Abstract
Background
Uptake of HIV testing and counseling (HTC) is lower among members of the poorest households in sub-Saharan countries, thereby creating significant inequalities in access to HTC and possibly ARV treatment.
Objectives
To measure uptake of home-based HTC and estimate HIV prevalence among members of the poorest households in a sub-Saharan population.
Methods
Residents of 6 villages of Likoma Island (Malawi) aged 18–35 and their spouses were offered home-based HTC services. Socioeconomic status, HIV testing history and HIV risk factors were assessed. Differences in HTC uptake and HIV infection rates between members of households in the lowest income quartile and the rest of the population were estimated using logistic regression.
Results
Members of households in the lowest income quartile were significantly less likely to have ever used facility-based HTC services than the rest of the population (OR = 0.60, 95% CI: 0.36–0.97). In contrast, they were significantly more likely to use home-based HTC services provided during the study (aOR = 1.70, 95% CI: 1.04–2.79). Socioeconomic differences in uptake of home-based HTC were not due to underlying differences in socioeconomic characteristics or HIV risk factors. The prevalence of HIV was significantly lower among members of the poorest households tested during home-based HTC than among the rest of the population (aOR=0.37, 95% CI: 0.14–0.96).
Conclusions
HTC uptake was high during a home-based HTC campaign on Likoma Island, particularly among the poorest. Home-based HTC has the potential to significantly reduce existing socioeconomic gradients in HTC uptake, and help mitigate the impact of AIDS on the most vulnerable households.
Keywords: HIV testing and counseling, HIV prevention, ARV treatment, poverty and inequality, Malawi, sub-Saharan Africa
The rapid expansion of HIV treatment is an important response to the HIV/AIDS epidemic in sub-Saharan countries. However, enrollment of infected individuals in treatment programs continues to be hampered by the low uptake of HIV testing and counseling (HTC). Furthermore, Demographic and Health Surveys (DHS) conducted in sub-Saharan African countries have identified a strong socioeconomic gradient in uptake of HTC.1–4 A significant fraction of the most vulnerable households may thus be excluded from the potential benefits stemming from the knowledge of one's HIV status—such as the adoption of strategies to reduce HIV transmission to partners and children—and the benefits associated with anti-retroviral treatment—such as increased survival and labor productivity.5–8
Poor accessibility of health facilities, fatalism, HIV-related stigma and confidentiality are the main barriers to use of HTC services in African countries.9 While several strategies to increase the uptake of HTC among sub-Saharan populations have been suggested,10 their impact on the poorest may be limited. Routine testing in hospitals and other health care facilities, for example, significantly increases uptake and case-finding among the attendees of these facilities,11–13 but cost and convenience issues often limit the use of healthcare facilities among the lower socioeconomic strata in sub-Saharan countries.1,2 HTC uptake is also increased through workplace-based initiatives,14 but such strategies similarly do not reach the poorest members of society who are often unemployed or employed in the informal sector. To overcome these limitations, the home-based provision of HTC services has recently been proposed as a strategy to attain “universal HIV testing and counseling” in Africa.15 Indeed, community-based approaches—like mobile HTC units or home-based HTC provision—have been shown to dramatically increase the uptake of testing services.16–20 However, previous studies have not documented whether the provision of home-based HTC may reduce the socioeconomic gradient in HTC uptake observed in sub-Saharan countries. The objectives of this study are to measure the uptake of home-based HTC among different socioeconomic groups on Likoma, and to assess whether the home-based provision of HTC services has the potential to reduce inequalities in access to, and uptake of, HTC.
1 Methods
Our analyses are based on the Likoma Network Study (LNS) that is conducted on a small island (Likoma) located in the northern region of Lake Malawi.21,22 In 2005, the island was inhabited by approximately 7,000 people residing in 12 villages. Facility-based HTC was offered on the island at the local hospital (operated by the Anglican diocese of Northern Malawi), and one NGO-operated HTC center. At the time of the study, HTC at these facilities was entirely free of charge, and was conducted on a patient-initiated (“opt-in”) basis only. More distant HTC centers were located on the mainland of Malawi. HTC was not offered in the bordering villages of the Mozambican shore.
