Abstract
Functional immunologic assays using cryopreserved peripheral blood mononuclear cells (PBMC) are influenced by blood processing, storage and shipment. The objective of this study was to compare the viability, recovery and ELISPOT results of PBMC stored and shipped in liquid nitrogen (LN/LN) or stored in LN and shipped on dry ice (LN/DI) or stored at −70°C for 3 to 12 weeks and shipped on DI (70/DI 3 to 12); and to assess the effect of donor HIV infection status on the interaction between storage/shipment and the outcome measures. PBMC from 12 HIV-infected and 12 uninfected donors showed that LN/LN conferred higher viability and recovery than LN/DI or 70/DI 3, 6, 9 or 12. LN/DI PBMC had higher viability than any 70/DI PBMC. The PBMC viability and recovery linearly decreased with the duration of storage at −70°C from 3 to 12 weeks. This effect was more pronounced in samples from HIV-infected than uninfected donors. Results of ELISPOT assays using CMV pp65, CEF and Candida albicans antigens were qualitatively and quantitatively similar across LN/LN, LN/DI and 70/DI 3. However, ELISPOT values significantly decreased with the duration of storage at −70°C both in HIV-infected and uninfected donors. ELISPOT results also decreased with PBMC viability <70%.
Introduction
The use of cryopreserved peripheral blood mononuclear cells (PBMC) for immunologic assays has increased in recent years in proportion to the globalization of medical research. The advantages of using cryopreserved PBMC for immunologic assays include the ability to consolidate testing in central laboratories and to analyze longitudinally collected samples in simultaneous runs, thus minimizing the effect of assay variability. Furthermore, targeted analysis can be performed on samples selected at the end of clinical trials using known clinical outcomes as a selection criterion.
Previous studies established the need to cryopreserve PBMC within 8 h of collection1 in order to maintain their functionality in ELISPOT assays. This observation was confirmed in a large study where cells cryopreserved on site on the day of blood collection generated higher ELISPOT and responder cell frequency results compared with cells cryopreserved after overnight shipment2. A deleterious effect of submitting the cryopreserved PBMC to large temperature variations, albeit all < 0°C, was also described3. To avoid multiple temperature changes during storage and transportation of cryopreserved PBMC, different algorithms have been proposed, including storing and shipping cells in liquid nitrogen (LN) and storing cells at −70°C for up to 3 weeks and shipping them on dry ice (DI)4 to the testing site or repository where cells are placed in LN for long term storage. Any additional shipping is done in LN in the latter shipping/storage algorithm.
This study examined for the first time in a systematic fashion the effect of shipping cryopreserved PBMC on DI after storage in LN. We also extended the existing information regarding the length of time for which cells can be stored at −70°C at the processing laboratory before being shipped on DI to the testing laboratory without loss of function and identified differences between samples from HIV-infected and uninfected donors with respect to cell viability, recovery and function. Using cells stored in LN at the processing laboratory and shipped to the testing laboratory in LN as a gold standard, we assessed the viability, recovery and function of cells stored at −70°C for 3 to 12 weeks or stored in LN and subsequently shipped on DI to the testing laboratories.
Subjects and Methods
Study Design
The Immunology Quality Assurance laboratory (IQA) of the Division of AIDS identified 12 HIV-infected and 12 uninfected donors; collected the cells by leukopheresis; cryopreserved them within 8 hours of collection and stored them in multiple replicates in LN or at −70°C. Replicate aliquots stored in LN were shipped to the testing laboratories on DI at 6 weeks and in LN at 7 weeks after collection, such that all subjects had cells shipped in both conditions. Replicate aliquots from the same donors stored at −70°C were shipped on DI at weekly intervals between 3 and 12 weeks after collection. There were 4 testing laboratories. Each laboratory received the full set of samples obtained from 3 HIV-infected and 3 uninfected volunteers. The laboratories also received centrally prepared reagents for ELISPOT assays. Samples from the same participant were tested in the same run. Results were entered in a study-specific excel spreadsheet and converted to SAS datasets for the statistical analysis.
