Abstract
Purpose
Prolonged survival after two-stage resection (TSR) of advanced colorectal liver metastases (CLM) may be the result of selection of best responders to chemotherapy. The impact of complete resection in this well-selected group is controversial.
Patients and Methods
Data on 890 patients undergoing resection and 879 patients who received only chemotherapy for CLM were collected prospectively. We used intent-to-treat analysis to evaluate the survival of patients who underwent TSR. Additionally, we evaluated a cohort of nonsurgically treated patients selected to mirror the TSR population: colorectal metastases with liver-only disease, objective response to chemotherapy, and alive 1 year after chemotherapy initiation.
Results
Sixty-five patients underwent the first stage of TSR; 62 patients fulfilled the inclusion criteria for the medical group. TSR patients had a mean of 6.7 ± 3.4 CLM with mean size of 4.5 ± 3.1 cm. Nonsurgical patients had a mean of 5.9 ± 2.9 CLM with mean size of 5.4 ± 3.4 cm (not significant). Forty-seven TSR patients (72%) completed the second stage. Progression between stages was the main cause of noncompletion of the second stage (61%). After 50 months median follow-up, the 5-year survival rate was 51% in the TSR group and 15% in the medical group (P = .005). In patients who underwent TSR, noncompletion of TSR and major postoperative complications were independently associated with worse survival.
Conclusion
TSR is associated with excellent outcome in patients with advanced CLM as a result of both selection by chemotherapy and complete resection of metastatic disease.
INTRODUCTION
Surgical resection is associated with long-term survival in patients with colorectal liver metastases (CLM).1,2 The majority of patients with CLM present with advanced metastases at the time of diagnosis and therefore receive systemic chemotherapy.3,4 In patients with advanced CLM who have a sufficient response to chemotherapy, an aggressive surgical approach including two-stage resection (TSR) has been proposed to improve outcome.5 Although TSR has been associated with prolonged survival in noncomparative surgical series,6–8 whether the survival benefit of this strategy results from complete resection of metastatic disease or selection for resection of the best responders to chemotherapy is controversial. In other words, is surgery really helpful in patients with advanced CLM who respond to chemotherapy? What makes this question especially relevant is that the TSR strategy has been introduced during the same period as new, more effective cytotoxic agents (oxaliplatin and irinotecan) and monoclonal antibodies targeting the vascular endothelial growth factor (bevacizumab) and the epidermal growth factor receptor (cetuximab and panitumumab).9–12 To date, the reported median overall survival of patients with advanced colorectal cancer who respond to these regimens has been as much as 31 months.13 A randomized trial comparing TSR and continuation of chemotherapy in patients with advanced bilateral CLM that respond to systemic therapy may be impractical. We therefore conducted this retrospective study to compare outcomes of patients undergoing at least the first stage of TSR with those of selected nonsurgically treated patients responding to modern chemotherapy.
PATIENTS AND METHODS
Patient Population
From June 2002 to February 2010, data were prospectively collected on 890 consecutive patients who underwent surgical resection of CLM at one institution and 879 consecutive patients with advanced colorectal cancer treated with systemic chemotherapy only at the same institution.
For this study, for the surgically treated group, we selected all patients who underwent at least the first stage of planned TSR of advanced bilateral CLM. For the nonsurgically treated group, we selected patients who were similar to the surgically treated patients in terms of performance status, absence of extrahepatic metastases, extent of hepatic metastases on pretreatment imaging, and objective response to chemotherapy. In other words, we tried to ensure similarity of the surgically treated and nonsurgically treated patient groups. A similar selection process has previously been used to compare surgical and medical populations when a prospective controlled trial is not possible.3,14 For the medical population, we selected patients treated with chemotherapy only who were alive at 1 year and who met the following inclusion criteria: age younger than 70 years, Eastern Cooperative Oncology Group performance status 0 to 2, liver-only metastases excluding patients with innumerable CLM, objective response to first-line chemotherapy consisting of irinotecan or oxaliplatin with or without bevacizumab or cetuximab, no evidence of bowel obstruction, and alive 1 year after chemotherapy initiation. Pre- and post-treatment archived imaging of the patients was systematically reviewed to ensure that they were potential candidates for TSR (J.N.V., E.K.A., and A.B.). The study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center.