1.1 Likoma Network Study
The Likoma Network Study is a study that investigates sexual networks and HIV transmission on the island.21,22 It enumerated 1,030 eligible adults aged 18–35 and their older spouses in 7 villages of the island, of which 923 (89%) completed a socioeconomic, health and sexual networks survey during January 2006 (Figure 1). Interviews were conducted using computer-assisted self-interviewing techniques23, and all questions were fixed-choice questions. Respondents were asked if they had ever been tested for HIV infection at a health facility and, if not, the main reason why they had not used facility-based HTC services. Sexual risk behaviors elicited during the survey included number of sexual partners over a 3-year period and number of concurrent partnerships at the time of the survey.21 Self-reported symptoms of STIs during the year prior to the survey were ascertained through a series of questions with yes/no answers. Such symptoms included painful urination, ulceration of the genital area or discharge from the penis/vagina. A dummy variable was created that equaled one if a respondent experienced any of these symptoms, and zero otherwise. Socioeconomic status of households was measured by household income. In particular, respondents were asked about the main income generating activity of each household member, and the typical income from this activity over a 1-month period. Respondents were then asked if there were other income-generating activities household members were engaged in, and how much they would earn from these sources over the course of a month. Total household income was calculated as the sum of incomes from these different activities. Per capita household income was obtained by dividing the total income by the number household members (average household size = 6.2, IQR: 4–8). Per capita household income is used to classify households into those belonging to lowest income quartile (households with a per capital income below the 25th percentile), and those belong to higher income quartiles (households with a per capital income above the 25th percentile). When several household members were interviewed, the total household income used in our analyses is the average of individual reports (the correlation among reports of household income across household members was 0.81). Average monthly income per household resident was 1,430 Kwachas (≈ 10USD), equivalent to 0.33USD per person and per day. Ninety percent of the LNS respondents had an income below the World Bank poverty line of 1USD per day and per person, and the poorest quartile of LNS respondents was earning under 0.15$ per person and per day. Other information allowing the measurement of household poverty were limited to characteristics of a family's house (e.g., roof and wall material). More detailed measures of household poverty (for example based on household expenditures) were not available in our data.
Figure 1.
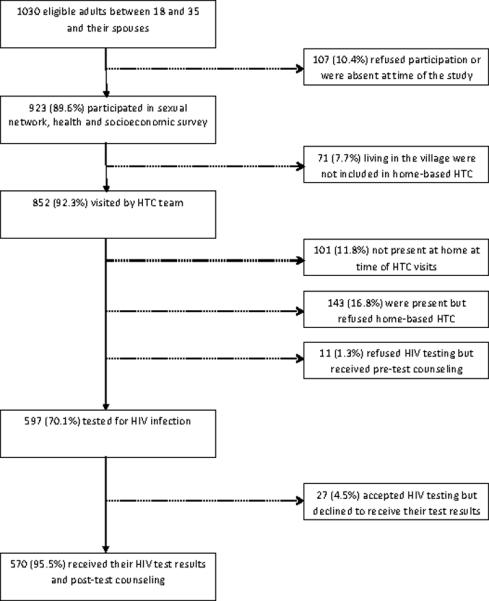
Flow chart of participation in home-based HTC provision during the Likoma Network Study
1.2 Home-based HIV testing and counseling procedures
In February 2006, two attempts were made by the research team to contact for HTC all participants of the LNS survey in six (out of seven) study villages. The seventh village was not included due to funding and timing constraints. Prior to the LNS, home-based HTC had not been available to Likoma residents. Residents were offered home-based HTC services irrespective of prior testing history. The research team responsible for the provision of home-based HTC was composed of 10 health counselors trained and certified in HTC by the Malawian Ministry of Health. All health counselors were recruited from the mainland to increase the confidentiality of HTC. HIV testing followed the Malawian Ministry of Health guidelines and was based on two parallel rapid tests for the detection of HIV antibodies (Determine and Unigold). Sensitization meetings were conducted in each village prior to the start of home-based HTC. Consent from traditional chiefs and government officials was obtained. The study protocol received clearance from institutional review boards at the University of Pennsylvania (Philadelphia, USA) and at the University of Malawi College of Medicine (Blantyre, Malawi).
Participation in home-based HTC was voluntary and based on informed consent. Typical counseling and testing sessions were to last between 40 and 60 minutes, with 15–20 minutes allocated for pre-test counseling, 15–20 allocated for testing, and the remainder of the session consisting of post-test counseling. Participants could refuse HIV testing after having completed pre-test counseling, and they could also opt not to learn their results after completing HIV testing. Testing and counseling were strictly individual, and we did not attempt to jointly counsel and test co-residing couples. Study participants in HTC were provided with a small bar of soap—valued at approximately 0.06USD—as a token of appreciation. Because participants may have been concerned about the privacy of home-based HTC,16,20 they finally had the option to be tested in a private room at the research team's offices on the island.
1.3 Statistical approach
All LNS data were double-entered into a MS Access database during fieldwork, and all analyses were performed using STATA 10. We computed odds ratios for the odds of using facility-based and home-based HTC services, as well as HIV infection risk, based on the presence of various sociodemographic and behavioral characteristics. Adjusted odds ratios were computed using logistic regression models that included adjustments for clustering of observations within households.24 Determinants of participation in home-based HTC were assessed first among all respondents visited by the HTC team; second, among respondents who were present at their homes at the time of the visit(s). We focus on respondents who accepted to be tested and learned their test results. Differences in the effect of low household income on HTC uptake by gender were assessed on the basis of an interaction term introduced in the logistic regression models. Predicted probabilities of participation in facility-based and home based HTC were derived from these models using the STATA 10 command.25 All models included village fixed-effects to control for distance to the testing facility as a determinant of HTC uptake.