Cryopreservation was performed as per the IMPAACT/ACTG/HPTN/HVTN cross-network consensus procedure (http://www.hanc.info/labs/Pages/SOPs.aspx).
Thawing procedure, viability and recovery
PBMC were thawed slowly as previously described5–7. Viability and viable recovery were counted manually by trypan blue exclusion on the day of thawing (Day 1) and after overnight incubation (Day 2). Overnight incubated cells were used in ELISPOT assays regardless of viability results provided enough cells were available to test at least one antigen. When the cells recovered were insufficient to test for all antigens, the following priority order was followed: medium control, CMV, candida, CEF and PHA.
ELISPOT
The general outline of the assay used in this study was previously optimized for CMV pp654. The same assay was used to investigate responses to CMV pp65 (NIH repository), CEF (NIH repository) and Candida albicans (candida; Greer). Two testing laboratories used MabTech kits (Immunotech) for the ELISPOT assays; one used BD kits (Becton Dickinson) and one an internally developed assay 4. Overnight incubated PBMC were plated at 200,000 cells/well in 100 μl RPMI containing 10% human AB or fetal calf serum. Cells were stimulated with CMV pp65 and CEF peptides at 1 μg/ml; candida antigen at 100 μg/ml8, PHA at 5 μg/ml and medium control. Spot forming centers (SFC) were counted using a computerized ELISPOT reader (ImmunoSpot, CTL) as previously described9–11. Results were reported as median SFC in antigen-stimulated wells minus median SFC in medium control wells. For CMV pp65 and CEF, which detect both CD4- and CD8- mediated responses, ELISPOT results were considered positive if the difference between stimulated and background wells was ≥50 SFC/106 PBMC and the ratio ≥2-fold. For candida, which detects CD4-mediated responses only, a positive result was defined by a difference between stimulated and unstimulated wells ≥20 SFC/106 PBMC and a ratio ≥2-fold.
Statistical analysis
An analysis of variance (ANOVA) with repeated-measures design was used to evaluate the trend of viability, viable recovery and ELISPOT results over the time of the storage at −70°C. Subjects with negative ELISPOT results in LN/LN were excluded from the ELISPOT data analyses. For the ELISPOT data analyses the data were log10 transformed to normalize their distributions. For Candida ELISPOT data which included values < 0 (due to subtraction of medium well readings from the sample well readings), a value of 6 was added to enable log transformation. The statistical significance of all paired comparisons was assessed by paired Student T test. The association of the proportions of subjects with maintained ELISPOT results (defined as >0.5 of the LN/LN gold standard results) with the proportions of subjects with Day 2 viability ≥70% was assessed using Fisher’s exact test. SAS Version 9.1.3 was used for statistical analysis; STATA 8 was used for graphic representations.
Results
Study participants
The 12 HIV-infected volunteers had a mean ± S.D. age of 42±6 years; included 1 woman; and 5 black and 7 white persons. Corresponding figures for the 12 uninfected individuals were age of 36±13 years; 4 women; all white. The HIV-infected individuals had mean ± S.D. CD4 numbers of 695±268 cells/μl and median (range) plasma HIV RNA of 134 (<40; 5010) copies/ml. The uninfected individuals had CD4 numbers of 855±275 cells/μl.
Effect of Storage and Shipment Conditions and of HIV Status on the Viability and Recovery of Cryopreserved PBMC
Viability and viable recovery of cryopreserved PBMC were measured on the day of thawing (Day 1) and after overnight incubation (Day 2). The analysis of the results obtained on Day 2 was the most relevant, because it reflected the status of the cells when the functional assays were set up. On Day 2, across all subjects, cells stored and transported in LN (LN/LN) had mean viability of 89% (95% CI: 85; 92) and viable recovery of 70% (63; 77). Cells stored in LN and shipped on DI (LN/DI) had mean viability and recovery of 84% (78; 90) and 65% (56; 74); p < 0.01 and 0.04, respectively, compared with LN/LN. Cells stored at −70°C for 3 weeks and shipped on DI (70/DI 3) had viability and recovery of 75% (66; 83) and 55% (46; 63), which represented a decrease compared with LN/LN results (p< 0.01 and <0.001, respectively) and with LN/DI results (p = 0.03 for both viability and viable recovery). Differences in the same direction were observed for Day 1 viability and recovery.
