Abstract
Purpose
Transplantation-related mortality (TRM) is a major barrier to the success of allogeneic hematopoietic cell transplantation (HCT).
Patients and Methods
We assessed changes in the incidence of TRM and overall survival from 1985 through 2004 in 5,972 patients younger than age 50 years who received myeloablative conditioning and HCT for acute myeloid leukemia (AML) in first complete remission (CR1) or second complete remission (CR2).
Results
Among HLA-matched sibling donor transplantation recipients, the relative risks (RRs) for TRM were 0.5 and 0.3 for 2000 to 2004 compared with those for 1985 to 1989 in patients in CR1 and CR2, respectively (P < .001). The RRs for all causes of mortality in the latter period were 0.73 (P = .001) and 0.60 (P = .005) for the CR1 and CR2 groups, respectively. Among unrelated donor transplantation recipients, the RRs for TRM were 0.73 (P = .095) and 0.58 (P < .001) for 2000 to 2004 compared with those in 1990 to 1994 in the CR1 and CR2 groups, respectively. Reductions in mortality were observed in the CR2 group (RR, 0.74; P = .03) but not in the CR1 group.
Conclusion
Our results suggest that innovations in transplantation care since the 1980s and 1990s have reduced the risk of TRM in patients undergoing allogeneic HCT for AML and that this reduction has been accompanied by improvements in overall survival.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is an effective therapy for a variety of malignant and nonmalignant diseases. However, it carries a significant risk for treatment-related mortality, stemming primarily from infection,1–3 conditioning regimen–related toxicities,4–6 and graft-versus-host disease (GVHD).7–9 The risk for transplantation-related mortality (TRM) is influenced by several factors, including patient age, donor type, and conditioning regimen intensity.10–14 The risk of TRM varies from < 10% in children younger than age 10 years receiving HLA-matched related donor (MRD) transplantations to 30% or higher in adolescents and adults receiving unrelated donor (URD) transplantations.10,11,13,14
Since the 1980s, several innovations have been implemented to reduce TRM. More effective approaches for prevention of GVHD,15 fungal infection, and cytomegalovirus (CMV) disease16 have been introduced. Pharmacokinetic-based targeting of busulfan dosing has been adopted.17 For patients receiving URD transplantations, enhancements have been made in HLA typing and matching.18 At the same time, relevant advances have occurred in related fields, including critical care medicine, nephrology, and transfusion medicine.19–21
The collective impact of these advances on patient outcome is unknown. To address this matter, we assessed the change in TRM after transplantations for acute myeloid leukemia (AML), the most common indication for allogeneic HCT,22 from 1985 to 2004.
PATIENTS AND METHODS
Patient-, Disease-, and Transplantation- Related Characteristics
Data on patients with AML who received mobilized peripheral blood or marrow HCT were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR). CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive HCTs to a statistical center located at the Medical College of Wisconsin (MCW) in Milwaukee, WI, and at the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis, MN. Participating centers are required to report all transplantations consecutively. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensured data quality. Observational studies conducted by the CIBMTR were performed in compliance with the Privacy Rule (Health Insurance Portability and Accountability Act [HIPAA]) as a public health authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants, as determined by continual review of the NMDP and MCW institutional review boards since 1985.
Patients age 50 years or younger with AML in first complete remission (CR1) or second complete remission (CR2) who received an HCT from an MRD from 1985 to 2004 or from a URD from 1990 to 2004 were eligible. All received bone marrow (BM) or peripheral blood progenitor cell (PBPC) grafts and myeloablative conditioning regimens based on busulfan/cyclophosphamide (BuCy) or cyclophosphamide/total-body irradiation (CyTBI).
End Points
The primary end point was TRM, defined as death during continuous complete remission. Overall survival (OS), leukemia-free survival (LFS), and leukemia relapse were also assessed.
Statistical Methods
Four groups defined by disease status at transplantation (CR1 and CR2) and donor type (MRD and URD) were formed. These groups, in turn, were separated into 5-year cohorts. Within each of the groups, patient-, disease-, and transplantation-related characteristics were compared by using the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of OS and LFS were calculated by using the Kaplan-Meier estimator.23 For survival analyses, death from any cause was considered an event, and data on surviving patients were censored at last follow-up. For LFS analyses, relapse or death were considered an event, and data for patients alive in CR were censored at last follow-up. Probabilities of TRM and leukemia relapse were calculated by using the cumulative incidence function.24 For TRM, relapse was the competing event and for relapse, TRM was the competing event. Data on patients without competing events were censored at last follow-up and CIs were calculated with a log transformation.24
The initial multivariate models were adjusted for patient characteristics only to avoid removing the effect of changes in practice. Cox proportional hazards regression25 was used with a stepwise forward selection technique, in which year of transplantation was forced into the model and a P value ≤ .05 was the criterion for other covariates to be included in the final model. Other patient characteristics considered in the analyses were comorbidities (the presence or absence of any comorbidity), recipient age, performance score at time of HCT, time from diagnosis to transplantation, sex, WBC count at diagnosis, and cytogenetics at diagnosis. Because of the possible confounding between unknown cytogenetics and year of transplantation, we fit models both with and without adjustment for cytogenetics; the results were similar in all cases. All possible risk factors were checked for proportional hazards by using a time-dependent covariate approach, and a stratified model was used when there were nonproportional hazards. First-order interactions between year of transplantation and other variables were assessed. Trend tests were used in the Cox model to test for the overall effect of year of transplantation. Adjusted probabilities of OS and LFS by year of transplantation were estimated by stratified Cox model. P values are two-sided. Analyses were done by using SAS software (SAS Institute, Cary, NC).