Systemic Chemotherapy
At the MD Anderson Cancer Center, patients with advanced CLM initially unsuitable for resection are seen by a surgeon before initiation of chemotherapy to determine whether the patient may be a surgical candidate with a chemosensitive tumor (one with a radiographic response to chemotherapy). In patients with advanced CLM who are identified before chemotherapy as being potential candidates for surgery, resection is considered only if it can be completed in stable disease (no change in size) or downsized CLM (decrease in size).15 In all patients receiving first-line chemotherapy, tumor response was assessed every three to four cycles by using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and classified as complete response, partial response, stable disease, or progressive disease16; beginning in 2009, tumor response was also evaluated according to morphologic criteria (none, incomplete, optimal).13
Two-Stage Liver Resection
TSR was proposed to patients with advanced bilateral CLM who responded to chemotherapy and in whom limited resection could clear the less affected side of the liver before the patient underwent a planned extended contralateral liver resection (Fig 1). TSR requires an area of the liver to be relatively spared by disease because the surgeon must be able to resect all CLM while preserving a sufficient future liver remnant (20% of total liver volume) and adequate vascular inflow and outflow.17 In the majority of patients who were candidates for and opted for TSR, the program included limited resection of CLM located in the left liver followed by right portal vein embolization 1 to 2 weeks later and then an extended right hepatectomy. Portal ligation was not used because most patients needed extended right hepatectomy and this type of resection requires embolization of segment IV branches which cannot be achieved by ligation.18,19 Interval chemotherapy was not used routinely to avoid the risk associated with extended chemotherapy.20–22 In patients who developed disease progression or recurrence after the first stage of liver resection, usually the second stage was postponed, systemic chemotherapy was restarted, and response was reevaluated after three or four cycles of interval chemotherapy to determine whether the second stage could eventually be performed. Postoperative 30-day and 90-day morbidity and mortality were recorded prospectively, and postoperative complications were classified according to their severity.23 Pathologic response was assessed as previously described.4 Briefly, complete pathologic response was defined as 0% viable tumor cells in residual tumor, major pathologic response 1% to 49%, and minor pathologic response ≥ 50%.4 After surgery, chemotherapy was usually reintroduced to complete a total of 12 cycles, including both preoperative and postoperative chemotherapy. Patients were reassessed every 4 months after completion of the second stage of liver resection, and further treatment was decided according to the findings at reassessment.
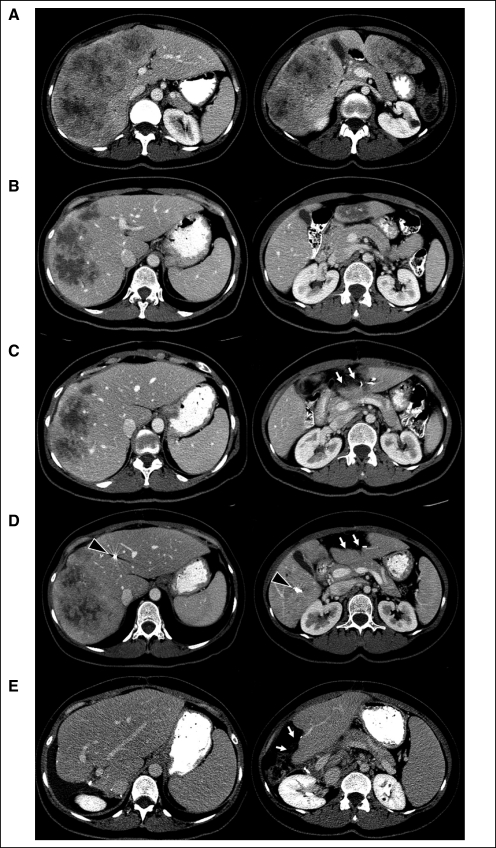
Fig 1.