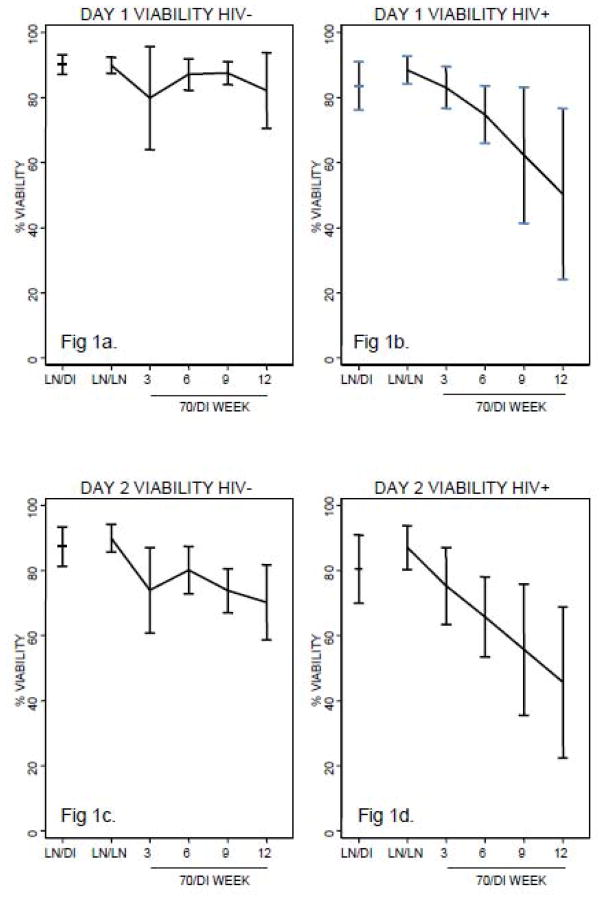
Figure 1 summarizes the viability and viable recovery separating the subjects according to their HIV status. The comparisons between samples from HIV-infected and uninfected donors did not reveal significant differences with respect to viability for the following conditions: LN/LN, LN/DI or 70/DI 3 on Day 2 or Day 1 except for the Day 1 viable recovery of LN/DI samples (p=0.04). There were significant linear trends representing a decrease of the viability and recovery of PBMC from baseline to 12 weeks of storage at −70°C in samples from HIV-infected individuals both on Day 2 and Day 1 (p ≤0.01; Fig 1b, 1d, 1f and 1h). In uninfected individuals the linear decrease over time reached statistical significance on Day 2 (p≤0.01; Fig 1c and 1g), but not on Day 1 (Fig 1a and 1e). The loss of viability over time of storage at −70°C was significantly steeper for samples from HIV-infected compared with uninfected donors on Day 2 and Day 1 (p=0.05 and 0.02, respectively). The viable recovery also had a significantly steeper decay in samples from HIV-infected compared with uninfected subjects on Day 1 (p=0.02), but not on Day 2 (p=0.11). A quadratic trend was tested across all time points, and it was not significant for Day 2 or Day 1 viability or for Day 1 recovery, indicating that the decay of viability and viable recovery over time of storage at −70°C was linear as opposed to step-wise. For Day 2 recovery, the quadratic test was positive (p=0.03), reflecting an accelerated decay from LN/LN to 70/DI 3 relative to changes at later time points.
Figure 1.