2 Results
2.1 Uptake of Facility-based HIV testing and counseling
Table 1 describes the (reported) uptake of facility-based HTC among the LNS study population. 23.5% of men and 22.4% of women reported having ever been tested for HIV at a health-care facility. This uptake of facility-based HTC varied considerably across socioeconomic groups. Table 1 documents significantly lower uptake of facility-based HTC among members of the poorest income quartile: while 25.4% of LNS respondents in the upper income quartiles had ever been tested for HIV at a health-care facility, this was the case for only 16.9% of the poorest. These socioeconomic differences in uptake of facility-based HTC remained even after controlling for underlying differences in sexual behaviors and co-factors of HIV infection (adjusted OR: 0.66, 95% CI: 0.41–1.07). Other important correlates of having ever used facility-based HTC services included older age, increased schooling, having ever resided on the mainland, and having had multiple sexual partners over the 3 years prior to the survey. The main reasons reported by respondents for never having used facility-based HTC services were distance to the testing center (24%), lack of confidentiality at the testing center (20%) and fear of diagnosis (15%). Reasons for never having been tested did not differ significantly by income quartile.
Table 1.
Determinants of participation in facility-based HIV counseling and testing. Likoma Island, February 2006.
| Ever tested for HIV at health facility |
||||
|---|---|---|---|---|
| N (% ever tested†) | Unadjusted OR (95% CI) | Adjusted OR‡ (95% CI) | ||
| Sociodemographic characteristics | ||||
| Sex | Men(Ref) | 370(23.5) | 1 | 1 |
| Women | 432(22.4) | 0.91 (0.67,1.24 | 1.09 (0.77,1.56) | |
| Age | < 20 (Ref) | 153(10.5) | 1 | 1 |
| 20–24 | 264(28.0) | 3.58(1.90,6.72)** | 3.62(1.83,7.14)** | |
| 25–29 | 196(31.0) | 3.72(1.95,7.09)** | 4.43(2.03,9.66)** | |
| 30–34 | 106(19.8) | 2.25(1.05,4.81)** | 2.73(1.11,6.72)** | |
| 35+ | 83(19.3) | 2.18(0.99,4.79)* | 2.72(1.02,7.21)** | |
| Marital status | Never married (Ref) | 343(22.7) | 1 | 1 |
| currently married | 402(22.4) | 0.99(0.69,1.42) | 0.82(0.51,1.31) | |
| widowed or divorced | 57(8.7) | 1.23(0.66,2.33) | 0.96(0.44,2.11) | |
| Schooling | Incomplete primary (Ref) | 353(15.6) | 1 | 1 |
| Completed primary school | 449(28.7) | 2.11(1.45,3.09)** | 1.79(1.18,2.71)** | |
| Religion | Anglican (Ref) | 671(21.9) | 1 | 1 |
| Other religions | 131(28.2) | 1.46(0.93,2.29)* | 1.38(0.85,2.25) | |
| Residence | Never on mainland (Ref) | 503(18.1) | 1 | 1 |
| Ever on mainland | 295(31.2) | 2.12(1.52,2.98)** | 1.65(1.14,2.39)** | |
| Income | Top income quartiles (25th percentile and above, Ref) | 587(25.4) | 1 | 1 |
| Bottom quartile (Below 25thpercentile) | 189(16.9) | 0.60(0.36,0.97)** | 0.66(0.41,1.07)* | |
| HIV behaviors and risk factors | ||||
| Number of sexual partners during 3-year period prior to survey | ||||
| 0–1 (Ref) | 247(16.6) | 1 | 1 | |
| 2 | 229(24.4) | 1.55(0.98,2.47)* | 1.14(0.71,1.83) | |
| 3 or more | 326(26.7) | 1.87(1.24,2.82)** | 1.31(0.83,2.08)* | |
| Concurrent partnerships at time of home-based HTC visit | ||||
| No (Ref) | 685(22.5) | 1 | 1 | |
| Yes | 117(25.6) | 1.16(0.74,1.84) | 1.20(0.73,1.97) | |
| Symptoms of STI over the last 12 months | ||||
| No symptoms (Ref) | 659(23.2) | 1 | 1 | |
| Any symptoms | 143(21.7) | 0.97(0.62,1.51) | 1.39(0.86,2.24) | |
Notes:
p < 0.1
p < 0.05
Figures in parentheses are row percentages indicating the proportion of participants in a given category who have ever been tested for HIV at a health-care facility.
Estimates presented in this column are derived from a logistic regression model that includes all variables listed in the table. The model is estimated among respondents with non-missing information on all variables.
2.2 Uptake of Home-based HIV testing and counseling
The LNS team attempted to enroll a total of 852 study participants for home-based HTC (Figure 1), of whom 101 (11.9%) were absent at the time of the visits, and 143 (19.0%) declined to participate during the informed consent process. 87% of men and 89.1% of women were available for HTC at one of the LNS team's visits. While older respondents were generally less likely to be present, other socioeconomic characteristics and HIV risk factors were not related to the probability of being successfully contacted by the research team. Eleven respondents received pre-test counseling, but subsequently refused to be tested for HIV infection. 597 (70.1%) respondents agreed to be tested for HIV during home-based HTC, and among those HIV prevalence was 8.0% (95% CI: 6.0–10.5). Of the 597 LNS participants tested for HIV infection, 585 (98%) preferred HTC to be conducted at their home and 12(2%) requested that HTC be conducted in a private room at the LNS offices instead. Among those tested, 570 (95.5%) chose to be informed about their results immediately. Seven respondents asked to learn their results at a later date but never returned to retrieve them.