Additional exploratory multivariate analyses were done to investigate the impact of changes in select transplantation characteristics on changes in outcomes by year of transplantation, including donor-recipient sex and CMV serologic status, graft type (BM v PBPC), conditioning regimen (BuCy v CyTBI), GVHD prophylaxis (cyclosporine v tacrolimus-based), and HLA matching in the URD group.
URD-recipient pairs were classified according to the Weisdorf Criteria,26 designed for use in retrospective studies that analyze HLA-matching data spanning many years. Weisdorf et al analyzed 21 subgroups of URD-recipient pairs whose matching varied from the three-loci low-resolution typing (A, B, DRB1) approach, common in the 1980s and early 1990s, to the four-loci high-resolution typing (A, B, C, DRB1) that is now standard. These subgroups clustered in three major groups according to survival analyses: well matched, partially matched, or mismatched.
Factors with sufficient overlap over time were included in the multivariate model in a stepwise fashion. Some factors (graft type, GVHD prophylaxis, HLA matching) changed dramatically over the study period. To avoid confounding, we conducted subgroup analyses examining the effect of year of transplantation in the largest groups of consistently treated patients over the years on the basis of those receiving BM, those receiving cyclosporine and methotrexate (CSA/MTX) for GVHD prophylaxis, and those receiving partially matched URD grafts.26
RESULTS
Patient-, Disease-, and Transplantation- Related Characteristics
Data were analyzed on 5,972 transplantations (3,704 MRD CR1, 750 MRD CR2, 738 URD CR1, 780 URD CR2; Table 1). Over time, changes in several patient, disease, and transplantation-related characteristics occurred. In the MRD CR1 group, patients were less likely to have a Karnofsky performance score (KPS) of at least 90 but were more likely to be > 6 months from diagnosis. There was a decrease over time in the proportion of transplantations in which both the recipient and donor were serologically negative for CMV. In the MRD CR2 group, patients were less likely to have a transplantation within 12 months of diagnosis but were more likely to not have a comorbid condition at time of HCT. In both the MRD CR1 and MRD CR2 groups, BuCy was used more frequently for conditioning, and CSA/MTX was used more frequently for GVHD prophylaxis regimens in later periods. In the URD CR1 group, patients were less likely to be > 6 months from diagnosis over time. In both the URD CR1 and URD CR2 groups, patients were less likely to have a KPS of at least 90 and to receive T-cell depleted grafts in later time periods; they were more likely have a comorbid condition, to receive TBI-based conditioning and to have a well-matched donor. In all four groups, patients were more likely to have received a PBPC graft but were less likely to have unknown cytogenetic testing results in later periods.
Table 1.
Characteristics of Patients With AML in CR1 or CR2 Who Received HLA-MRD or Matched URD Allogeneic HCT From 1985 to 2004
| Characteristic | 1985-1989 |
1990-1994 |
1995-1999 |
2000-2004 |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| MRD/CR1 HCT | |||||||||
| No. of patients | 1,124 | 1,283 | 901 | 460 | — | ||||
| Age, years | < .01 | ||||||||
| Median | 27 | 30 | 31 | 31 | |||||
| Range | 1-50 | 1-50 | 1-50 | 1-50 | |||||
| KPS ≥ 90 | 984 | 87 | 1,081 | 84 | 739 | 81 | 376 | 81 | < .01 |
| No comorbid conditions* | 962 | 86 | 1,020 | 80 | 664 | 73 | 350 | 76 | < .01 |
| Cytogenetics† | < .01 | ||||||||
