Abstract
Purpose
This study compared the occurrence rates for and severity ratings of sleep disturbance in patient-family caregiver (FC) dyads.
Patients and Methods
In total, 102 dyads were recruited from two radiation therapy (RT) departments. Patients and their FCs completed the Pittsburgh Sleep Quality Index (PSQI) and the General Sleep Disturbance Scale (GSDS) and wore wrist actigraphs to obtain subjective and objective measures of the occurrence and severity of sleep disturbance at the initiation of RT. Match paired t tests were used to evaluate for dyadic differences.
Results
No differences were found in the occurrence of clinically significant levels of sleep disturbance between patients and their FCs that ranged between 40% and 50% using subjective and objective measures. Few differences were found in the severity of any of the sleep-wake parameters between patients and FCs using both the subjective and objective measures of sleep disturbance.
Conclusion
The findings from this study suggest that patients with cancer and their FCs experience similar levels of sleep disturbance and that both groups could benefit from interventions that aim to promote restful sleep. In addition to routine and systematic assessment of sleep disturbance by oncology clinicians, interventions are needed that take into account the specific needs of the patient and the FC as well as the potential for partners' sleep patterns to influence one another.
INTRODUCTION
Poor sleep is linked to negative health outcomes, including impaired cognitive, psychological, and physical functioning and a lower quality of life.1–5 Prevalence of sleep disturbances among patients with cancer ranges from 30% to 55%, about twice the rate found in the general population.6 Patients with cancer are at high risk for sleep disturbances because of the physiological and/or psychological stressors associated with the disease and its treatments as well as the day-to-day burden of living with cancer. Also at risk are the family caregivers (FC) of patients with cancer, given that FCs are assuming more and more responsibility for the care of their loved ones as health care delivery grows increasingly more complex and outpatient focused. While patients with cancer report significant distress as a result of poor sleep, the magnitude and severity of sleep disturbance is not routinely assessed and managed by oncology clinicians. The prevalence and severity of sleep disturbances in FCs of oncology patients has received even less attention despite serious health and safety implications for both the patient and their FCs.7–9
While it is clear that both oncology patients and their FCs experience sleep disturbance, it is unclear how or to what degree the sleep patterns of patients and FCs within dyads differ and how partners' sleep patterns may influence one another. Only one study simultaneously measured the sleep habits of patients with advanced cancer and their FCs and found a similar prevalence of poor sleep in both groups (47% and 42%, respectively).10 However, only 60 dyads were evaluated. Given the paucity of research on sleep disturbance in oncology patient-FC dyads, the purposes of this study were to evaluate for differences in the occurrence of sleep disturbance as well as for differences in the severity of self-reported sleep disturbance and objective measures of nocturnal sleep/rest and daytime wake/activity in oncology patients and their FCs at the initiation of radiation therapy (RT).
PATIENTS AND METHODS
Participants and Settings
This descriptive, correlational study is part of a larger, longitudinal study that evaluated multiple symptoms in patients who underwent primary or adjuvant RT and their FCs.11–14 Participants were recruited from two RT departments located in a Comprehensive Cancer Center and a community-based oncology program at the time of the patient's simulation visit.
Patients were eligible to participate if they were ≥ 18 years of age; were scheduled to receive primary or adjuvant RT for one of four cancer diagnoses (ie, breast, prostate, lung, brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky performance score (KPS) of ≥ 60. Patients were excluded if they had metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder. FCs were eligible to participate if they were an adult (≥ 18 years of age); were able to read, write, and understand English; gave written informed consent; had a KPS score of ≥ 60; were living with the patient; and did not have a diagnosed sleep disorder.
Instruments
The study instruments included a demographic questionnaire, the KPS scale,15 the Pittsburgh Sleep Quality Index (PSQI),16 and the General Sleep Disturbance Scale (GSDS).17 To compare these subjective responses with a more objective measure, data on nocturnal sleep/rest and daytime wake/activity were obtained by continuous noninvasive monitoring of activity over 48 hours using a wrist motion sensor (Mini Motionlogger Actigraph, Ambulatory Monitoring, Ardsley, NY).18–20
The demographic questionnaire obtained information on age, sex, marital status, education, ethnicity, employment status, and the presence of a number of comorbid conditions.