The determinants of participation in home-based HTC are described in Table 2. Consistent with earlier studies,16–20 the uptake of home-based HTC is considerably higher than that of facility-based HTC: 62.5% of eligible men, and 70.7% of eligible women participated learned their test result. Among respondents who were successfully contacted, these figures increased to 71.9 and 79.3%, respectively. The uptake of home-based HTC was significantly higher among members of households in the lowest income quartile relative to the rest of the population (OR: 1.45, 95% CI: 0.97–2.17). Among respondents who were found at home, the odds of learning one's HIV status during home-based HTC among the poorest respondents were 1.6 times those of respondents in the upper income quartiles (95% CI: 1.04,2.46). These differences remained, and even increased in magnitude, in multivariate analyses that controlled for underlying differences in sexual risk behaviors and co-factors of HIV infection (adjusted OR: 1.70, 95% CI: 1.04,2.79). In addition, participation in home-based HTC was significantly higher among younger respondents, respondents who had completed primary schooling, respondents with multiple sexual partners over the last 3 years, and respondents who had experienced symptoms of sexually transmitted infections during the year prior to the survey. Participation in home-based HTC was however lower among respondents who were engaged in multiple concurrent sexual partnerships at the time of the survey.
Table 2.
Probability of being tested and learning HIV status during home-based HIV testing and counseling. Likoma Island, February 2006.
| Among all respondents visited by HTC team |
Among respondents successfully contacted |
||||||
|---|---|---|---|---|---|---|---|
| N* (%§) | Unadjusted OR† (95% CI) | Adjusted OR‡ (95%CI) | N** (%§) | Unadjusted OR† (95% CI) | Adjusted OR†‡ (95% CI) | ||
| Sociodemographic characteristics | |||||||
| Sex | Men(Ref) | 392(62.5) | 1 | 1 | 341(71.9) | 1 | 1 |
| Women | 460(70.7) | 1.43(1.09,1.86)** | 1.33(0.97,1.83)* | 410(79.3) | 1.48(1.07,2.03)** | 1.42(0.97,2.08)* | |
| Age | < 20 (Ref) | 165(79.4) | 1 | 1 | 154(85.1) | 1 | 1 |
| 20–24 | 276(72.5) | 0.69(0.44,1.10) | 0.65(0.39,1.08)* | 245(81.6) | 0.80(0.47,1.36) | 0.69(0.37,1.29) | |
| 25–29 | 208(64.4) | 0.47(0.29,0.76)** | 0.51(0.28,0.94)** | 183(73.2) | 0.48(0.28,0.82)** | 0.46(0.22,0.96)** | |
| 30–34 | 115(52.2) | 0.29(0.18,0.49)** | 0.29(0.15,0.55)** | 97(61.9) | 0.29(0.16,0.53)** | 0.25(0.11,0.54)** | |
| 35+ | 88(51.1) | 0.39(0.23,0.65)** | 0.27(0.13,0.57)** | 72(62.5) | 0.36(0.20,0.65)** | 0.28(0.11,0.67)** | |
| Marital status | Never married (Ref) | 364(71.1) | 1 | 1 | 330(78.5 | 1 | 1 |
| currently married | 426(64.3) | 0.73(0.53,1.01)* | 1.01(0.65,1.57) | 367(74.7) | 0.80(0.55,1.15) | 1.15(0.67,1.98) | |
| widowed or divorced | 61(59.0) | 0.57(0.33,0.99)** | 0.92(0.46,1.85) | 53(67.9) | 0.57(0.31,1.04)* | 0.94(0.42,2.09) | |
| Schooling | Incomplete primary (Ref) | 377(62.6) | 1 | 1 | 332(71.1) | 1 | 1 |
| Completed primary school | 475(70.3) | 1.37(1.01,1.84)** | 1.28(0.90,1.81) | 419(79.7) | 1.55(1.09,2.22)** | 1.35(0.88,2.09) | |
| Religion | Anglican (Ref) | 709(66.6) | 1 | 1 | 624(75.6) | 1 | 1 |
| Other religions | 143(68.5) | 1.09(0.70,1.72) | 1.11(0.68,1.81) | 127(77.2) | 1.13(0.66,1.93) | 1.17(0.66,2.06) | |
| Residence | Never on mainland (Ref) | 538(66.7) | 1 | 1 | 478(75.1) | 1 | 1 |
| Ever on mainland | 308(67.5) | 1.04(0.77,1.41) | 1.02(0.73,1.44) | 268(77.6) | 1.14(0.79,1.64) | 1.06(0.70,1.59) | |
| Income | Top income quartiles (25th percentile and above, Ref) | 627(64.6) | 1 | 1 | 550(73.6) | 1 | 1 |
| Bottom quartile (Below 25th percentile) | 197(72.6) | 1.45(0.97,2.17)* | 1.45(0.97,2.17)* | 175(81.7) | 1.60(1.04,2.46)** | 1.70(1.04,2.79)** | |
| HIV behaviors and risk factors | |||||||
| Number of sexual partners during 3-year period prior to survey | |||||||
| 0–1 (Ref) | 270(63.7) | 1 | 1 | 245(70.2) | 1 | 1 | |