Viability and viable recovery of cryopreserved PBMC from HIV-infected and uninfected subjects according to shipment and storage conditions. Data derived from 12 HIV+ (HIV-infected) and 12 HIV− (HIV-uninfected) subjects are presented as mean and 95% CI of the viability and viable recovery on Day 1= day of thawing and on Day 2= after overnight incubation, when functional assays were set up. Cells were cryopreserved at a central laboratory and shipped to the testing laboratories in the conditions indicated on each graph: LN/DI=stored in liquid nitrogen and shipped on dry ice; LN/LN=stored and shipped in liquid nitrogen; 70/DIstored at −70°C and shipped on dry ice. 3, 6, 9, 12 indicate the numbers of weeks of storage at −70°C before shipment. The testing laboratories thawed and assayed samples from each subject in the same run. Figures 1a and 1c show that the viability of samples from HIV-uninfected individuals did not differ among LN/LN, LN/DI and 70/DI 3 samples on Day 1. On Day 2, there were no significant differences in LN/LN and LN/DI sample viability, but 70/DI 3 samples had significantly lower viability than LN/LN or LN/DI samples (p of 0.03 and 0.04, respectively). The Day 2 viability of 70/DI samples significantly decreased with the time of storage at −70°C (p=0.01), but the Day 1 did not (p=0.56). Figures 1b and 1d show that both Day 1 and Day 2 viability of cells from HIV-infected individuals was significantly higher in LN/LN compared with 70/DI 3 samples (p of 0.05 and 0.04, respectively). Viability of LN/DI samples was similar with that of LN/LN samples on Day 1, but lower on Day 2 (p of 0.1 and 0.03, respectively). No differences were observed in LN/DI and 70/DI 3 samples. There were sharp linear decreases of viability over time of storage at −70°C on Day 1 and Day 2 (p of 0.01 and 0.004, respectively). Figures 1e and 1g show that the viable recovery of samples from HIV-uninfected individuals did not differ among LN/LN, LN/DI and 70/DI 3 samples on Day 1. On Day 2, there were no significant differences in LN/LN and LN/DI sample viable recovery, but 70/DI 3 samples had significantly lower viable recovery than LN/LN or LN/DI samples (p of 0.02 and 0.05, respectively). The Day 2 viable recovery of 70/DI samples significantly decreased with the time of storage at −70°C (p =0.007), but the Day 1 did not (p=0.60). Figures 1f and 1h show that both Day 1 and Day 2 viable recovery of cells from HIV-infected individuals was significantly higher in LN/LN compared with 70/DI 3 samples (p of <0.01 and 0.02, respectively). Viable recovery of LN/DI samples was lower than that of LN/LN samples on Day 1, but similar on Day 2 (p of 0.01 and 0.09, respectively). No differences were observed in LN/DI and 70/DI 3 samples. There were sharp linear decreases of viable recovery over time of storage at −70°C on Day 1 and Day 2 (p of 0.0098 and 0.0078, respectively).
A qualitative analysis was performed to assess the proportion of samples that met acceptability criteria for functional assays, defined as viability ≥70% or viable recovery ≥50% (Table 1). Overall, samples in LN/LN had the lowest (0 to 9%) and samples in 70/DI the highest (17 to 73%) failure rates. Samples in LN/DI had intermediate failure rates of 8 to 27%.
Table 1.
Effect of shipping and storage conditions on the proportion of cryopreserved PBMC samples that fail viability or viable recovery acceptability criteria for functional assays
| Outcome | HIV Status | LN/LN | 70/DI 3 | 70/DI 6 | 70/DI 9 | 70/DI 12 | LN/DI |
|---|---|---|---|---|---|---|---|
| Day 2 Viability<70% | HIV− | 0% (0/12) | 25% (3/12) | 17% (2/12) | 42% (5/12) | 42% (5/12) | 8% (1/12) |
| HIV+ | 9% (1/11) | 36% (4/11) | 64% (7/11) | 64% (7/11) | 73% (8/11) | 27% (3/11) | |
| Day 2 Viable recovery<50% | HIV− | 8% (1/12) | 33% (4/12) | 25% (3/12) | 25% (3/12) | 33% (4/12) | 17% (2/12) |
| HIV+ | 0% (0/11) | 45% (5/11) | 64% (7/11) | 64% (7/11) | 73% (8/11) | 18% (2/11) | |
| Day 2 Viability<70% or Viable recovery<50% | HIV− | 8% (1/12) | 42% (5/12) | 33% (4/12) | 50% (6/12) | 50% (6/12) | 17% (2/12) |
| HIV+ | 9% (1/11) | 45% (5/11) | 73% (8/11) | 73% (8/11) | 73% (8/11) | 27% (3/11) |
Results did not differ across all 4 testing laboratories, but there were lab/sample-specific outliers.