The PSQI consists of 19 items designed to assess the quality of sleep in the past month. The global PSQI score is the sum of the seven component scores (ie, subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, daytime dysfunction). Each component score ranges from 0 to 3, and the global PSQI score ranges from 0 to 21. Higher global and component scores indicate more severe complaints and a higher level of sleep disturbance. A global PSQI score of > 5 indicates a significant level of sleep disturbance.16 A cutoff score of 8 was found to discriminate poor sleep quality in oncology patients.21 The PSQI has established internal consistency, test-retest reliability, and construct validity.16,21,22 In this study, the Cronbach's alpha for the global PSQI score was .72 for patients and .68 for FCs.
The GSDS consists of 21 items designed to assess the quality of sleep in the past week. Each item was rated on a 0 (never) to 7 (every day) numeric rating scale. The GSDS total score is the sum of the seven subscale scores (ie, quality of sleep, quantity of sleep, sleep onset latency, midsleep awakenings, early awakenings, medications for sleep, excessive daytime sleepiness) that can range from 0 (no disturbance) to 147 (extreme sleep disturbance). Each mean subscale score can range from 0 to 7. Higher total and subscale scores indicated higher levels of sleep disturbance. Subscales scores of ≥ 3 and a GSDS total score of ≥ 43 indicates a significant level of sleep disturbance.13 The GSDS has well-established validity and reliability in shift workers, pregnant women, and patients with cancer and HIV.17,23,24 In this study, the Cronbach's alpha for the GSDS total score was .84 for patients and .79 for FCs.
Objective data on sleep-wake parameters were obtained by continuous noninvasive monitoring of activity over 48 hours using wrist actigraphy. Seven nocturnal sleep/rest and four daytime wake/activity variables were selected that were identified by a National Cancer Institute–sponsored conference,7 an expert panel that recommended a standard set of research assessments in insomnia,25 and recently published studies.26,27 Wrist actigraphy has been validated with EEG measures of sleep and awakening in men and women with both healthy and disturbed sleep patterns.18,20,25 It provides continuous motion data using a battery-operated wristwatch-size microprocessor that senses motion with a piezoelectric beam and detects movement in all three axes. The accompanying Action 4 software (Ambulatory Monitoring, Ardsley, NY) allows analysis of activity and nonactivity as well as automatic scoring of sleep and wake episodes in minutes.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco, and at the second site. At the time of the simulation visit (ie, approximately 1 week before the initiation of RT), patients and their FCs were approached by a research nurse to discuss participation in the study. After providing written informed consent, patients and FCs completed the demographic questionnaire, KPS scale,15 PSQI,16 and GSDS.17 Medical records were reviewed for disease and treatment information.
Actigraphy data were collected for two consecutive days before the start of RT. Wrist actigraphy data were collected on weekdays to avoid confounding data with weekend sleep patterns. Data were collected for only 48 hours to reduce respondent burden, maximize the number of eligible participants, and minimize the amount of missing data.
Participants wore the wrist actigraph on their nondominant wrist. The epoch length for the actigraph was set at 30 seconds. Patients and FCs were asked to use the event marker on the wrist actigraph to indicate “lights out” and “lights on” time. Participants reported no difficulty wearing the wrist actigraph. Since the actual time is important in the calculation of the amount of sleep obtained in the amount of time designated for sleep, having an additional source of information about nap times, bed times, and wake times is important. This information was recorded by patients and FCs in a 2-day diary. On awakening, the patients and FCs used the diary to indicate the number of awakenings during the night. Patients and FCs returned the questionnaires and actigraphs to the research nurse in the RT department at the completion of the 2 days of data collection.
Data Analysis
Data were analyzed using SPSS version 15 (SPSS, Chicago, IL). Descriptive statistics and frequency distributions were generated for the sample characteristics.
Actigraphy files in zero-crossing mode were analyzed by two of the researchers (K.L. and C.W.) who used the Cole-Kripke algorithm in the Action 4 software. First, the file was scanned for missing data. If more than 4 hours of day data or 2 hours of night data were missing, that day's or night's data were not used in the analyses. Time limits were set for the 48-hour period. The file was reviewed, and intervals were individually set for each day and night period using, in order of priority as decision guides, the event marker, diary data, channel data, and cascading movement data. Because no differences were found in the various actigraphy parameters between the 2 days of data collection, means were calculated and used in the analyses.