Advanced bilateral colorectal liver metastases at diagnosis (A), after preoperative chemotherapy with infusional fluorouracil, leucovorin, and oxaliplatin plus bevacizumab for five cycles (B), after the first-stage hepatectomy (C), after right portal vein embolization extended to segment IV (D), and after the second stage hepatectomy (E). White arrows indicate surgical defect after the first-stage hepatectomy; black arrowheads indicate the coils after right portal vein embolization extended to segment IV.
Statistical Analysis
Quantitative and qualitative variables were expressed as mean ± standard deviation, median and range, and frequency. Overall and disease-free survival were calculated by using the Kaplan-Meier method and compared by using the log-rank test. Overall survival was calculated from the beginning of chemotherapy in both surgically treated and medically treated patients. Analysis of survival in surgical patients was done on an intent-to-treat basis and included patients who died after surgery and patients who underwent only the first stage of liver resection. For the detection of factors associated with survival in patients with advanced bilateral CLM undergoing at least the first stage of TSR, univariate and multivariate analyses were used to examine the relationship between overall survival and the following variables: type of primary tumor (rectum v colon), regional node metastases (v no metastasis), carcinoembryonic antigen plasma level, synchronous (v metachronous) CLM, number of CLM (up to 7 v > 7 CLM) as previously reported,7 maximum CLM size (< 5 cm v ≥ 5 cm), duration of preoperative chemotherapy (> 3 months v ≤ 3 months), type of chemotherapy, cumulative postoperative morbidity, and noncompletion (v completion) of TSR.
RESULTS
Clinicopathologic Features of Patients With Advanced Bilateral CLM Who Underwent at Least the First Stage of TSR
Clinicopathologic characteristics of the 65 patients with advanced bilateral CLM who underwent at least the first stage of TSR are summarized in Table 1. Most of the patients (52 of 65; 80%) had synchronous CLM. All patients received an oxaliplatin- or irinotecan-containing preoperative chemotherapy regimen, and the median number of chemotherapy cycles before first-stage liver resection was six (range, two to 26 cycles). Only 23 patients (35%) received more than six preoperative chemotherapy cycles.
Table 1.
Clinicopathologic Features of 65 Patients Who Underwent at Least the First Stage of Two-Stage Resection and 62 Patients Treated by Chemotherapy Only
| Feature | Two-Stage Resection Strategy (n = 65) |
Chemotherapy Only(n = 62) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | N/S | ||||
| Mean ± SD | 52 ± 9 | 55 ± 9 | |||
| Median | 52 | 54 | |||
| Range | 35-69 | 32-68 | |||
| Sex | N/S | ||||
| Male | 47 | 37 | |||
| Female | 18 | 25 | |||
| Rectal primary tumor | 18 | 28 | 11 | 18 | N/S |
| Node positive | 47 | 72 | 43 | 69 | N/S |
| Synchronous liver metastases | 52 | 80 | 52 | 84 | N/S |
| No. of lesions | N/S | ||||
| Mean ± SD | 6.7 ± 3.4 | 5.9 ± 2.9 | |||
| Median | 6 | 5.5 | |||
| Range | 2-18 | 2-14 | |||
| Tumor size, cm | N/S | ||||
| Mean ± SD | 4.5 ± 3.1 | 5.4 ± 3.4 | |||
| Median | 4 | 4 | |||
| Range | 1-15 | 1-17 | |||
| First-line chemotherapy regimen | |||||
| Fluorouracil + oxaliplatin | 46 | 71 | 41 | 66 | N/S |
| Fluorouracil + irinotecan | 19 | 29 | 21 | 34 | N/S |
| Bevacizumab-containing regimen | 48 | 74 | 38 | 61 | N/S |
| Multiple lines of preoperative chemotherapy | 6 | 9 | — | — | |
| No. of cycles of preoperative chemotherapy | — | — | |||
| Mean ± SD | 8 ± 5.6 | ||||
| Median | 6 | ||||
| Range | 2-26 | ||||
| CEA plasma level, ng/mL (preoperative or after initial response) | N/S | ||||
| Mean ± SD | 397 ± 2,232 | 255 ± 1,254 | |||
| Median | 6 | 13 | |||
| Range | 1-17,000 | 1-9,740 | |||
| Radiologic response using RECIST | |||||
| Partial or complete response | 28 | 62 | < .001 | ||
| Stable disease | 37 | 0 | |||
| Portal vein embolization | 45 | 70 | — | — | |
Abbreviations: N/S, not significant; SD, standard deviation; CEA, carcinoembryonic antigen; RECIST, Response Evaluation Criteria in Solid Tumors.