| 2 | 244(67.6) | 1.18(0.81,1.72) | 1.22(0.80,1.86) | 211(77.7) | 1.48(0.96,2.35)* | 1.57(0.96,2.57)* | |
| 3 or more | 339(69.0) | 1.24(0.86,1.78) | 1.26(0.81,1.97) | 295(79.3) | 1.55(1.03,2.35)** | 1.56(0.93,2.64)* | |
| Concurrent partnerships at time of home-based HTC visit | |||||||
| No (Ref) | 731(67.6) | 1 | 1 | 646(76.5) | 1 | 1 | |
| Yes | 121(62.8) | 0.78(0.52,1.17) | 0.60(0.38,0.96)** | 105(72.4) | 0.78(0.50,1.22) | 0.52(0.31,0.88)** | |
| Symptoms of STI over the last 12 months | |||||||
| No symptoms (Ref) | 664(64.9) | 1 | 1 | 580(74.3) | 1 | 1 | |
| Any symptoms | 144(77.1) | 1.80(1.18,2.76)** | 1.91(1.19,3.09)** | 131(84.7) | 1.84(1.11,3.07)** | 1.94(1.11,3.40)** | |
| HIV testing history | |||||||
| Never been tested for HIV prior to study (Ref) | 618(67.15) | 1 | 1 | 551(75.3) | 1 | 1 | |
| Ever tested prior to study | 184(66.3) | 0.94(0.65,1.36) | 0.93(0.62,1.41)** | 154(79.2) | 1.23(0.79,1.91) | 1.27(0.76,2.12) | |
Notes:
p < 0.1
p < 0.05
* Number of respondents for whom contact attempt was made
** Number of respondents who were successfully contacted by HTC team and who were offered home-based HTC
Conditional on being found at home by the HTC team.
Estimates presented in this column are derived from a logistic regression model that includes all variables listed in the table, as well as a series of indicator variables for village of residence. The model is estimated among respondents with non-missing information on all variables. Standard errors are adjusted for clustering of observations within households.
Figures in parentheses are row percentages indicating the proportion of participants in a given category who were found at home by the HTC team/got tested and learned their results.
The effects of low income on the use of HTC services varied significantly by sex and by type of HTC delivery. For facility-based HTC, an interaction term between sex and income introduced in the logistic regression model was significant at the .05 level. While men in the poorest income quartile were as likely to use facility-based HTC services as men in the upper quartiles of the income distribution (Figure 2), the poorest women were only half as likely to have ever been tested at a health facility than women in upper income quartiles (adjusted proportions: 12.2% vs. 24.0%). Patterns of use of home-based HTC were almost opposite: uptake was highest among women, but differences in uptake by income quartile among women were not significant; among men, on the other hand, socioeconomic differences in uptake of home-based HTC were large and favored men in the lowest income quartile. Men in upper income quartiles were less likely to participate in home-based HTC than all other population groups, even after accounting for the probability of being found at home by the HTC team (p < 0.01).
Figure 2.
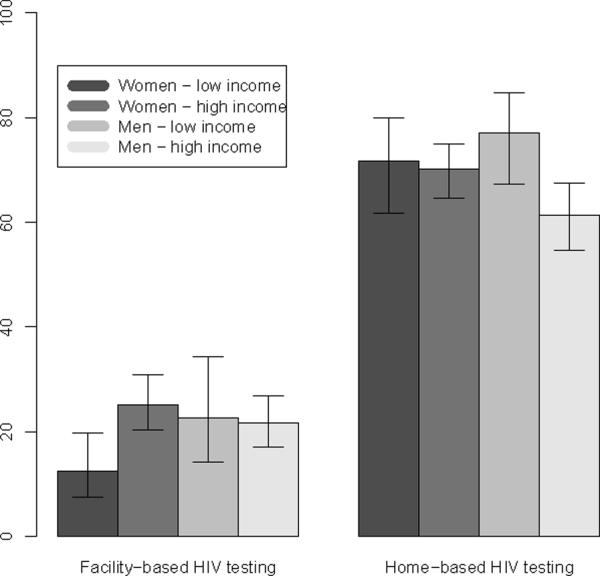
Predicted proportions of respondents participating in home-based and facility-based HTC services by sex and income. Error bars represent 95% CI. For home-based HTC, predicted probabilities refer to all respondents visited by HTC team
2.3 Prevalence of HIV infection among participants in home-based HTC
Table 3 shows the association between characteristics of home-based HTC participants and risk of HIV infection. Among residents of Likoma who used home-based HTC services, the prevalence of HIV infection was significantly higher among women, older respondents, and divorced or widowed individuals21. Being in an ongoing marital union at the time of the survey and having completed primary schooling were significant determinants of HIV risk in bivariate analyses, but not in multivariate analyses. After controlling for demographic characteristics and underlying differences in HIV-related behaviors, the odds of HIV infection among members of the poorest households were close to 3 times lower than among the rest of the population (aOR=0.37, 95%CI=0.14,0.96).
Table 3.