To evaluate for differences in occurrence rates for the subjective and objective measures, the PSQI global score (> 5), GSDS total score (≥ 43), and total sleep time; < 420 minutes) were dichotomized using clinically meaningful cut points. McNemar tests were done to evaluate for differences between patients and their FCs in occurrence rates using these newly created categorical variables. Differences between dyads for continuous data were evaluated using match-paired t tests. Differences between dyads in categorical data were evaluated using the McNemar test. Relationships between patients' and FCs' subjective and objective sleep data were evaluated using correlations derived from the match-paired t tests. All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of available data across groups. On the basis of recommendations of Rothman,28 no adjustments were made for multiple testing. A P value < .05 was considered statistically significant.
RESULTS
Patient and FC Characteristics
Table 1 presents a summary of the demographic characteristics of the 102 patient-FC dyads. No differences in demographic characteristics were found between the dyads except for age and sex. Patients were significantly older (P < .001) and more likely to be male (P < .001). Patients were diagnosed with prostate (59.2%), breast (26.2%), lung (7.8%), or brain (6.8%) cancers. The majority of the FCs (91.2%) were the patient's spouse or partner.
Table 1.
Demographic and Clinical Characteristics of Patients and Their Family Caregivers (n = 102)
| Characteristic | Patient |
Family Caregiver |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | % | Clock Time | No. | % | Clock Time | ||
| Age, years | .00 | ||||||
| Mean | 64.41 | 61.69 | |||||
| SD | 10.22 | 10.37 | |||||
| Education, years | .10 | ||||||
| Mean | 16.29 | 15.73 | |||||
| SD | 3.10 | 3.02 | |||||
| KPS score | .17 | ||||||
| Mean | 91.83 | 93.87 | |||||
| SD | 11.51 | 10.64 | |||||
| Number of comorbidities | .26 | ||||||
| Mean | 4.57 | 4.18 | |||||
| SD | 2.57 | 2.92 | |||||
| Sex | |||||||
| Male | 70 | 68.6 | 29 | 28.4 | < .001 | ||
| Female | 32 | 31.4 | 73 | 71.6 | |||
| Marital status | |||||||
| Married/partnered | 97 | 95.1 | 97 | 95.1 | 1.00 | ||
| Not married | 5 | 4.9 | 5 | 4.9 | |||
| Race/ethnicity | |||||||
| White | 81 | 80.2 | 79 | 78.2 | .73 | ||
| Nonwhite | 20 | 19.8 | 22 | 21.8 | |||
| Work for pay | |||||||
| Yes | 42 | 42.4 | 46 | 46.5 | .62 | ||
| Children living at home | |||||||
| Yes | 14 | 18.9 | 14 | 18.9 | 1.00 | ||
| Parent living at home | |||||||
| Yes | 1 | 1.3 | 1 | 1.3 | 1.00 | ||
| Relationship to patient | |||||||
| Spouse/partner | 93 | 91.2 | |||||
| Significant other | 5 | 4.9 | |||||
| Child | 2 | 2.0 | |||||
| Friend | 2 | 2.0 | |||||
| Bed partner or roommate | |||||||
| Partner in same bed | 79 | 79.0 | 80 | 80.8 | |||
| Partner in same room but different bed | 5 | 5.0 | 5 | 5.1 | |||
| Partner in different room | 6 | 6.0 | 7 | 7.1 | |||
| Bedtime | .13 | ||||||
| Mean | 21:39 | 20:38 | |||||
| SD | 4:26 | 6:14 | |||||
| Wake time | .62 | ||||||
| Mean | 6:26 | 6:22 | |||||
| SD | 1:13 | 1:04 | |||||
| Time got out of bed | .69 | ||||||
| Mean | 6:56 | 6:59 | |||||
| SD | 1:16 | 1:13 | |||||
Abbreviations: SD, standard deviation; KPS, Karnofsky performance score.
Differences Between Patients and FCs in the Occurrence of Sleep Disturbance
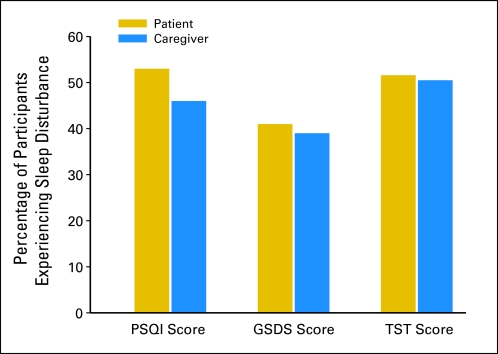
As shown in Figure 1, while occurrence rates were between 40% and 50%, no differences were found between patients and their FCs in the occurrence rates for clinically significant levels of sleep disturbance on the basis of cutoff scores for the PSQI global score (P = .40), the GSDS global score (P = .89), or total sleep time (P = 1.00).