Feasibility of Two-Stage Strategy
Intraoperative characteristics and postoperative outcomes of the 65 patients who underwent at least the first stage of TSR are summarized in Table 2. No patient died within 90 days after the first stage hepatectomy. Two patients (3%) developed major postoperative complications: one required re-operation for severe wound infection after rectal resection performed at the same time as the first stage, and one patient developed severe postoperative renal insufficiency. Nineteen patients (29%) had resection of the primary tumor combined with the first-stage hepatectomy. The postoperative complication rate was significantly increased in patients undergoing resection of the primary tumor combined with the first stage of liver resection (eight of 19 [42%] v eight of 46 [17%]; P = .03). Histopathologic examination of CLM resected at the first stage indicated that 52 patients (80%) had a major response to chemotherapy.
Table 2.
Perioperative Characteristics in Patients Undergoing First-Stage and Second-Stage Resection
| Characteristic | No. | % |
|---|---|---|
| First-stage resection | 65 | |
| Major liver resection (> three contiguous liver segments) | 2 | 3 |
| Radiofrequency ablation | 2 | 3 |
| Associated resection of primary tumor | 19 | 29 |
| No. of lesions resected during first stage | ||
| Mean ± SD | 2 ± 1.7 | |
| Median | 1 | |
| Range | 1-8 | |
| Largest diameter of tumors resected during first stage in cm | ||
| Mean ± SD | 2 ± 2 | |
| Median | 1.4 | |
| Range | 0.4-15 | |
| Estimated blood loss, mL | ||
| Mean ± SD | 215 ± 222 | |
| Median | 150 | |
| Range | 50-1,300 | |
| Transfusion | 1 | 2 |
| Postoperative 90-day deaths | 0 | 0 |
| Postoperative complications | 16 | 25 |
| Major postoperative complications | 2 | 3 |
| Length of hospital stay, days | ||
| Mean ± SD | 7 ± 2.4 | |
| Median | 6 | |
| Range | 4-14 | |
| Positive margins | 10 | 15 |
| Second-stage resection | 47 | |
| Major liver resection (> three contiguous liver segments) | 40 | 85 |
| Extended liver resection (> four contiguous liver segments) | 32 | 68 |
| Type of resection | ||
| Right hemihepatectomy | 8 | |
| Left hemihepatectomy | 1 | |
| Extended right hepatectomy | 22 | |
| Extended left hepatectomy | 10 | |
| Segmental resection | 6 | |
| Estimated blood loss, mL | ||
| Mean ± SD | 500 ± 350 | |
| Median | 400 | |
| Range | 50-1,950 | |
| Transfusion | 6 | 13 |
| Postoperative 90-day deaths | 3 | 6.4 |
| Complications | 23 | 49 |
| Major complications | 12 | 26 |
| Hepatic insufficiency* | 4 | 6 |
| Fluid collection | 13 | 27 |
| Length of hospital stay in days | ||
| Mean ± SD | 8 ± 6.6 | |
| Median | 7 | |
| Range | 4-50 | |
| Positive margins | 10 | 21 |
Abbreviation: SD, standard deviation.