Effect of Storage and Shipment Conditions and of HIV Status on ELISPOT Results
ELISPOT values for each antigen were compared across storage and shipment conditions for samples that had a positive result in LN/LN. The exclusion of samples with negative ELISPOT results in the gold standard increased the ability to detect a decrease in responses in the conditions that were being investigated. In LN/LN samples, CMV pp65 ELISPOT results had a mean of 766 SFC/106 PBMC (95% CI = 382,1534; N=16; Fig 2). There were no significant differences between LN/DI or 70/DI 3 and LN/LN gold standard samples. However, there was a significant linear decay of SFC in 70/DI 3, 6, 9 and 12 samples over the time of storage at −70°C (p=0.03). Similar results were obtained for CEF, except that values were generally higher (mean and 95% CI in LN/LN samples of 1237, 627 and 2439 SFC/106 PBMC, respectively; N=13) and the linear decrease in 70/DI over time of storage did not reach statistical significance (p=0.08). For candida, the mean (95% CI) of LN/LN samples was 265 SFC/106 PBMC (114, 605; N=14). The results of LN/DI and 70/DI 3 samples did not significantly differ from the LN/LN sample results with mean (95% CI) of 164 (57; 447) SFC/106 PBMC, p=0.1 and 217 (76 and 559) SFC/106 PBMC, p=0.35, respectively. There was a highly significant linear decrease of SFC with time of storage at −70°C (p=0.007). For all antigens, the quadratic test did not show significant trends, indicating that the loss in SFC over time was linear. The overall linear trends did not reveal significant differences by donor HIV status and they were evident across all testing laboratory.
Figure 2.
Effect of storage and shipment conditions on ELISPOT results. Data were derived from cryopreserved PBMC of 12 HIV-infected and 12 uninfected subjects. Cells were cryopreserved at a central laboratory and shipped to the testing laboratories in the conditions indicated on each graph: LN/DI=stored in liquid nitrogen and shipped on dry ice; LN/LN=stored and shipped in liquid nitrogen; 70/DIstored at −70°C and shipped on dry ice. 3, 6, 9, 12 indicate the numbers of weeks of storage at −70°C before shipment. The testing laboratories thawed and assayed samples from each subject in the same run. Results were pooled and presented as means and 95% CI of the entire population, because there were no differences by HIV status (p>0.5). Fig 2a shows that CMV pp65 peptide- stimulated ELISPOT values were not significantly different in LN/LN, LN/DI and 70/DI 3 samples. There was a linear decrease of ELISPOT values with the time of storage at −70°C (p=0.03). Fig 2b shows that CEF peptide-stimulated ELISPOT values were similar in LN/LN, LN/DI and 70/DI 3 samples. There was a trend linear decrease of ELISPOT values with the time of storage at −70°C that did not reach statistical significance (p=0.08). Fig 2c shows that candida- stimulated ELISPOT values were similar in LN/LN, LN/DI and 70/DI 3 samples. There was a linear decrease of ELISPOT values with the time of storage at −70°C (p=0.007).
