV-tuberculosis treatment |
||
| CD4+ | CD8+ | CD4+ | CD8+ | |
| Naive, % RO–/62L+ (IQR) | ||||
| Baseline | 47 (42.1–53.5) | 25.4 (17.2–31.4) | 44.1 (36.0–58.0) | 26.6 (19.1–36.5) |
| 6 months | 47.5 (38.8–53.1) | 24.5b (18.0–30.0) | 46.5 (36.3–59.0) | 35.2b (22.7–52.3) |
| 12 months | 49.1 (39.6–57.4) | 24.3 (20.1–36.6) | 48.3 (36.8–59.4) | 29.5 (21.8–38.3) |
| Memory, % RO+/62L+ (IQR) | ||||
| Baseline | 38.6 (32.9–41.8) | 15.7 (12.6–21.3) | 39.3 (30.2–43.9) | 17.7 (15.0–21.1) |
| 6 months | 41.5 (34.9–49.1) | 16.9 (10.9–20.8) | 38.3 (30.2–45.2) | 15.2 (10.8–18.5) |
| 12 months | 35.3 (32.7–43.9) | 13.2 (9.5–19.0) | 36.3 (27.2–44.7) | 15.2 (13.1–20.4) |
| Effector, % 62L– (IQR) | ||||
| Baseline | 12.8 (9.5–17.3) | 59.3 (50.2–65.2) | 14.8 (9.1–19.0) | 54.8 (45.8–62.0) |
| 6 months | 11.3 (8.9–14.2) | 58.6b (50.0–64.2) | 13.7 (7.0–20.6) | 45.7b (35.0–62.3) |
| 12 months | 15.7 (7.3–17.8) | 60.5 (50.6–66.7) | 11 (8.7–16.9) | 54.8 (47.2–61.0) |
NOTE. IQR, interquartile range.
bP < .05, nonparametric cross-sectional comparison between treatment arms.
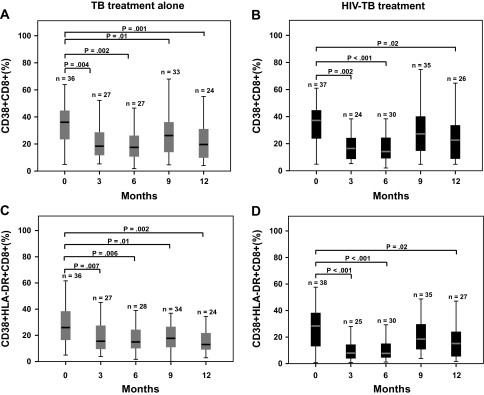
Tuberculosis Treatment Alone and Combination HIV-Tuberculosis Therapy Both Significantly Reduce CD4+ and CD8+ T Cell Activation
T cell activation was assessed by measuring the expression of CD38 and HLA-DR on CD4+ and CD8+ T cells by means of flow cytometry at enrollment and every 3 months for 12 months. Among patients treated for tuberculosis alone, there was a significant reduction in median proportion of CD38+/CD8+ and CD38+/HLA-DR+/CD8+ T cells at all time points, compared with baseline (Figure 2A,C). The median proportion of HLA-DR+/CD8+ T cells was not significantly altered at any time point among control arm participants (data not shown). Participants who received combined HIV-tuberculosis therapy had a significant reduction in median proportion of CD38+/CD8+ and CD38+/HLA-DR+/CD8+ T cells at 3, 6, and 12 months, compared with baseline (Figure 2B,D). Among intervention participants, the median proportion of HLA-DR+/CD8+ T cells was significantly decreased at 3 and 6 months, compared with baseline (data not shown). Comparison of the change in proportion of CD38+/HLA-DR+/CD8+ T cells from baseline between the control and intervention arms revealed a more significant reduction in median proportion of CD38+/HLA-DR+/CD8+ T cells among the intervention arm at 3 (P = .01) and 6 (P = .007) months. However, this reduction was no longer significantly different between arms by 9 months (P = .70).
Figure 2.
Changes in CD8+ T cell activation markers in response to tuberculosis treatment alone and combined human immunodeficiency virus (HIV)–tuberculosis treatment. Percentage expression of CD38 and CD38/HLA-DR on CD8+ T cells at baseline and 3, 6, 9, and 12 months was measured for participants receiving tuberculosis treatment alone (A,C) and combined HIV-tuberculosis treatment (B,D). The boxes indicate the interquartile ranges, the horizontal lines transecting the boxes indicate the medians, and the whiskers indicate the highest and lowest values. Nonparametric rank tests were performed to compare median changes in percentage of CD38+ and CD38+/HLA-DR+ CD8+ T cells within each treatment group from baseline to months 3, 6, 9, and 12. Significant P values are indicated. TB, tuberculosis.