Postoperative hepatic insufficiency was defined as bilirubin peak > 7 mg/dL.23
Among the 65 patients who underwent the first-stage hepatectomy, 47 patients (72%) underwent the second-stage hepatectomy, a mean of 8 ± 4 weeks after the first stage. Intraoperative characteristics and postoperative outcomes of these 47 patients are summarized in Table 2. Of these 47 patients, nine (19%) developed progression of CLM between the first and second stages and were restarted on chemotherapy before completion of the second stage. In these patients, the mean time interval between stages was significantly longer than in patients who did not receive interval chemotherapy (17 ± 2 weeks v 8 ± 3 weeks, respectively; P = .04). Thirty patients (64%) had complete resection with negative margins at both stages. Postoperative 30-day mortality rate was 2%. Three patients (6%) died within 90 days after the second-stage hepatectomy from irreversible postoperative liver failure. Of these three patients, one patient had future liver remnant growth from 19% to 21% (hypertrophy < 5%), one patient was expected to undergo a right hepatectomy but underwent an unplanned extended right hepatectomy with a future liver remnant volume of 19% because of intraoperative findings of more advanced disease, and the remaining patient had an adequate future liver remnant volume but had significant intraoperative blood loss necessitating polytransfusion. Overall, major complications after first- or second-stage resection occurred in 14 patients (22%).
Eighteen (33%) of the 65 patients who underwent the first stage did not undergo the second stage. Of these 18 patients, 11 (61%) had progression or recurrence, including disease progression of the CLM to be resected (n = 7), recurrence in the future liver remnant (n = 3), or progression of both CLM and extrahepatic metastases (n = 1). Imaging reevaluation after initiation of interval chemotherapy showed that complete resection could not be performed. In the other seven patients (39%) who did not undergo the second-stage hepatectomy, the second stage could not be performed for technical reasons, including an inadequate increase in future liver remnant volume after portal vein embolization (n = 3), an inadequate recovery after first-stage hepatectomy (n = 2), and postoperative (n = 1) or postportal vein embolization (n = 1) partial portal vein thrombosis.
Influence of the Two-Stage Strategy on Outcome of Patients With Advanced Bilateral CLM Who Responded to Chemotherapy
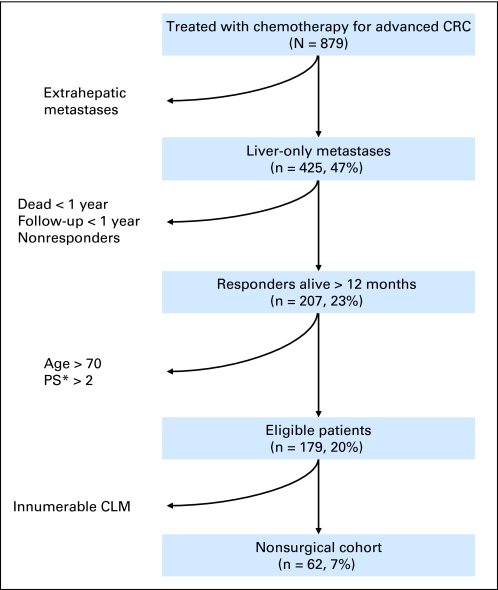
From June 2002 to February 2010, among 879 consecutive patients who received only chemotherapy for advanced colorectal cancer, 62 (7%) fulfilled this study's inclusion criteria for comparison with the patients who underwent at least the first stage of TSR (Fig 2). Clinicopathologic features of these 62 patients are summarized in Table 1. These patients were not statistically different from patients who underwent at least one stage of the TSR except for RECIST response to systemic therapy with all patients in the chemotherapy-only group having partial response (Table 1).
Fig 2.
Selection of patients with advanced bilateral colorectal liver metastases (CLM) treated with chemotherapy for inclusion in the nonsurgically treated population for this study. CRC, colorectal cancer. (*) Performance status (PS) according to the Eastern Cooperative Oncology Group scoring system.
