Abstract
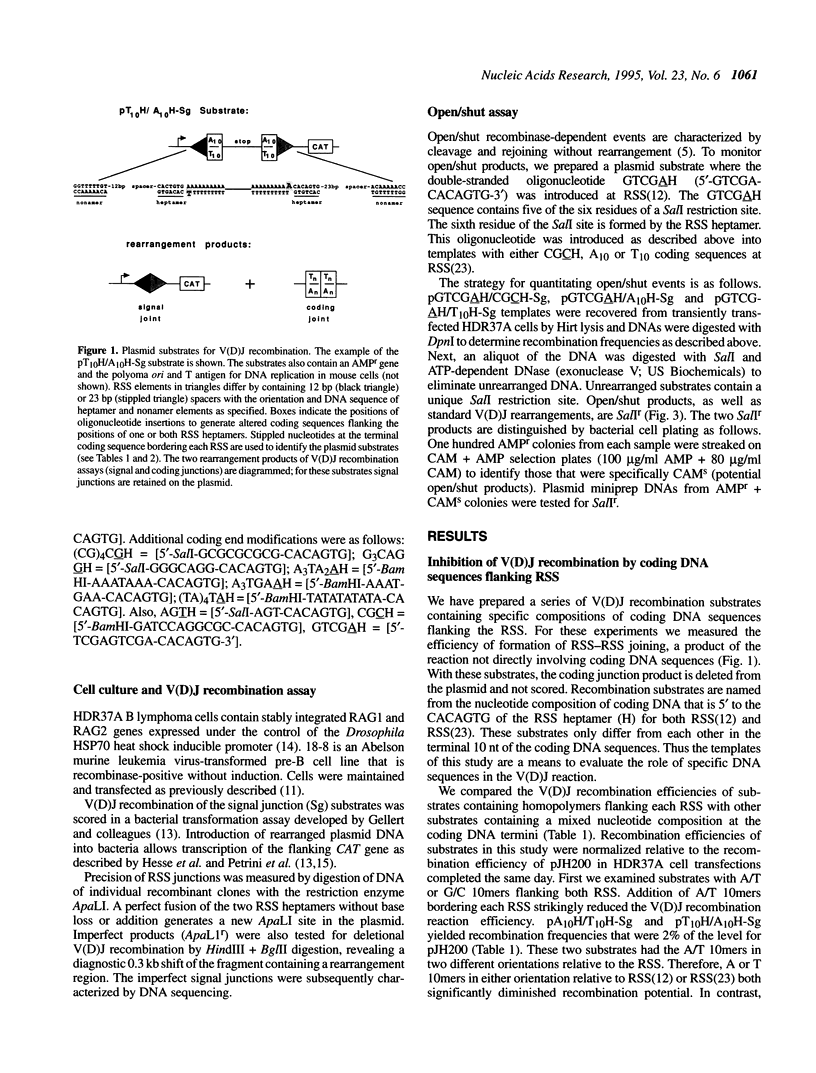
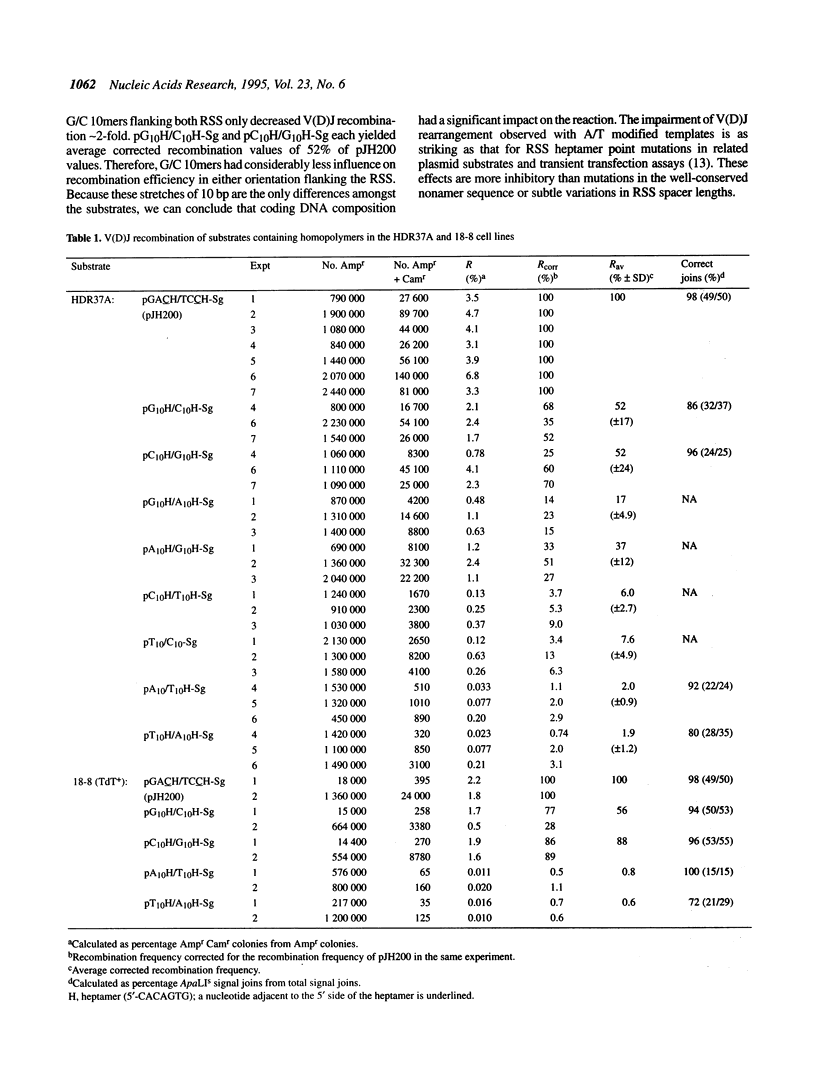
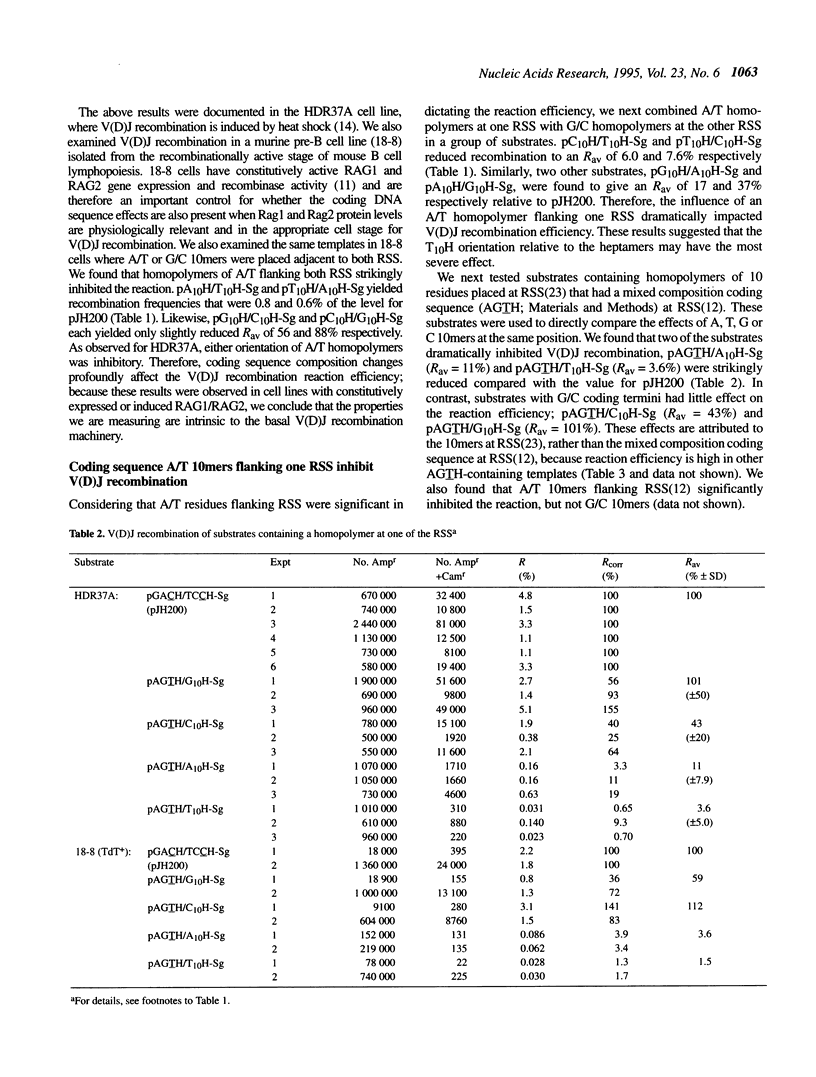
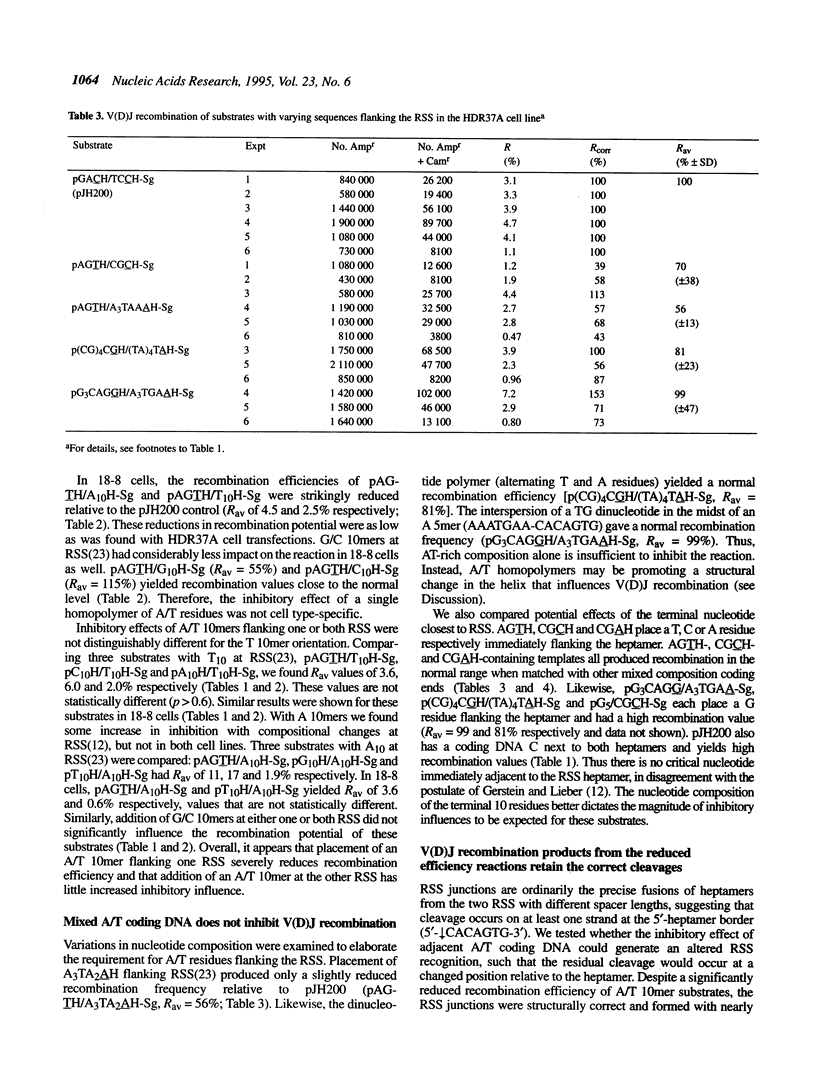
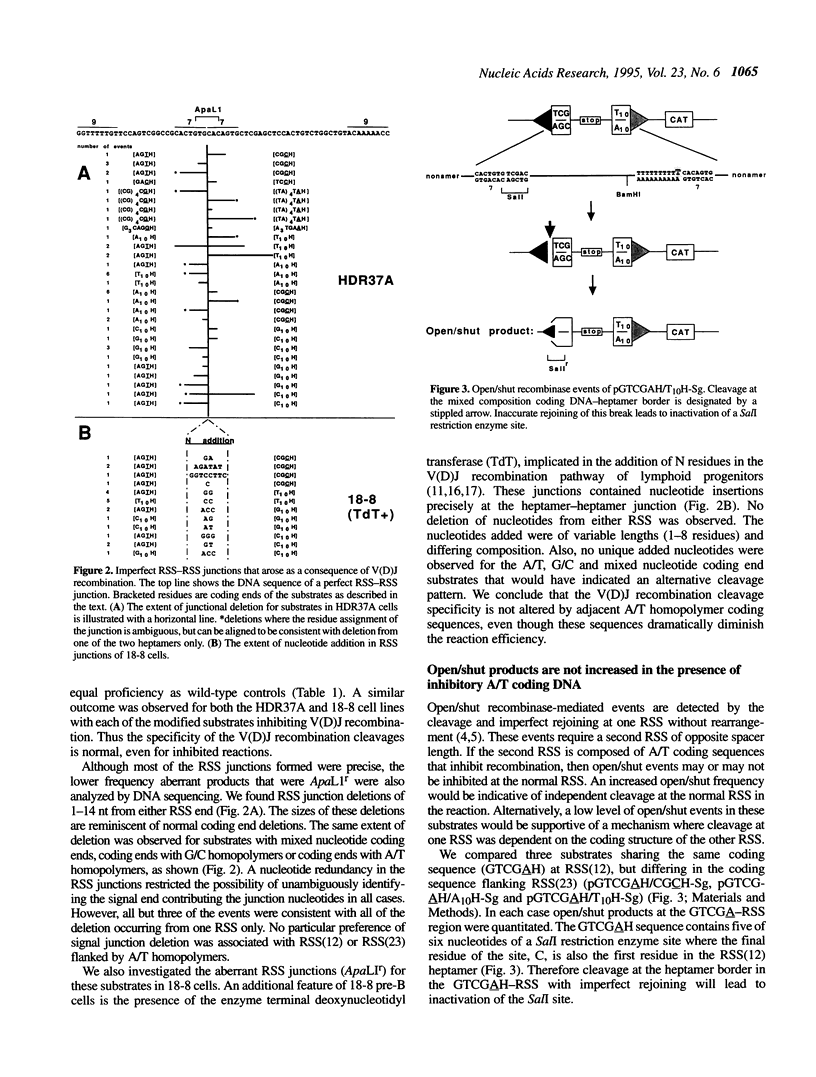
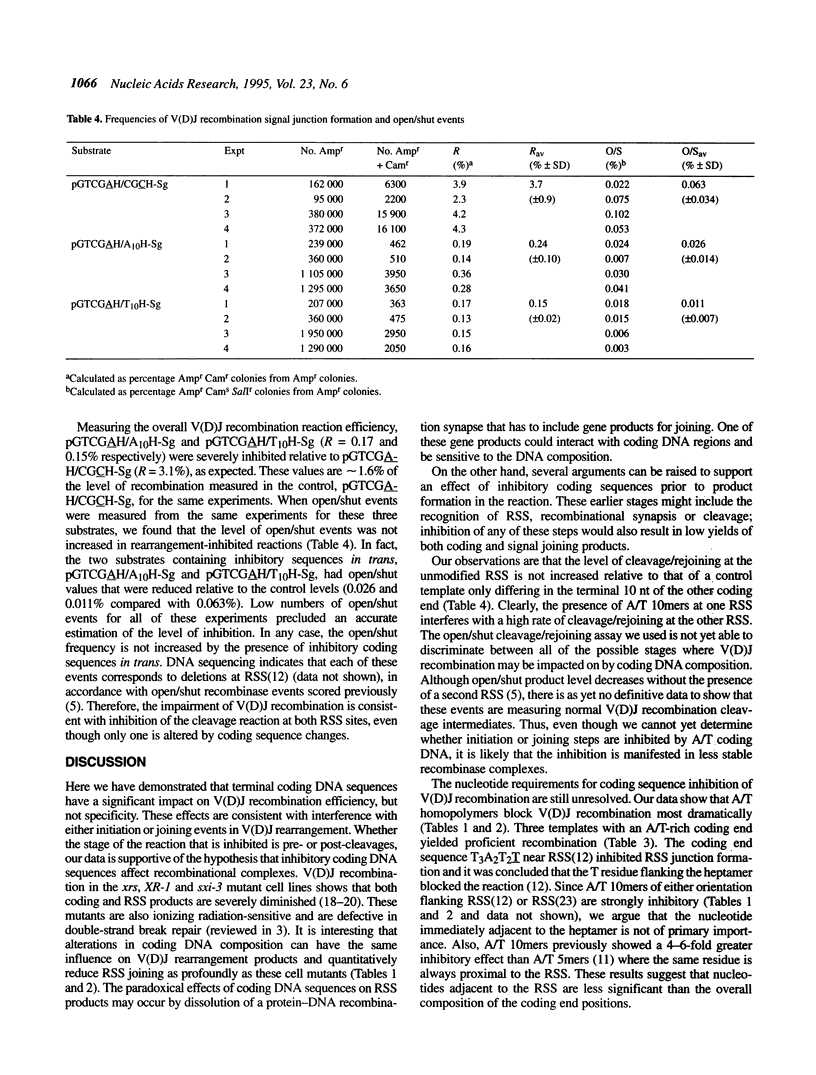
Lymphoid V(D)J rearrangement is targeted by recombination signal sequences (RSS) bordering V, D or J exons. We demonstrate that the DNA composition of flanking coding positions, particularly poly(A) or poly(T) stretches at one or both RSS, diminishes V(D)J recombination up to 100-fold. Positionally correct cleavages occur in the inhibited reactions, since the junctions formed show the same frequency of precision as uninhibited reactions. Open/shut cleavage/rejoining is not increased at a normal RSS in substrates containing inhibitory A/T homopolymers versus random sequence at a second RSS. Thus recombinase action at both cleavage sites is severely disrupted by modified