Determinants of HIV infection risk. Likoma Island, February 2006.
| Probability of HIV infection |
||||
|---|---|---|---|---|
| N (% ever tested†) | Unadjusted OR (95% CI) | Adjusted OR‡ (95% CI) | ||
| Sociodemographic characteristics | ||||
| Sex | Men(Ref) | 257(4.7) | 1 | 1 |
| Women | 340(10.6) | 2.91 (1.38,6.14)** | 2.61(1.12,6.07)** | |
| Age | < 20 (Ref) | 138(0.7) | 0.16(0.02,1.30)* | 0.17(0.02,1.57) |
| 20–24 | 210(4.3) | 1 | 1 | |
| 25–29 | 139(13.0 | 3.42(1.48,7.92)** | 2.93 (1.07,8.06)** | |
| 30–34 | 62(16.1) | 4.21(1.61,10.97)** | 3.52 (1.03,12.0)** | |
| 35+ | 48(20.8) | 5.91(2.26,15.45)** | 6.13 (1.76,21.4)** | |
| Marital status | Never married (Ref) | 275(2.6) | 1 | 1 |
| currently married | 284(9.1) | 3.90(1.63,9.37** | 1.02(0.32,3.16) | |
| widowed or divorced | 37(40.4) | 26.8(9.87,73.0)** | 6.88(1.98,23.9)** | |
| Schooling | Incomplete primary (Ref) | 246(10.6) | 1 | 1 |
| Completed primary school | 351(6.3) | 0.57(0.31,1.04)* | 0.94(0.44,1.96) | |
| Religion | Anglican (Ref) | 495(7.7) | 1 | 1 |
| Other religions | 102(9.8) | 1.35(0.65,2.87) | 1.17(0.47,2.94) | |
| Residence | Never on mainland (Ref) | 376(8.0) | 1 | 1 |
| Ever on mainland | 217(8.3) | 1.03(0.55,1.93) | 0.98(0.46,2.08) | |
| Income | Top income quartiles (25th percentile and above, Ref) | 426(9.4) | 1 | 1 |
| Bottom quartile (Below 25thpercentile) | 149(5.4) | 0.54(0.24,1.23) | 0.37(0.14,0.96)** | |
| HIV behaviors and risk factors | ||||
| Number of sexual partners during 3-year period prior to survey | ||||
| 0–1 (Ref) | 183(8.2) | 1 | 1 | |
| 2 | 172(9.3) | 1.16(0.55,2.46) | 1.39(0.52,3.74) | |
| 3 or more | 244(7.8) | 0.99(0.47,2.05) | 1.37(0.52,3.64)* | |
| Concurrent partnerships at time of home-based HTC visit | ||||
| No (Ref) | 518(8.3) | 1 | 1 | |
| Yes | 79(6.3) | 0.76(0.29,2.02) | 0.81(0.29,2.23) | |
| Symptoms of STI over the last 12 months | ||||
| No symptoms (Ref) | 453(7.5) | 1 | 1 | |
| Any symptoms | 114(10.5) | 1.49(0.74,3.01) | 1.42(0.63,3.17) | |
| HIV testing history | ||||
| Never been tested for HIV prior to study (Ref) | 434(8.3) | 1 | 1 | |
| Ever tested prior to study | 128(7.8) | 0.94(0.44,2.01) | 0.55(0.24,1.26) | |
Notes:
p < 0.1
p < 0.05
Figures in parentheses are row percentages indicating the proportion of participants in a given category who were found to be infected with HIV during home-based HTC.
Estimates presented in this column are derived from a logistic regression model that includes all variables listed in the table. The model is estimated among respondents with non-missing information on all variables.
Standard errors were adjusted for clustering of observations within households.
3 Discussion
Our study documents that residents of households in the lowest income quartile were significantly less likely to have ever used facility-based HTC services than the rest of the population of Likoma (Malawi). These disparities in access to facility-based HTC were particularly severe among women: women in the poorest households were only half as likely to have ever been tested for HIV at a health-care facility than women in more affluent households. Several factors potentially contribute to this inequality in HTC uptake at health-care facilities. This may be due, for example, to the lower burden of HIV and associated infections among the poorest (section 2.3). Among respondents tested during home-based HTC, the prevalence of HIV was significantly lower among members of the poorest households. This pattern of HIV infection has been reported elsewhere.26,27In addition, poorer women may be more dependent on their husband's approval to attend facility-based HTC testing services because they need to seek money for transport.10 Women are also often tested for HIV in the context of antenatal care (ANC), and ANC attendance is lower among women in poor households.1 Independent of the underlying causes, the inability of facility-based HTC services to reach the poorest has potentially far-reaching implications, which may turn HIV into a “disease of the poor”.27 By constraining access to HIV prevention and treatment services that effectively mitigate the transmission and the impact of the disease, low HTC uptake at health facilities among the poorest may lead to increased incidence of the virus and lower survival rates. It may also broaden economic inequalities and further jeopardize already fragile livelihoods in the poorest households.