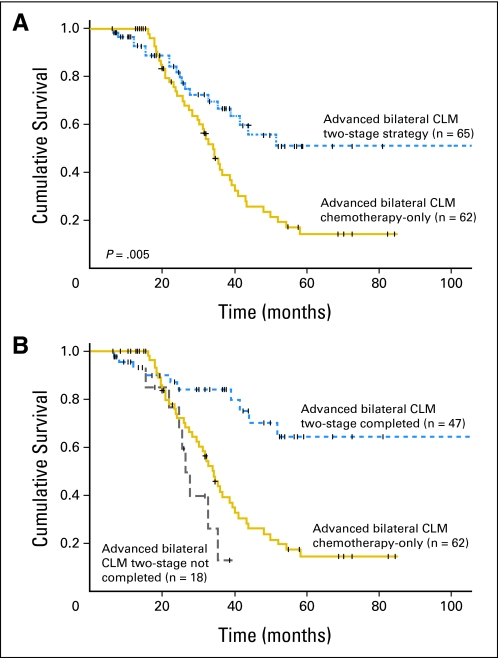
After a median of 50 months follow-up, patients who underwent at least the first stage of TSR had significantly improved survival compared with patients who underwent only chemotherapy for advanced bilateral CLM (67% v 41% 3-year overall survival rate and 51% v 15% 5-year overall survival rate; P = .005; Fig 3A). The survival advantage was prominent for patients who completed the second stage of TSR (84% v 42% 3-year survival rate and 64% v 15% 5-year survival rate, respectively; P < .001). In contrast, there was no survival advantage for patients who underwent only the first stage (3-year survival rate 13% for first stage only v 42% for chemotherapy only; P = .12; Fig 3B). Twenty-nine of the 47 patients who underwent complete TSR (62%) had developed recurrences by the time of last follow-up. One-year, 3-year, and 5-year disease-free survival rates in these patients were 39%, 20%, and 20%, respectively.
Fig 3.
Overall survival in patients with advanced bilateral colorectal liver metastases (CLM) responding to chemotherapy enrolled in two-stage strategy (intent-to-treat analysis including patients undergoing only the first stage of two-stage hepatectomy) or receiving chemotherapy only (A) and stratified on the basis of whether two-stage resection was completed (B).
Factors Associated With Survival in Patients Who Underwent at Least the First Stage of TSR
Results of univariate and multivariate analyses for predictors of survival in patients with advanced bilateral CLM who underwent at least the first stage of TSR are summarized in Table 3. Univariate analysis showed that total number of CLM greater than seven and noncompletion of the two-stage strategy were associated with shorter survival. In multivariate analysis, the occurrence of major postoperative complications after the first- or second-stage hepatectomy and noncompletion of the two-stage strategy were independently associated with worse survival.
Table 3.