coding sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender J., Kleckner N. Tn10 insertion specificity is strongly dependent upon sequences immediately adjacent to the target-site consensus sequence. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7996–8000. doi: 10.1073/pnas.89.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubnov N. V., Wills Z. P., Weaver D. T. V(D)J recombination coding junction formation without DNA homology: processing of coding termini. Mol Cell Biol. 1993 Nov;13(11):6957–6968. doi: 10.1128/mcb.13.11.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Lieber M. R. Coding end sequence can markedly affect the initiation of V(D)J recombination. Genes Dev. 1993 Jul;7(7B):1459–1469. doi: 10.1101/gad.7.7b.1459. [DOI] [PubMed] [Google Scholar]
- Gilfillan S., Dierich A., Lemeur M., Benoist C., Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993 Aug 27;261(5125):1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Annu Rev Biochem. 1990;59:755–781. doi: 10.1146/annurev.bi.59.070190.003543. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Liu V. F., Weaver D. T. Strand breaks without DNA rearrangement in V (D)J recombination. Mol Cell Biol. 1991 Jun;11(6):3155–3162. doi: 10.1128/mcb.11.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Huisman O., Faelen M., Girard D., Jaffé A., Toussaint A., Rouvière-Yaniv J. Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol. 1989 Jul;171(7):3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Charretier E., Kochoyan M., Guéron M. Evidence from base-pair kinetics for two types of adenine tract structures in solution: their relation to DNA curvature. Biochemistry. 1988 Dec 13;27(25):8894–8898. doi: 10.1021/bi00425a004. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Hesse J. E. Cutting and closing without recombination in V(D)J joining. EMBO J. 1991 Dec;10(12):3631–3639. doi: 10.1002/j.1460-2075.1991.tb04929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J. G., Crothers D. M. Structural basis for DNA bending. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2622–2626. doi: 10.1073/pnas.86.8.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltz E. M., Alt F. W., Lin W. C., Chen J., Taccioli G., Desiderio S., Rathbun G. A V(D)J recombinase-inducible B-cell line: role of transcriptional enhancer elements in directing V(D)J recombination. Mol Cell Biol. 1993 Oct;13(10):6223–6230. doi: 10.1128/mcb.13.10.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J. H., Donovan J. W., Dimare C., Weaver D. T. Normal V(D)J coding junction formation in DNA ligase I deficiency syndromes. J Immunol. 1994 Jan 1;152(1):176–183. [PubMed] [Google Scholar]
- Roth D. B., Zhu C., Gellert M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10788–10792. doi: 10.1073/pnas.90.22.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Constantinescu A., Morrow T., Baxter M., Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5'-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993 Dec;7(12B):2520–2532. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Cheng H. L., Varghese A. J., Whitmore G., Alt F. W. A DNA repair defect in Chinese hamster ovary cells affects V(D)J recombination similarly to the murine scid mutation. J Biol Chem. 1994 Mar 11;269(10):7439–7442. [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Weaver D. T. V(D)J recombination and double-strand break repair. Adv Immunol. 1995;58:29–85. doi: 10.1016/s0065-2776(08)60619-7. [DOI] [PubMed] [Google Scholar]
- Wu Z., Chaconas G. Flanking host sequences can exert an inhibitory effect on the cleavage step of the in vitro mu DNA strand transfer reaction. J Biol Chem. 1992 May 15;267(14):9552–9558. [PubMed] [Google Scholar]