The Likoma Network Study provided, for the first time on the island, home-based HTC services as part of a broader study on sexual networks and HIV transmission.21,22 Despite the fact that less than one quarter of the study population had previously participated in facility-based HTC, the home-based provision of HTC was very well accepted in this population: when present at home at the time of the HTC team's visit, more than 75% of respondents accepted to be tested and immediately retrieved their HIV test results at home. Uptake was even higher among the poorest, suggesting a strong unmet need for HTC among the most disadvantaged subgroups of the population. Home-based HTC thus has the potential to increase access of under-served populations to a series of services (e.g., ARV treatment) that may help the poorest households cope with the consequences of AIDS.
Participation in home-based HTC was also associated with several risk factors for HIV transmission/acquisition: inhabitants of Likoma with multiple sex partners over the last 3 years and inhabitants who presented recent symptoms of sexually transmitted infections were more likely to participate in home-based HTC. These findings suggest that the “yield” (i.e., the number of newly identified HIV cases) of a home-based HTC intervention might be high. Respondents involved in multiple concurrent partnerships at the time of the survey, however, were less likely to participate in home-based HTC in multivariate models. Such partnerships play a key role in HIV transmission,28 thus even though home-based HTC may significantly enhance case finding for HIV its impact on the onward transmission of HIV during non-marital partnerships may be more limited.
There are several limitations to our study. First, our assessment of prior use of facility-based HTC services is admittedly somewhat crude: we have no information about the last time a respondent got tested (recently or not), in which context testing was conducted (e.g., voluntary counseling and testing versus diagnostic testing), or whether the respondent retrieved his/her test results. Second, our measure of household poverty (household income in the lower quartile) is also crude. More precise measures of poverty often rely on detailed accounts of household expenditure, for example. Unfortunately such data were not available in our study. Third, our measures of sexual activity were limited to a small number of variables. As such, they may not have captured important differences in sexual behaviors between members of the poorest households and the rest of the population. Fourth, the provision of home-based HTC services during our study was limited to a narrow age range (ages 18–35), preventing this study to investigate the acceptability of home-based HTC among younger and older age groups. Fifth, our study is also not able to assess the potential impact of home-based HTC on the identification of cases of paediatric HIV,19 and it did not investigate the effect of home-based HTC on the communication of HIV test results among couples, which could lead to lower infection risks in discordant couples.15 Finally, the provision of a small bar of soap as a token of appreciation for participation in HTC may have provided stronger incentives for the individuals in the poorest households to participate in home-based HTC as compared to individuals in more affluent households. However, if the remuneration—rather than the ability to learn one's HIV status—had provided the primary motivation for HTC participation among the poorest individuals, one would have expected a higher rate of stopping HTC after the pre-test counseling among the poorest individuals. This was not the case.
In summary, the analyses presented in this paper confirm earlier studies showing large socioeconomic differences in uptake of HIV testing and counseling services provided at health facilities (e.g., hospitals, health centers). For the first time, however, this study documents that the home-based provision of HTC services has the potential of not only increasing the uptake of HTC services among the general population, but also to substantially reduce the socioeconomic gradient in HTC utilization observed in several African countries. While the logistical issues associated with large-scale home-based provision of HTC services are important,10 the home-based provision of HTC thus has the potential to satisfy a strong unmet need for testing and counseling among the poorest segments of sub-Saharan populations. It may also subsequently allow the poorest to gain greater access to life-extending ARV treatment, as well as to other prevention, care and support services. The impact of home-based HTC on uptake of testing and counseling, socioeconomic inequalities in access to treatment and HIV transmission should thus be tested rigorously, through controlled trials in which local communities are randomly assigned to facility-based or home-based schemes of HTC provision29.
Acknowledgments
We gratefully acknowledge the support for this research through NIH grants RO1 HD044228 and RO1 HD/MH41713, and a PARC/Boettner/PSC Pilot Grant by the Population Studies Center, University of Pennsylvania.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Malawi National Statistical Office . Malawi demographic and health survey 2004 — preliminary report. Malawi National Statistical Office; Zomba, Malawi and Measure DHS, Claverton, MD: 2004. Available online at http://www.nso.malawi.net/data_on_line/demography/dhs2004/dhs2004.html. [Google Scholar]
- [2].Uganda Bureau of Statistics (UBOS) Macro International Inc . Uganda Demographic and Health Survey 2006. UBOS and Macro International Inc.; Calverton, Maryland, USA: 2007. [Google Scholar]