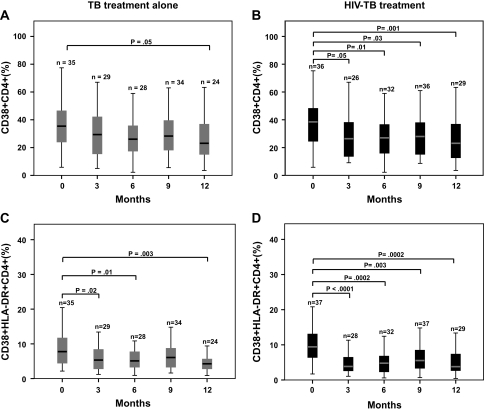
Patients in both arms showed significant reductions in CD38 and HLA-DR expression on CD4+ T cells, although this was more pronounced in the intervention group. The median proportion of CD38+/CD4+ T cells was significantly reduced from baseline only at 12 months among control patients, yet in the intervention arm, significant reductions from baseline were seen at 3, 6, 9, and 12 months (Figure 3A,B). A significant reduction in the median proportion of CD38+/HLA-DR+/CD4+ T cells from baseline was seen in the control arm at 3, 6, and 12 months (Figure 3C). The median proportion of CD38+/HLA-DR+/CD4+ cells was significantly decreased, compared with baseline, at all study time points among intervention participants (Figure 3D). The median proportion of HLA-DR+/CD4+ T cells was significantly decreased from baseline only at 12 months in the control group, yet in the intervention arm, significant decreases from baseline were seen at all study time points (data not shown).
Figure 3.
Changes in CD4+ T cell activation markers in response to tuberculosis treatment alone and combined human immunodeficiency virus (HIV)–tuberculosis treatment. Percentage expression of CD38 and CD38/HLA-DR on CD4+ T cells at baseline and at 3, 6, 9, and 12 months was measured among participants receiving tuberculosis treatment alone (A,C) and combined HIV-tuberculosis treatment (B,D). The boxes indicate the interquartile ranges, the horizontal lines transecting the boxes indicate the medians, and the whiskers indicate the highest and lowest values. Nonparametric rank tests were performed to compare median changes in percentage of CD38+ and CD38+/HLA-DR+ CD4+ T cells within each treatment group from baseline to months 3, 6, 9, and 12. Significant P values are indicated. TB, tuberculosis.
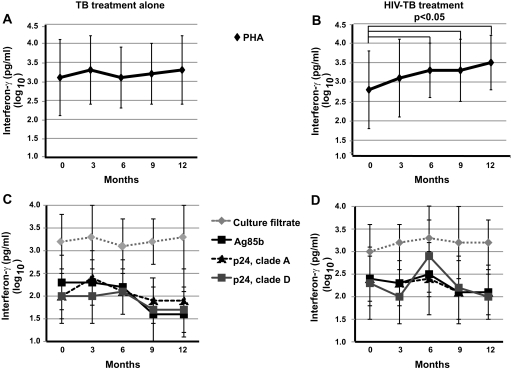
Combination HIV-Tuberculosis Treatment Improves T Cell Response to Mitogen but Has No Effect on HIV or M. tuberculosis–Specific Responses
T cell effector function was assessed by means of IFN-γ production in response to 7-day incubation of whole blood with mitogen PHA, as well as M. tuberculosis–specific (M. tuberculosis culture filtrate, Ag85B) and HIV-specific (p-24 protein from HIV clade A and clade D) proteins. Whole-blood IFN-γ responses were measured with use of ELISA. Participants in the intervention arm had significant increases in IFN-γ production, compared with baseline (2.8 log10 pg/mL), in response to PHA at 6 (3.3 log10 pg/mL; P = .002), 9 (3.3 log10 pg/mL; P = .01), and 12 (3.5 log10 pg/mL; P = .002) months (Figure 4B). Control patients did not show significant changes in IFN-γ production in response to PHA (Figure 4A).
Figure 4.
Changes in whole-blood IFN-γ production in response to phytohemagglutinin (PHA), M. tuberculosis culture filtrate, M. tuberculosis antigen 85B, p24 protein/ human immunodeficiency virus (HIV) clade A, and p24 protein/HIV clade D in response to tuberculosis treatment alone (A,C) and to combined HIV-tuberculosis treatment (B,D). Median values ± standard deviations are shown. Nonparametric rank tests were performed to compare median changes in IFN-γ production within each treatment group from baseline to months 3, 6, 9, and 12. Significant P values are indicated.