Fig 1.
Differences between patients and family caregivers (FCs) in the occurrence of significant levels of sleep disturbance using clinically significant cut points on the Pittsburgh Sleep Quality Index (PSQI), General Sleep Disturbance Scale (GSDS), and total sleep time (TST). All values are plotted as percentages of patients and FCs with clinically significant levels of sleep disturbance. By using McNemar tests, no significant differences were found in the percentage of patients and FCs with significant levels of sleep disturbance using all three measures.
Differences Between Patients and FCs in the Severity of Sleep Disturbance Subjective Data
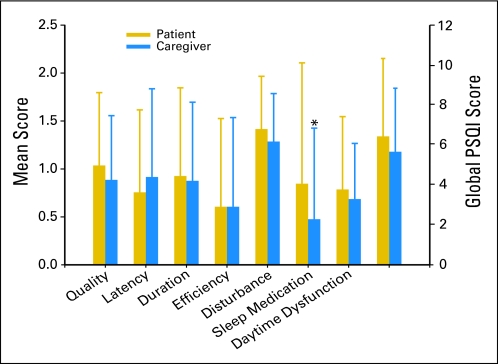
As shown in Figure 2, no differences were found between patients and their FCs in any of the PSQI global or subscale scores except for the use of sleep medications. Patients reported significantly higher use of sleep medication scores than their FCs (P = .01). As shown in Table 2, the majority of the correlations between patients' and FCs' PSQI subscale and global scores were not significant.
Fig 2.
Differences between patients and family caregivers in mean subscale and global Pittsburgh Sleep Quality Index (PSQI) scores. (*) Significant differences between the groups at P ≤ .05.
Table 2.
Correlations Between PSQI and GSDS Scores Between Patients and Their Family Caregivers (n = 102)
| Instrument | r | P |
|---|---|---|
| PSQI | ||
| Sleep quality | −0.03 | .76 |
| Sleep latency | 0.13 | .20 |
| Sleep duration | 0.22 | .04 |
| Sleep efficiency | 0.13 | .21 |
| Sleep disturbance | −0.09 | .41 |
| Use of sleep medication | 0.26 | .009 |
| Daytime dysfunction | 0.24 | .02 |
| PSQI global score | 0.04 | .71 |
| GSDS | ||
| Sleep quality | 0.21 | .03 |
| Sleep latency | −0.05 | .65 |
| Sleep quantity | 0.14 | .16 |
| Midsleep awakening | 0.09 | .37 |
| Early awakenings | 0.22 | .03 |
| Use of sleep medication | 0.19 | .06 |
| Excessive daytime sleepiness | 0.06 | .54 |
| GSDS total score | 0.14 | .18 |
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; GSDS, General Sleep Disturbance Scale.
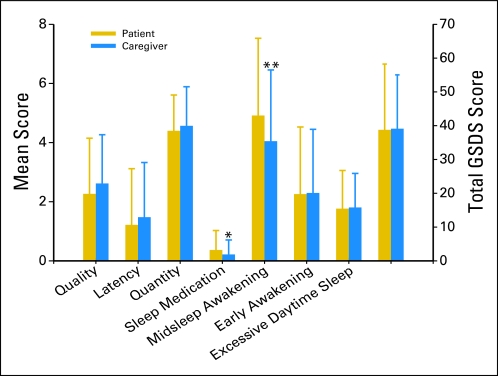
In contrast to the PSQI, which assesses participants' sleep quality over the past month, the GSDS assesses the quality of sleep over the past week. As shown in Figure 3, patients scored significantly higher than their FCs on the GSDS subscales of midsleep awakenings (P = .01) and use of sleep medications (P = .04). As shown in Table 2, most of the correlations between patients' and their FCs' GSDS subscale and total scores were not significant.
Fig 3.
Differences between patients and family caregivers mean subscale and total General Sleep Disturbance Scale (GSDS) scores. (*) Significant differences between the groups at P = .04 and (**) P = .01.