- [3].Central Statistical Office (Zimbabwe) Macro International Inc . Zimbabwe Demographic and Health Survey 2005–06. UBOS and Macro International Inc.; Calverton, Maryland, USA: 2007. [Google Scholar]
- [4].Central Bureau of Statistics (Kenya) Macro International Inc . Kenya Demographic and Health Survey 2003. UBOS and Macro International Inc.; Calverton, Maryland, USA: 2004. [Google Scholar]
- [5].Bunnell R, Ekwaru J, Solberg P, Wamai N, Bikaako-Kajura W, Were W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20(1):85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- [6].Mermin J, Were W, Ekwaru J, Moore D, Downing R, Behumbiize JP, Lule, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371(9614):752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- [7].Jahn A, Floyd S, Crampin A, Mwaungulu F, Mvula H, Munthali F, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in malawi. Lancet. 2008;371(9624):1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Larson B, Fox M, Rosen S, Sigei C, Shaffer D, Sawe F, et al. Early effects of antiretroviral therapy on work performance: preliminary results from a cohort study of Kenyan agricultural workers. AIDS. 2008;22(3):421–425. doi: 10.1097/QAD.0b013e3282f3cc0c. [DOI] [PubMed] [Google Scholar]
- [9].Makhlouf Obermeyer C, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. American journal of public health. 2007;97(10):1762–1774. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matovu J, Makumbi F. Expanding access to voluntary HIV counselling and testing in sub-saharan Africa: alternative approaches for improving uptake, 2001–2007. Tropical medicine and international health. 2007;12(11):1315–1322. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- [11].Creek T, Ntumy R, Seipone K, Smith M, Mogodi M, Smit M, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. Journal of acquired immunodeficiency syndromes. 2007;45(1):102–107. doi: 10.1097/QAI.0b013e318047df88. [DOI] [PubMed] [Google Scholar]
- [12].Chandisarewa W, Stranix-Chibanda L, Chirapa E, Miller A, Simoyi M, Mahomva A, et al. Routine offer of antenatal HIV testing (“opt-out” approach) to prevent mother-to-child transmission of HIV in urban Zimbabwe. Bulletin of world health organization. 2007;85(11):843–850. doi: 10.2471/BLT.06.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bassett I, Giddy J, Nkera J, Wang B, Losina E, Lu Z, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. Journal of acquired immunodeficiency syndromes. 2007;46(2):181–186. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corbett E, Dauya E, Matambo R, Cheung Y, Makamure B, Bassett M, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLOS Medicine. 2006;3(7) doi: 10.1371/journal.pmed.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bunnell R, Cherutich P. Universal HIV testing and counseling in Africa. Lancet. 2008;371:2148–2150. doi: 10.1016/S0140-6736(08)60929-0. [DOI] [PubMed] [Google Scholar]
- [16].Bateganya M, Abdulwadud O, Kiene S. Home-based HIV voluntary counseling and testing in developing countries. Cochrane Database systematic reviews. 2007;17(4) doi: 10.1002/14651858.CD006493.pub2. [DOI] [PubMed] [Google Scholar]
- [17].Mbopi-Kéou F, Ongolo-Zogo P, Angwafo F, Ndumbe P, Bélec L. High impact of mobile units for mass HIV testing in Africa. AIDS. 2007;21(14):1994–1996. doi: 10.1097/QAD.0b013e3282f006c3. [DOI] [PubMed] [Google Scholar]
- [18].Wolff B, Nyanzi B, Katongole H, Ssesanga D, Ruberantwari A, Whitworth J. Evaluation of a home-based voluntary counseling and testing intervention in rural Uganda. Health policy and planning. 2005;20(2):109–116. doi: 10.1093/heapol/czi013. [DOI] [PubMed] [Google Scholar]
- [19].Were W, Mermin J, Wamai N, Awor A, Bechange S, Moss S, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. Journal of acquired immunodeficiency syndromes. 2006;43(1):91–95. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- [20].Fykesnes K, Siziya S. A randomised trial on acceptability of voluntary HIV counseling and testing. Tropical medicine and international health. 2004;9:566–572. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- [21].Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: Evidence from Likoma Island, Malawi. AIDS. 2007;21(17):2323–2332. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- [22].Helleringer S, Kohler HP, Chimbiri A, Chatonda P, Mkandawire J. The Likoma network study: Context, data collection and survey instruments. University of Pennsylvania, Population Studies Center; 2006. Unpublished working paper. [Google Scholar]
- [23].Mensch BS, Hewett PC, Erulkar A. The reporting of sensitive behavior among adolescents: A methodological experiment in Kenya. Demography. 2003;40(2):247–268. doi: 10.1353/dem.2003.0017. [DOI] [PubMed] [Google Scholar]
- [24].Agresti A. Categorical Data Analysis. Wiley; Cambridge: 1990. [Google Scholar]
- [25].Garrett J. Predxcat: predicted probabilities from logistic regressions. 2006 [Google Scholar]
- [26].Mishra V, Bignami S, Grenner R, Vaessen M, Hong R, Ghys P, et al. HIV infection does not disproportionately affect the poorer in sub-saharan africa. AIDS. 2007;21(S7):s17–28. doi: 10.1097/01.aids.0000300532.51860.2a. [DOI] [PubMed] [Google Scholar]
- [27].Lopman B, Lewis J, Nyamukapa C, Mushati P, Chandiwana S, Gregson S. HIV incidence and poverty in Manicaland, Zimbabwe: is HIV becoming a disease of the poor? AIDS. 2007;21(S7):S57–S66. doi: 10.1097/01.aids.0000300536.82354.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- [29].Pope D, Deluca A, Kali P, Hausler H, Sheard C, Hoosain E, et al. A cluster-randomized trial of provider-initiated (opt-out) HIV counseling and testing of tuberculosis patients in South Africa. Journal of Acquired Immune Deficiency Syndrome. 2008;48(2):190–195. doi: 10.1097/QAI.0b013e3181775926. [DOI] [PMC free article] [PubMed] [Google Scholar]