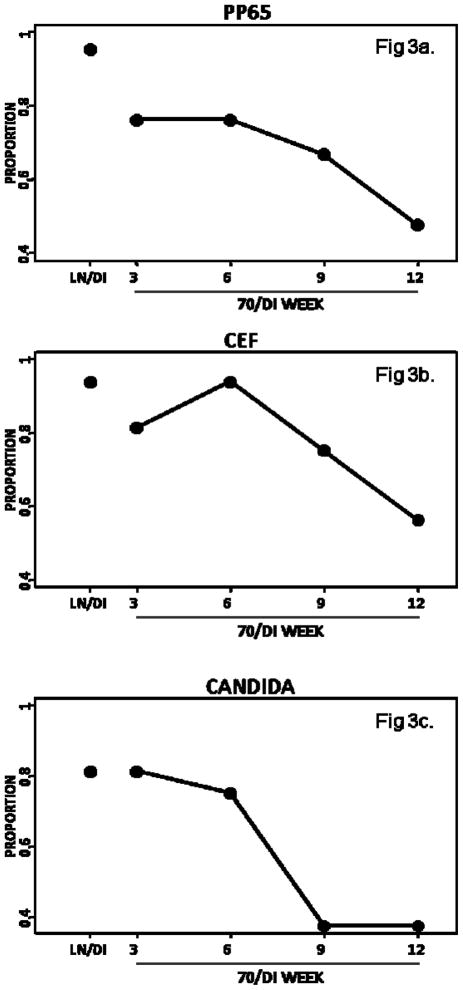
A qualitative analysis was performed to determine the proportion of samples in each condition for which the ELISPOT result would be considered different from the result obtained in LN/LN gold standard conditions. A score=0 was ascribed if the samples had a ≥2-fold decrease of SFC compared with the LN/LN gold standard or if there were too few cells to perform the assay. A score=1 was ascribed to samples with < 2-fold decrease relative to the LN/LN gold standard (Figure 3). The data showed that 95% and 94% of LN/DI samples reached a passable score of 1 for pp65 and CEF, respectively. Among 70/DI 3 samples, 76% and 81% had a passable score of 1, but among 70/DI 12 samples only 48% and 56%, respectively, reached the passable score. For candida, 81% of samples had a score of 1 both in LN/DI and 70/DI 3, but only 38% of the 70/DI 12 samples achieved this mark.
Figure 3.
Effect of shipment and storage of cryopreserved PBMC on the reproducibility of ELISPOT results relative to the LN/LN gold standard. Data were derived from PBMC obtained from 12 HIV-infected and 12 uninfected individuals. Cells were cryopreserved at a central laboratory and shipped to the testing laboratories in the conditions indicated on each graph: LN/DI=stored in liquid nitrogen and shipped on dry ice; LN/LN=stored and shipped in liquid nitrogen; 70/DIstored at −70°C and shipped on dry ice. 3, 6, 9, 12 indicate the numbers of weeks of storage at −70°C before shipment. The testing laboratories thawed and assayed samples from each subject in the same run. Data are presented as the proportion of samples in each storage/shipment condition that had a <2-fold difference in SFC compared with the gold standard results obtained in the LN/LN samples. 95, 94 and 81% of LN/DI samples had SFC within 2 fold of the LN/LN sample SFC for CMV pp65, CEF and candida, respectively. For 70/DI 3, corresponding numbers were 76, 81 and 81%, respectively. The proportion of samples with ELISPOT results within 2 fold of the LN/LN gold standard decreased with longer time of storage at −70°C.
Effect of Viability on ELISPOT Results
A viability threshold of 70% below which results of lymphoproliferative assays lose interpretability was experimentally determined in previous studies5, 12. Similar studies were not previously performed for ELISPOT. We examined the relationship between acceptable viability, defined as ≥70%, with a passable ELISPOT score of 1 (described above). With one exception, passable ELISPOT results were associated with acceptable viabilities across antigens and time periods. However, this association varied by antigen, such that it was relatively weak for candida, which registered the lowest ELISPOT-measured responses in this study, it was stronger but not statistically significant for CEF and it was strongest and statistically significant for CMV pp65 in most conditions (Table 2).
Table 2.