In both study arms, there were no significant changes in IFN-γ production in response to M. tuberculosis culture filtrate, M. tuberculosis Ag85b, HIV p-24 protein/clade A, or HIV p-24 protein/clade D during the study period (P > .05) (Figure 4 C,D). In addition, changes in IFN-γ production in response to these antigens were not significantly different between the 2 arms at any time point (P > .05).
DISCUSSION
In this prospective cohort study of immunologic changes associated with tuberculosis therapy alone in comparison with combined HIV-tuberculosis treatment among HIV-infected Ugandan adults with pulmonary tuberculosis and preserved CD4+ T cell counts, we have found that tuberculosis therapy alone produces significant reduction in T cell activation and maintains stable HIV viral load and CD4+ T cell counts. A punctuated course of ART given concurrently with tuberculosis treatment suppressed HIV replication, as expected, and allowed for a more significant reduction in CD8+ T cell activation than did tuberculosis therapy alone. However, these virologic and immunologic benefits were not sustained once ART was withdrawn. Significant improvements in T cell cytokine production in response to M. tuberculosis– and HIV-specific antigens were not seen in either group. Thus, it seems that tuberculosis treatment alone has a substantial effect on immune activation and that the addition of ART during tuberculosis treatment does not result in additional sustained improvements in T cell activation or antigen-specific T cell function for HIV-1 infected persons with preserved immune function.
Reports on the effects of tuberculosis treatment on immune activation in HIV-tuberculosis coinfection are conflicting. Two studies from sub-Saharan Africa reported reduction in serum markers of immune activation among ART-naive, HIV-1–infected adults undergoing treatment for pulmonary tuberculosis, and we previously reported that tuberculosis treatment alone results in decreased T cell activation in HIV-tuberculosis coinfection in persons with less advanced HIV disease [31, 34, 35]. However, Morris et al found that markers of T cell activation did not decrease among HIV-tuberculosis coinfected South African patients with advanced immunosuppression treated for tuberculosis [36]. Although the exact mechanism(s) of increased T cell activation in HIV is not understood, there is a well-recognized correlation between viral replication and CD38 expression on CD8+ T cells [20, 37], and reduction of viral load with ART clearly reduces immune activation in HIV infection alone [29, 30]. Studies comparing T cell activation among individuals with tuberculosis or HIV alone with individuals with HIV-tuberculosis coinfection reveal that dual infection is associated with higher levels of CD38 and HLA-DR expression on T cells [26, 27]. Therefore, simultaneous treatment of both infections would be expected to maximize reduction in T cell activation.
The comparative analysis performed in this study reveals that combined HIV-tuberculosis treatment allows for a more significant reduction in CD38/HLA-DR coexpression on CD8+ T cells than does tuberculosis therapy alone. However, this benefit over tuberculosis therapy alone is lost once ART is withdrawn and viral load rebounds to levels equivalent to those in the control arm. Although viral load was maintained at baseline levels in the control arm, a significant reduction in T cell activation was still observed. Moreover, at month 12, participants in both study arms maintained significant reductions in T cell activation. Thus, the relationship between viral replication and immune activation in HIV-tuberculosis coinfection seems not to be linear. In HIV-tuberculosis coinfection, active mycobacterial disease is a major driver of immune activation, and treatment of tuberculosis alone results in substantial reduction in immune activation.
Several groups have investigated the mechanism(s) of increased T cell activation in HIV-tuberculosis coinfection. M. tuberculosis can induce HIV replication in lymphocytes from HIV-infected individuals with M. tuberculosis infection, suggesting that an antigen-specific response against M. tuberculosis induces T cell activation and results in enhanced HIV replication [23]. The excess of proinflammatory cytokines, such as tumor necrosis factor–α, that are characteristic of tuberculosis drives viral replication [38]. Chemokines upregulated at sites of tuberculosis disease, such as monocyte chemotactic protein–1, increase HIV replication and are associated with advanced HIV disease [24, 39, 40]. Therefore, treatment of tuberculosis alone may provide sufficient reduction in systemic inflammation to reduce drivers of T cell activation and thus HIV replication.
It is unclear whether tuberculosis treatment has a significant impact on other markers of HIV disease progression, such as T cell counts and changes in T cell subsets. ART partially restores naive T cell populations in individuals with HIV infection, and in some studies tuberculosis treatment increased CD4+ T cell counts among persons with HIV-tuberculosis coinfection [41, 42]. In our study, neither treatment arm experienced significant improvements in absolute CD4+ T cell counts or restoration of the naive T cell population. Importantly, however, median CD4+ T cell counts did not decline during the 12-month study period in either treatment arm, which may be a reflection of the relatively preserved CD4+ T cell counts (>350 cells/mm3) of both groups at enrollment.