Univariate and Multivariate Analyses of Factors Associated With Survival in 65 Patients Who Underwent at Least the First Stage of Two-Stage Liver Resection
| Factor | No. of Patients | 5-Year Survival (%) | Univariate Analysis P | Multivariate Analysis P | HR | 95% CI |
|---|---|---|---|---|---|---|
| Primary tumor | .55 | |||||
| Rectal | 18 | 48 | ||||
| Colon | 47 | 52 | ||||
| Node | .4 | |||||
| Positive | 47 | 42 | ||||
| Negative | 12 | 65 | ||||
| Unknown | 6 | 75 | ||||
| CLM | .1 | .9 | ||||
| Synchronous | 52 | 58 | ||||
| Metachronous | 13 | 22 | ||||
| Total number of CLM | .05 | .18 | ||||
| ≤ 7 | 41 | 65 | ||||
| > 7 | 24 | 26 | ||||
| Size of CLM, cm | .3 | |||||
| ≤ 5 | 45 | 58 | ||||
| > 5 | 20 | 31 | ||||
| Preoperative chemotherapy regimen | .3 | |||||
| Fluorouracil + irinotecan | 19 | 59 | ||||
| Fluorouracil + oxaliplatin | 46 | 48 | ||||
| Bevacizumab | .9 | |||||
| No | 17 | 50 | ||||
| Yes | 48 | 53 | ||||
| Preoperative chemotherapy duration, months | .1 | .7 | ||||
| ≤ 3 | 43 | 65 | ||||
| > 3 | 22 | 15 | ||||
| CEA plasma level, ng/dL | .3 | |||||
| ≤ 200 | 5 | 0 | ||||
| > 200 | 60 | 54 | ||||
| No. of lesions resected during first stage | .6 | |||||
| 1 | 29 | 54 | ||||
| Multiple | 36 | 46 | ||||
| Major postoperative complication after first or second resection | .1 | .04 | 3.1 | 1.1 to 9.2 | ||
| Yes | 14 | 55 | ||||
| No | 51 | 33 | ||||
| Two-stage resection | < .001 | .001 | 7.2 | 2.4 to 22.4 | ||
| Noncompletion | 18 | 64 | ||||
| Completion | 47 | 13 |
Abbreviations: HR, hazard ratio; CLM, colorectal liver metastases; CEA, carcinoembryonic antigen.
DISCUSSION
This study indicates that complete TSR is associated with excellent outcome in patients with advanced bilateral CLM who respond to chemotherapy. Our findings demonstrate that the survival benefit associated with this aggressive surgical approach is due not only to selection by preoperative chemotherapy of patients with favorable tumor biology but also to complete resection of metastatic disease. Candidates for TSR are selected on the basis of the presenting extent and location of bilateral CLM and the response to first-line chemotherapy. In the current study, all patients selected to undergo TSR had an objective response to chemotherapy on imaging, and the great majority of them (80%) also had a major pathologic response, which is recognized as a strong predictor of survival.4 We found that TSR was completed in 47 (72%) of the 65 patients in whom it was attempted. This result is consistent with data from previously reported smaller series,6,7,24,25 in which rates of completion of both stages ranged from 69% to 78%.
In this study, we observed a 64% 5-year overall survival rate in the patients with advanced bilateral CLM who underwent complete TSR. To the best of our knowledge, our series of 47 patients is the largest series of patients with advanced bilateral CLM undergoing complete TSR reported to date. The survival rate for this group in our study compares favorably with previously reported rates, which range from 35% to 50%.6,7,24,25 Additionally, we observed a 5-year survival rate of 15% in patients with advanced bilateral CLM who received chemotherapy only. This is the highest survival rate reported in a nonsurgical cohort of patients with CLM. Although the study population represents a highly selected sample of patients with advanced colorectal cancer, it highlights the overall improvement of prognosis with the introduction of new chemotherapy regimens used in this setting.3,13,14 This improvement in survival rates after chemotherapy probably contributes to the good outcomes reported in TSR series, including ours, because TSR is offered only to responders to chemotherapy.6,7,24 However, our finding that patients who completed TSR had higher survival rates than those in patients treated with chemotherapy only shows that progress of chemotherapy and selection of good responders do not fully account for the survival benefit observed. In patients who underwent resection of advanced CLM, complete resection of metastatic disease also contributed to the observed survival benefit. Thus, there was no benefit of attempted TSR if the strategy could not be completed.