Objective Data
As shown in Table 3, actigraphy data revealed that patients and their FCs had similar nocturnal sleep/rest and daytime wake/activity parameters. For the objective measures, the only significant difference between patients and their FCs was for sleep efficiency. The mean sleep efficiency for patients was significantly less than that for their FCs (P = .03). Strong and statistically significant correlations were found between patients and FCs on most of the sleep/rest and wake/activity parameters (Table 3).
Table 3.
Differences in and Correlations Between Objective Sleep/Rest and Wake/Activity Parameters Between Patients and Their Family Caregivers
| Characteristic | Patient |
Family Caregiver |
r | P | t | P | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Nocturnal sleep/rest | ||||||||
| Sleep onset latency | 17.33 | 26.06 | 13.06 | 10.68 | 0.60 | < .001 | 1.90 | .06 |
| Awake after sleep onset as % of total sleep time | 14.64 | 13.46 | 12.49 | 10.03 | 0.56 | < .001 | 1.81 | .07 |
| No. of awakenings | 17.97 | 9.32 | 17.60 | 9.50 | 0.49 | < .001 | 0.38 | .71 |
| Awake duration | 4.26 | 6.85 | 3.37 | 2.15 | 0.45 | < .001 | 1.37 | .18 |
| Total sleep time | 405.50 | 85.83 | 408.50 | 78.74 | 0.41 | < .001 | −0.32 | .75 |
| Time in bed | 496.51 | 67.37 | 485.91 | 78.48 | 0.42 | < .001 | 1.28 | .21 |
| Sleep efficiency index as % of time in bed asleep | 81.36 | 14.79 | 84.17 | 10.90 | 0.57 | < .001 | −2.16 | .03 |
| Daytime wake/activity | ||||||||
| Total day sleep time | 57.60 | 92.13 | 44.49 | 69.50 | 0.19 | .11 | 1.07 | .29 |
| Total awake time per day | 662.41 | 92.13 | 675.51 | 69.50 | 0.19 | .11 | −1.07 | .29 |
| Day sleep as % of day asleep from 0900 to 2059 | 8.00 | 12.80 | 6.18 | 9.65 | 0.19 | .11 | 1.07 | .29 |
| Wake day as % of day awake from 0900 to 2059 | 92.00 | 12.80 | 93.82 | 9.65 | 0.19 | .11 | −1.07 | .29 |
NOTE. Values are listed in minutes unless otherwise specified.
Abbreviation: SD, standard deviation.
DISCUSSION
This study is the first to evaluate for differences in the occurrence and severity of sleep disturbance within oncology patient-FC dyads using both subjective and objective measures. Across both types of measures, rates of sleep disturbance in both patients and their FCs ranged from 40% to 50%. These results are consistent with a previous study of patients with advanced cancer and their FCs10 that reported sleep disturbance in 47% of the patients and 42% of the FCs. This high prevalence of sleep disturbance may be an underestimation of the magnitude of the problem. It should be noted that this level of sleep disturbance was found at the initiation of RT. This high level of sleep disturbance requires more systematic assessments by clinicians because sleep disturbance is associated with poorer health outcomes.29–31 In addition, the prevalence of sleep disturbance may increase over the course of RT as patients experience the adverse effects of treatment.
Across both the subjective and objective measures, few differences were found between patients' and FCs' sleep-wake parameters. On the basis of these data, both patients and FCs had a significant problem with sleep maintenance. While the actigraphy data suggest that patients and their FCs slept 6.75 hours per night, actigraphy tends to overestimate sleep time.18 Of note, both patients and their FCs were found to have approximately 18 awakenings per night that lasted 3 to 4 minutes. This level of sleep disturbance warrants further investigation because recent data suggest that sleep fragmentation is associated with metabolic disorders and chronic inflammation.30,32
Use of medication for sleep was the only subscale on both the PSQI (P = .01) and GSDS (P = .04) on which patients reported higher scores than FCs. This finding is consistent with a previous study4 in which FCs reported reluctance to use sleeping medications because of concerns that these medications might interfere with their ability to perform caregiving duties at night. In terms of the GSDS data, patients reported a higher number of midsleep awakenings (P = .01) compared with their FCs. This finding may be partially explained by the large number of patients with prostate cancer who were awakened with urinary symptoms (unpublished data). In terms of objective data and consistent with the increased number of awakenings in patients, sleep efficiency was the only parameter in which patients had lower scores than their FCs (P = .03).