Effect of Day 2 viable recovery ≥70% on CMV pp65 ELISPOT results
| N | Sample ID | Elispot Score=0* | Elispot Score=1** | Fisher’s exact p-value |
|---|---|---|---|---|
| 21 | LN/DI | 0% (0/1) | 85% (17/20) | 0.19 |
| 21 | 70/DI 3 | 20% (1/5) | 81% (13/16) | 0.03 |
| 21 | 70/DI 6 | 0% (0/5) | 75% (12/16) | <0.01 |
| 21 | 70/DI 9 | 0% (0/7) | 64% (9/14) | <0.01 |
| 21 | 70/DI 12 | 27% (3/11) | 60% (6/10) | 0.20 |
Discussion
We showed that LN/DI or 70/DI storage/shipment of cryopreserved PBMC are associated with a decrease in viability and viable recovery compared with LN/LN. Although statistically significant, the decrease in viability for LN/DI or 70/DI 3 was small enough not to translate into loss of function. Likewise, although the viability of LN/DI PBMC was significantly higher than that of 70/DI 3 PBMC, there was no difference between these conditions with respect to ELISPOT results. The most likely explanation for the discrepancy between the effects of storage/shipment conditions on viability compared with function of cryopreserved PBMC is that the viable cell count is used to set up functional assays, thus attenuating the effect of decreased viability. In addition, functional assays are more variable and require larger sample sizes to demonstrate significant differences. Our study had 80% power to detect 2.3-, 1.8-and 2.5-fold differences between LN/DI and 70/DI 3 conditions for CMVpp65, CEF and candida ELISPOT values, respectively. In contrast, due to the lower variability of viability counts, the study had 80% power to detect 10%differences in Day 1 and Day 2 viability and 13.5% and 12% differences in Day 1 and Day 2 viable recovery.
Storage of cryopreserved PBMC at −70°C for 3 to 12 weeks prior to shipment was associated with a continuous decrease over time of storage of both viability and function of the PBMC. Although differences in PBMC viability were easily demonstrable between each 70/DI time point and the LN/LN gold standard, this was not the case for PBMC function. However, the test of trend clearly showed the decay of both viability and function over time of storage. A previous study4 that used only 3 weeks of storage at −70°C did not have the tools to detect the linear effect of time of −70°C storage on viability or function, explaining the difference in findings between this previous study and ours. The corollary of our observation is that cell storage at −70°C for variable periods of time may introduce a bias in the result of functional assays solely due to the difference in the time of storage. The linearity of the functional decay suggests that even small differences in time of storage, if consistent across samples, may introduce a significant bias.
Our study compared for the first time the effect of storage and shipment conditions of cryopreserved PBMC obtained from HIV-infected and uninfected subjects in parallel assays. There were no significant differences between the viability of cryopreserved PBMC from HIV-infected and uninfected donors in LN/LN, LN/DI or 70/DI 3 conditions. However, the viability of cryopreserved PBMC from HIV-infected individuals decreased significantly faster with time of storage at −70°C than the viability of PBMC from uninfected donors. It is conceivable that PBMC from HIV+ individuals contained a sizable amount of apoptotic cells that were not detected by trypan blue exclusion even before cryopreservation. These cells may have been responsible for the rapid decay in viability of HIV+ PBMC stored at −70°C. Irrespective of the mechanism that accounts for the loss of viability of PBMC from HIV+ individuals, our findings indicate that the bias introduced by the time of storage of cryopreserved PBMC at −70°C may be higher in studies that use samples from HIV-infected subjects than in those that use exclusively uninfected individuals. Of note, some participants in longitudinal studies including HIV vaccine clinical trials may change HIV status during the study, complicating the analysis of functional assays if PBMC are stored at −70°C before shipment.