One of the limitations of TSR is the complexity of the approach, which combines the difficulties associated with repeat hepatectomy with the difficulties associated with surgery on a regenerative liver. We report a 6% 90-day mortality rate and a 49% morbidity rate after the second stage of hepatectomy. These results are consistent with previously reported mortality and morbidity rates after TSR, which range from 0% to 9% and from 26% to 59%, respectively.6–8,24 The three postoperative deaths in this series occurred as a consequence of liver failure after second-stage extended right hepatectomy in patients who underwent preoperative portal vein embolization. Two of the three patient deaths occurred early in our experience in patients with either an inadequate future liver remnant volume (intraoperative change in treatment plan) or insufficient hypertrophy (< 5%) after portal vein embolization. These undesirable events reaffirm the importance of preoperative assessment and standardized requirements for minimal volume and hypertrophy cutoffs for extended liver resection and have since been incorporated into revised guidelines for major hepatectomy.26 The morbidity after the first stage of resection is low,7 and the first stage is generally not a threat to patients' lives because major complications are uncommon. However, maintaining a low complication rate after the first stage clearly must be a surgical priority since cumulative major complication after first- or second-stage resection was a strong predictor of survival. Major complications after the first stage delay recovery, disrupt the treatment strategy, and eventually compromise completion of the second stage. We also found that combined resection of the primary tumor with the first stage of liver resection was associated with an increased risk of complications. An alternative in patients in whom the primary tumor is nonobstructive is a reverse strategy in which the primary tumor is resected after completion of the two-stage strategy.27,28
This study has some limitations. We retrospectively analyzed two subsets of patients treated medically and surgically and we cannot exclude more advanced disease in patients treated medically despite well-defined selection criteria. However, a randomized controlled trial in this setting is unlikely because of the small subset of patients who are potential candidates for this approach (< 10% of patients with advanced colorectal cancer). Taken together, our findings cannot be used to define surgery and chemotherapy as opposing treatments; rather, our findings can be used to define the impact of a sequenced approach on a subset of patients with multiple bilateral CLM and sufficient parenchymal sparing to a allow for a two-stage strategy.
In conclusion, this study showed that complete TSR is associated with excellent outcome in patients with advanced bilateral CLM who respond to chemotherapy. The survival benefit associated with this aggressive surgical approach is due to (1) selection of patients with favorable tumor biology by preoperative chemotherapy, (2) selection of patients with adequate liver hypertrophy and good performance status, and (3) complete resection of metastatic disease. To further improve the survival rate in patients who complete TSR, efforts should focus on improved patient selection to increase the rate of success and to lower morbidity.
Acknowledgment
We thank Stephanie P. Deming and Ruth J. Haynes for editing.
Footnotes
Supported in part by MD Anderson Cancer Center Support Grant No. CA016672 from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Scott Kopetz, Roche (C); Michael J. Overman, Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Scott Kopetz, Roche; Cathy Eng, Genentech; Jean-Nicolas Vauthey, sanofi-aventis, Roche Research Funding: Scott Kopetz, Roche; Michael J. Overman, sanofi-aventis; Jean-Nicolas Vauthey, sanofi-aventis, Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Antoine Brouquet, Eddie K. Abdalla, Scott Kopetz, Jean-Nicolas Vauthey
Provision of study materials or patients: Eddie K. Abdalla, Christopher R. Garrett, Michael J. Overman, Cathy Eng, David C. Madoff, Steven A. Curley, Jean-Nicolas Vauthey
Collection and assembly of data: Antoine Brouquet, Eddie K. Abdalla, Scott Kopetz, Jean-Nicolas Vauthey
Data analysis and interpretation: Antoine Brouquet, Eddie K. Abdalla, Scott Kopetz, Christopher R. Garrett, Michael J. Overman, Cathy Eng, Evelyne M. Loyer, David C. Madoff, Steven A. Curley, Jean-Nicolas Vauthey
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 7.Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. discussion 1049-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: Perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–1504. doi: 10.1007/s11605-007-0272-2. discussion 1504-1505. [DOI] [PubMed] [Google Scholar]
- 9.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 10.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 13.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 15.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 18.Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–751. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: Improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 20.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 21.Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–2876. doi: 10.1245/s10434-010-1166-1. [DOI] [PubMed] [Google Scholar]
- 22.Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–286. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamecha V, Nedjat-Shokouhi B, Gurusamy K, et al. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolisation and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig Surg. 2008;25:387–393. doi: 10.1159/000176063. [DOI] [PubMed] [Google Scholar]
- 26.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: Evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 27.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: Classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]