Patients and their FCs had similar sleep/rest and wake/activity parameters, but their objective measures suggest that the severity of sleep disturbance was worse than that demonstrated in self-report measures. This finding is consistent with a previous study33 in which FCs of oncology patients underreported sleep disturbance compared with actigraphy data. Strong positive correlations were found within dyads for almost all of the objective measures, which suggests that if a patient slept poorly, so did his or her FC and vice versa. Strong correlations between patients and their FCs were not observed with the self-report data. This discrepancy may be attributed to the fact that subjective measures reflecting individuals' perceptions are not always consistent with objective measures of the same phenomenon and are more prone to external influences and variability. This finding warrants replication in future studies.
Several study limitations need to be acknowledged. Because participants were asked to reflect back and report on their sleep habits over the past month and week using the two self-report measures, responses were subject to recall bias. However, this limitation was partially mitigated by the collection of 48 hours of objective measurements using wrist actigraphy. In addition, the homogeneity of the participants in terms of ethnicity and education limits the generalizability of the study findings. Finally, in this study, actigraphy was measured for 48 hours instead of at least the recommended 72 hours to minimize respondent burden and reduce the amount of missing data.18 Therefore, the actigraphy data warrant replication with larger populations who are evaluated for longer periods of time.
Despite these limitations, findings from this study suggest that occurrence of clinically significant sleep disturbance was high for both oncology patients and their FCs. In addition, given the similarities in the severity of sleep disturbance across both subjective and objective sleep parameters, oncology patients and their FCs appear to be at similar risk for the development of other symptoms and negative outcomes as a result of sleep disturbance, including depression, anxiety, fatigue, impaired functional status, and reduced quality of life.34 Associations between sleep disturbance and these symptoms and outcomes have been documented in studies of both oncology patients and FCs.8,35–39
In addition, the findings from this study suggest potential implications for patient and FC well-being and safety. In light of current trends toward more outpatient-focused health care delivery that places increasing burden on informal caregivers, more research is needed on understanding sleep disturbance in FCs and its impact on their health and functioning. The effect of sleep disturbance on the ability of FCs to carry out caregiving duties has not been adequately addressed and is an important area for investigation. In addition, specific causes for the sleep disturbance warrant investigation.
In addition to routine and systematic assessment of sleep disturbance by oncology clinicians, interventions are needed that take into account the specific needs of individual patients and FCs as well as the potential for partners' sleep patterns to influence one another. Unfortunately, studies that evaluated the effectiveness of interventions for sleep disturbance in oncology patients are limited, and only one intervention study40 was done with FCs of oncology patients. Additional research is warranted to increase our understanding of the causes and characteristics of sleep disturbance in oncology patients and FCs and to develop interventions that promote restful sleep.
Footnotes
Supported by Grant No. NR04835 from the National Institute of Nursing Research, by the American Cancer Society (C.M.), by Grant No. KL2 RR624130 from the National Institutes of Health Roadmap for Medical Research (B.E.A.), and by the Mount Zion Health Fund and the University of California, San Francisco, Academic Senate (L.D.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Sara Carney, Theresa Koetters, Claudia West, Steven M. Paul, Marylin Dodd, Christine Miaskowski
Administrative support: Claudia West, William Wara, Patrick Swift
Provision of study materials or patients: William Wara, Patrick Swift
Collection and assembly of data: Christine Miaskowski
Data analysis and interpretation: Sara Carney, Theresa Koetters, Steven M. Paul, Bradley E. Aouizerat, Marylin Dodd, Bruce Cooper, Kathryn Lee, Christine Miaskowski
Manuscript writing: Sara Carney, Theresa Koetters, Maria Cho, Claudia West, Steven M. Paul, Laura Dunn, Bradley E. Aouizerat, Marylin Dodd, Bruce Cooper, Kathryn Lee, William Wara, Patrick Swift,Christine Miaskowski
Final approval of manuscript: Sara Carney, Theresa Koetters, Maria Cho, Claudia West, Steven M. Paul, Laura Dunn, Bradley E. Aouizerat, Marylin Dodd, Bruce Cooper, Kathryn Lee, William Wara, Patrick Swift, Christine Miaskowski