Both tuberculosis and HIV infection can inhibit T cell effector functions, such as production of IFN-γ and interleukin-2, and coinfection is associated with more profound suppression of type-1 cytokine responses [12, 14, 28, 43]. Therefore, we investigated the effect of tuberculosis treatment alone in comparison with combined HIV-tuberculosis treatment on T cell production of IFN-γ in response to the mitogen PHA, and M. tuberculosis– and HIV-specific antigens. Participants who received ART had significant increases in IFN-γ production in response to mitogen at 6 months that persisted throughout the study, suggesting a global improvement in T cell effector function. However, there was no significant change in cytokine production in response to M. tuberculosis– and HIV-specific antigens in either group. These results are consistent with those of longitudinal studies of tuberculosis patients who have depressed purified protein derivative–induced IFN-γ production for 18 months or more after completion of treatment [12].
Our study has several limitations. The relatively preserved immunologic function of subjects in this study limits our ability to generalize our findings to HIV-tuberculosis coinfected persons with more advanced immunosuppression. Inclusion of comparison groups with tuberculosis or HIV infection alone on therapy would provide valuable information on the contribution of each individual infection to immune dysfunction. In addition, because subjects in our intervention arm received only 6 months of ART, we cannot determine whether continued ART would have provided more long-term immunologic benefit, ie, increased CD4+ T cell counts or improved T cell effector function. The triple nucleoside reverse transcriptase inhibitor treatment regimen used in this study minimized the possibility of drug-drug interactions, but may not have provided optimal virologic suppression in comparison with regimens with a protease inhibitor or nonnucleoside reverse transcriptase inhibitor. In addition, because immunologic follow-up was limited to 12 months, we may have missed delayed improvements in immune phenotype or function. Subjects in both treatment arms received cotrimoxazole and rifampin, so the extent to which these medications with known anti-inflammatory properties contributed to decreasing immune activation cannot be excluded. Despite these limitations, to our knowledge, this is the first randomized study to compare immunologic changes associated with tuberculosis treatment alone with those associated with tuberculosis treatment plus ART among HIV-infected individuals with pulmonary tuberculosis and preserved immunologic function.
ART has revolutionized the care of individuals with HIV by substantially reducing mortality and progression to AIDS and thus the many opportunistic infections associated with very low CD4+ T cell counts [44–46]. However, tuberculosis remains the major opportunistic infection worldwide in part because it occurs at all levels of HIV-induced immunodeficiency, as emphasized by our study population, in which 25% of participants had CD4+ T cell counts >350 cells/mm3. Our study comparing the immunologic effects of tuberculosis treatment alone with the effects of tuberculosis treatment combined with punctuated ART suggests that treatment of tuberculosis alone has a major effect on immune activation that lasts at least 1 year from the time of initiation of tuberculosis treatment. Despite recent recommendations that all HIV-infected individuals with tuberculosis receive ART, access to these drugs in places where tuberculosis is endemic remains highly variable and unpredictable. Therefore, if immune activation is a major driver of HIV disease progression, our findings suggest that, for patients with HIV and preserved (>350 cells/mm3) CD4+ T cells who receive a diagnosis of and successful treatment for pulmonary tuberculosis, delaying the initiation of ART for up to 12 months may not accelerate a decline in immunologic function.
Funding
This work was supported by the National Institutes of Health (grants AI079847-01 to C.L., AI051219 to C.W., and T32 HL07889 to C.S.M.); the Rainbow Babies and Children's Hospital Fellowship Research Award (to C.L.); the Center for AIDS Research Developmental Pilot Grant Award (to C.S.M.); and grant HHSN266200700022C/NO1-AI-70022 for the Tuberculosis Research Unit (to W.H.B.).
Acknowledgments
This study was completed only through the dedicated work of many individuals. We thank the staff of the Uganda–Case Western Reserve University Research Collaboration TB Project Clinic and the Joint Clinical Research Center in Kampala, Uganda. In particular, we would like to thank Pierre Peters, Joy Baseke, Phineas Gitta, Jalia Birabwa, Abdunulu Mbabaali, Caroline Kumukama, Michael Odie, Michael George Mujwiga, Harriet Kose-Kayanja, Ezekiel Mupere, and Dennis Dobbs for their roles in the conduct of the study. We are most grateful to the patients who participated in the study.