We assessed the effect of the viability of cryopreserved PBMC on ELISPOT results. For this analysis, we used a viability threshold of 70%, previously shown to affect lymphocyte proliferation assays5, 12 and CMV ELISPOT results as the primary outcome measure, because the ELISPOT assay used in this study was previously optimized for CMV pp65, but not for the other antigens. The data showed a significant correlation between the two variables, indicating that cells with viability < 70% may introduce a bias in the ELISPOT-measured responses. A correlation between ELISPOT results for the other antigens and viability was present in some storage and shipment conditions, but not in all.
A clear limitation of this study was the application of ELISPOT conditions optimized for one antigen to all antigens. For example, we previously determined that optimal candida ELISPOT results are obtained at a concentration of 500,000 PBMC/well, 2.5-fold higher than the one used in this study. The low frequency of candida-specific responders may explain the significant loss of SFC under conditions that did not detect a difference in CMV-specific SFC. Since the study already had a complicated design, we opted for the use of a single ELISPOT format rather than different formats for each antigen. Another limitation of this study was the sample size of 24 subjects, which further decreased to 23 at two time points due to delays in transportation. An advantage of this study, however, was the use of four independent testing laboratories. The fact that the results were homogeneous across labs attests to their reproducibility in the field.
In conclusion, LN/LN storing and shipping grants a quality of cryopreserved PBMC that is not matched by either LN/DI or 70/DI 3. However, due to its high cost, LN/LN may not be practical for large clinical trials. Under limited funding conditions, LN/DI has the advantage that samples can be stored for variable periods of time in LN and shipments staggered at long intervals without any detriment to the assay results. There is a cost, however, associated with the provision of LN tanks or −150°C freezers to processing laboratories. 70/DI 3, which was the only 70/DI condition that may be acceptable, requires frequent shipments, but avoids the investment in LN tanks or −150°C freezers. If cells are stored and shipped in 70/DI, we recommend that the precise time of storage of each sample at −70°C be carefully monitored and factored into the statistical analysis to rule out potential biases that may be solely introduced by this variable.
Acknowledgments
We thank Ms. Daniella Livnat and Dr s. Susan Fiscus and Patricia Reichelderfer for continuous support of this project, advice and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Kierstead LS, Dubey S, Meyer B, et al. Enhanced rates and magnitude of immune responses detected against an HIV vaccine: effect of using an optimized process for isolating PBMC. AIDS Res Hum Retroviruses. 2007 Jan;23(1):86–92. doi: 10.1089/aid.2006.0129. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009 Oct 1;200(7):1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JG, Joseph HR, Green T, et al. Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin Vaccine Immunol. 2007 May;14(5):527–537. doi: 10.1128/CVI.00435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull M, Lee D, Stucky J, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007 Apr 30;322(1–2):57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg A, Song LY, Wilkening C, et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol. 2009 Aug;16(8):1176–1186. doi: 10.1128/CVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boaz MJ, Hayes P, Tarragona T, et al. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin Vaccine Immunol. 2009 Feb;16(2):147–155. doi: 10.1128/CVI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maecker HT, Moon J, Bhatia S, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg A, Dickover R, Britto P, et al. Continuous improvement in the immune system of HIV-infected children on prolonged antiretroviral therapy. AIDS. 2008 Nov 12;22(17):2267–2277. doi: 10.1097/QAD.0b013e3283189bb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buseyne F, Catteau A, Scott-Algara D, et al. A vaccinia-based elispot assay for detection of CD8+ T cells from HIV-1 infected children. J Immunol Methods. 2005 Mar;298(1–2):105–118. doi: 10.1016/j.jim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Gorse GJ, Baden LR, Wecker M, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008 Jan 10;26(2):215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg A, Pott GB. Immunity to human immunodeficiency virus (HIV) in children with chronic HIV infection receiving highly active antiretroviral therapy. Clin Diagn Lab Immunol. 2003 Sep;10(5):821–825. doi: 10.1128/CDLI.10.5.821-825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg A, Zhang L, Brown D, et al. Viability and functional activity of cryopreserved mononuclear cells. Clin Diagn Lab Immunol. 2000 Jul;7(4):714–716. doi: 10.1128/cdli.7.4.714-716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]