REFERENCES
- 1.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw. 2008;6:3–13. doi: 10.6004/jnccn.2008.0002. [DOI] [PubMed] [Google Scholar]
- 3.Byar KL, Berger AM, Bakken SL, et al. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–E26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 4.Carter PA, Chang BL. Sleep and depression in cancer caregivers. Cancer Nurs. 2000;23:410–415. doi: 10.1097/00002820-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wielgus KK, Berger AM, Hertzog M. Predictors of fatigue 30 days after completing anthracycline plus taxane adjuvant chemotherapy for breast cancer. Oncol Nurs Forum. 2009;36:38–48. doi: 10.1188/09.ONF.38-48. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM. Update on the state of the science: Sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009;36:E165–E177. doi: 10.1188/09.ONF.E165-E177. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Parker KP, Young-McCaughan S, et al. Sleep wake disturbances in people with cancer and their caregivers: State of the science. Oncol Nurs Forum. 2005;32:E98–E126. doi: 10.1188/05.ONF.E98-E126. [DOI] [PubMed] [Google Scholar]
- 8.Carter PA. Caregivers' descriptions of sleep changes and depressive symptoms. Oncol Nurs Forum. 2002;29:1277–1283. doi: 10.1188/02.ONF.1277-1283. [DOI] [PubMed] [Google Scholar]
- 9.Swore Fletcher BA, Dodd MJ, Schumacher KL, et al. Symptom experience of family caregivers of patients with cancer. Oncol Nurs Forum. 2008;35:E23–E44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- 10.Gibbins J, McCoubrie R, Kendrick AH, et al. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009;38:860–870. doi: 10.1016/j.jpainsymman.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher BA, Schumacher KL, Dodd M, et al. Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Res Nurs Health. 2009;32:125–139. doi: 10.1002/nur.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 14.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnofsky D, Abelmann WH, Craver LV, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 19.Berger AM, Wielgus KK, Young-McCaughan S, et al. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 22.Beck SL, Schwartz AL, Towsley G, et al. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19:208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 24.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 26.Berger AM, Farr LA, Kuhn BR, et al. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger AM, Wielgus K, Hertzog M, et al. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. doi: 10.1007/s00520-009-0636-0. [e-pub ahead of print on April 19, 2009] [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 29.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: A review of the literature. Prog Cardiovasc Nurs. 2004;19:56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 30.Bixler E. Sleep and society: An epidemiological perspective. Sleep Med. 2009;10(suppl 1):S3–S6. doi: 10.1016/j.sleep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Kripke DF. Sleep and mortality. Psychosom Med. 2003;65:74. doi: 10.1097/01.psy.0000039752.23250.69. [DOI] [PubMed] [Google Scholar]
- 32.Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter PA. Family caregivers' sleep loss and depression over time. Cancer Nurs. 2003;26:253–259. doi: 10.1097/00002820-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Lee KA, Landis C, Chasens ER, et al. Sleep and chronobiology: Recommendations for nursing education. Nurs Outlook. 2004;52:126–133. doi: 10.1016/j.outlook.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Sato R, Kanda K, Anan M, et al. Sleep EEG patterns and fatigue of middle-aged and older female family caregivers providing routine nighttime care for elderly persons at home. Percept Mot Skills. 2002;95:815–829. doi: 10.2466/pms.2002.95.3.815. [DOI] [PubMed] [Google Scholar]
- 36.Flaskerud JH, Carter PA, Lee P. Distressing emotions in female caregivers of people with AIDS, age-related dementias, and advanced-stage cancers. Perspect Psychiatr Care. 2000;36:121–130. doi: 10.1111/j.1744-6163.2000.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 37.Pal PK, Thennarasu K, Fleming J, et al. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson's disease and in their caregivers. Parkinsonism Relat Disord. 2004;10:157–168. doi: 10.1016/j.parkreldis.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Cho MH, Dodd MJ, Lee KA, et al. Self-reported sleep quality in family caregivers of gastric cancer patients who are receiving chemotherapy in Korea. J Cancer Educ. 2006;21(suppl 1):S37–S41. doi: 10.1207/s15430154jce2101s_8. [DOI] [PubMed] [Google Scholar]
- 39.McKibbin CL, Ancoli-Israel S, Dimsdale J, et al. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28:1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- 40.Carter PA. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nurs. 2006;29:95–103. doi: 10.1097/00002820-200603000-00003. [DOI] [PubMed] [Google Scholar]