References
- 1.Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 2.Lado Lado FL, Barrio Gomez E, Carballo Arceo E, Cabarcos Ortiz de Barron A. Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis. 1999;31:387–91. doi: 10.1080/00365549950163842. [DOI] [PubMed] [Google Scholar]
- 3.Gilks CF, Brindle RJ, Otieno LS, et al. Extrapulmonary and disseminated tuberculosis in HIV-1-seropositive patients presenting to the acute medical services in Nairobi. AIDS. 1990;4:981–5. doi: 10.1097/00002030-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357:1519–23. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 5.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–12. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 6.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5:225–32. [PubMed] [Google Scholar]
- 7.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 8.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–28. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Gatell H, Cole SR, Hessol NA, et al. Effect of tuberculosis on the survival of women infected with human immunodeficiency virus. Am J Epidemiol. 2007;165:1134–42. doi: 10.1093/aje/kwk116. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Gatell H, Cole SR, Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22:1869–73. doi: 10.1097/QAD.0b013e32830e010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Global tuberculosis control: WHO report 2009. http://www.who.int/tb/publications/global_report/2009/en/index.html. Accessed 2 June 2010. [Google Scholar]
- 12.Hirsch CS, Toossi Z, Othieno C, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–73. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 14.Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–72. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues DS, Medeiros EA, Weckx LY, Bonnez W, Salomao R, Kallas EG. Immunophenotypic characterization of peripheral T lymphocytes in Mycobacterium tuberculosis infection and disease. Clin Exp Immunol. 2002;128:149–54. doi: 10.1046/j.1365-2249.2002.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 17.Carbone J, Gil J, Benito JM, et al. Increased levels of activated subsets of CD4 T cells add to the prognostic value of low CD4 T cell counts in a cohort of HIV-infected drug users. AIDS. 2000;14:2823–9. doi: 10.1097/00002030-200012220-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bofill M, Mocroft A, Lipman M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10:827–34. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Hultin LE, Cumberland WG, et al. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 21.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 22.Toossi Z, Johnson JL, Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28:1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 24.Toossi Z, Mayanja-Kizza H, Hirsch CS, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–8. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakata K, Rom WN, Honda Y, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 26.Hertoghe T, Wajja A, Ntambi L, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:350–7. doi: 10.1046/j.1365-2249.2000.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–4. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisset LR, Cone RW, Huber W, et al. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities: Swiss HIV Cohort Study. AIDS. 1998;12:2115–23. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Plana M, Garcia F, Gallart T, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14:1921–33. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 31.Mahan CS, Walusimbi M, Johnson DF, et al. Tuberculosis treatment in HIV infected Ugandans with CD4 counts >350 cells/mm3 reduces immune activation with no effect on HIV load or CD4 count. PLoS One. 2010;5:e9138. doi: 10.1371/journal.pone.0009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 33.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalou M, Sassan-Morokro M, Abouya L, et al. Changes in HIV RNA viral load, CD4+ T-cell counts, and levels of immune activation markers associated with anti-tuberculosis therapy and cotrimoxazole prophylaxis among HIV-infected tuberculosis patients in Abidjan, Cote d'Ivoire. J Med Virol. 2005;75:202–8. doi: 10.1002/jmv.20257. [DOI] [PubMed] [Google Scholar]
- 35.Lawn SD, Shattock RJ, Acheampong JW, et al. Sustained plasma TNF-alpha and HIV-1 load despite resolution of other parameters of immune activation during treatment of tuberculosis in Africans. AIDS. 1999;13:2231–7. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 36.Morris L, Martin DJ, Bredell H, et al. Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patients. J Infect Dis. 2003;187:1967–71. doi: 10.1086/375346. [DOI] [PubMed] [Google Scholar]
- 37.Benito JM, Lopez M, Lozano S, Martinez P, Gonzalez-Lahoz J, Soriano V. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20:227–33. doi: 10.1089/088922204773004950. [DOI] [PubMed] [Google Scholar]
- 38.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–55. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 39.Vicenzi E, Alfano M, Ghezzi S, et al. Divergent regulation of HIV-1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP-1alpha, and MCP-3, and enhancement by MCP-1. J Leukoc Biol. 2000;68:405–12. [PubMed] [Google Scholar]
- 40.Lin Y, Gong J, Zhang M, Xue W, Barnes PF. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect Immun. 1998;66:2319–22. doi: 10.1128/iai.66.5.2319-2322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohler T, Walcher J, Holzl-Wenig G, et al. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. AIDS. 1999;13:779–89. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 42.Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland R, Yang H, Scriba TJ, et al. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS. 2006;20:821–9. doi: 10.1097/01.aids.0000218545.31716.a4. [DOI] [PubMed] [Google Scholar]
- 44.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 46.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]