| Favorable | 115 | 10 | 192 | 15 | 94 | 10 | 41 | 9 | |
| Intermediate | 326 | 39 | 506 | 39 | 508 | 56 | 304 | 66 | |
| Poor | 47 | 4 | 76 | 6 | 76 | 8 | 59 | 13 | |
| Unknown | 636 | 56 | 509 | 40 | 224 | 250 | 56 | 12 | |
| Time from diagnosis to transplantation < 6 months | 634 | 57 | 669 | 52 | 561 | 62 | 344 | 75 | < .01 |
| Bone marrow | 1,124 | 100 | 1,279 | 99 | 662 | 73 | 199 | 43 | < .01 |
| Negative donor-recipient CMV match | 319 | 28 | 371 | 29 | 287 | 32 | 97 | 21 | < .01 |
| BuCy | 278 | 26 | 679 | 53 | 553 | 61 | 327 | 71 | |
| GVHD prophylaxis | < .01 | ||||||||
| T-cell depletion | 257 | 23 | 182 | 14 | 44 | 5 | 6 | 1 | |
| CSA + MTX ± other | 460 | 41 | 829 | 64 | 665 | 74 | 325 | 71 | |
| CSA ± other (not MTX) | 302 | 27 | 232 | 18 | 125 | 14 | 61 | 13 | |
| Tacrolimus ± other | — | 6 | < 1 | 25 | 3 | 49 | 12 | ||
| Other | 105 | 9 | 57 | 4 | 42 | 5 | 19 | 4 | |
| Follow-up, months | |||||||||
| Median | 148 | 112 | 83 | 35 | |||||
| Range | 3-257 | 3-207 | 2-141 | 3-85 | |||||
| MRD/CR2 HCT | |||||||||
| No. of patients | 202 | 232 | 202 | 124 | |||||
| Age, years | < .01 | ||||||||
| Median | 28 | 30 | 34 | 29 | |||||
| Range | 1-49 | 1-50 | 1-9 | 2-50 | |||||
| KPS ≥ 90 | 166 | 82 | 174 | 75 | 147 | 73 | 98 | 79 | .26 |
| No comorbid conditions* | 160 | 79 | 182 | 78 | 138 | 68 | 89 | 72 | .03 |
| Time from diagnosis to transplantation < 12 months | 81 | 40 | 72 | 31 | 44 | 22 | 32 | 26 | < .01 |
| Cytogenetics† | < .01 | ||||||||
| Good | 12 | 6 | 37 | 16 | 61 | 30 | 46 | 37 | |
| Intermediate | 43 | 21 | 69 | 30 | 80 | 40 | 52 | 42 | |
| Poor | 7 | 3 | 8 | 3 | 16 | 8 | 9 | 7 | |
| Unknown | 140 | 69 | 118 | 51 | 45 | 22 | 17 | 14 | |
| Bone marrow | 202 | 230 | 99 | 132 | 65 | 36 | 29 | < .01 | |
| BuCy | 70 | 35 | 127 | 55 | 131 | 65 | 92 | 74 | < .01 |
| Donor-recipient CMV match negative/negative | 46 | 23 | 48 | 21 | 51 | 25 | 29 | 23 | < .01 |
| GVHD prophylaxis | < .01 | ||||||||
| T-cell depletion | 47 | 23 | 22 | 9 | 13 | 6 | 2 | 2 | |
| CSA + MTX | 84 | 42 | 143 | 62 | 148 | 74 | 90 | 73 | |
| CSA ± other (not MTX) | 53 | 26 | 57 | 25 | 30 | 15 | 13 | 10 | |
| Tacrolimus ± other | — | 1 | < 1 | 8 | 4 | 14 | 12 | ||
| Other | 18 | 9 | 9 | 4 | 2 | 1 | 5 | 4 | |
| Follow-up, months | |||||||||
| Median | 146 | 115 | 93 | 48 | |||||
| Range | 3-251 | 5-200 | 3-151 | 3-86 | |||||
| Matched URD/CR1 HCT | |||||||||
| No. of patients | — | 82 | 230 | 440 | — | ||||
| Age, years | — | < .01 | |||||||
| Median | 26 | 29 | 30 | ||||||
| Range | 1-50 | 1-50 | 1-50 | ||||||
| KPS ≥ 90 | — | 67 | 82 | 184 | 80 | 330 | 75 | .20 | |
| No comorbid conditions* | — | 69 | 84 | 181 | 79 | 268 | 61 | < .01 | |
| Cytogenetics† | .08 | ||||||||
| Good | — | 6 | 7 | 12 | 5 | 29 | 7 | ||
| Intermediate | — | 43 | 52 | 140 | 61 | 232 | 53 | ||
| Poor | — | 15 | 18 | 47 | 20 | 122 | 28 | ||
| Unknown | — | 18 | 22 | 31 | 14 | 57 | 13 | ||
| Bone marrow | — | 82 | 100 | 223 | 97 | 259 | 59 | < .01 | |
| Negative donor-recipient CMV | — | 33 | 40 | 65 | 28 | 127 | 29 | < .01 | |
| BuCy | — | 41 | 50 | 96 | 42 | 216 | 49 | .19 | |
| HLA match status‡ | < .01 | ||||||||
| Well matched | — | 4 | 5 | 38 | 17 | 189 | 43 | ||
| Partially matched | — | 30 | 37 | 140 | 61 | 215 | 49 | ||
| Mismatched | — | 48 | 59 | 52 | 23 | 36 | 8 | ||
| GVHD prophylaxis | < .01 | ||||||||
| T-cell depletion | — | 29 | 35 | 56 | 24 | 45 | 10 | ||
| CSA + MTX | — | 46 | 56 | 151 | 66 | 239 | 54 | ||
| CSA ± other (not MTX) | — | 5 | 6 | 4 | 2 | 25 | 5 | ||
| Tacrolimus ± other | — | 1 | 1 | 17 | 8 | 123 | 28 | ||
| Other | — | 1 | 1 | 2 | < 1 | 7 | 3 | ||
| FU, months | — | ||||||||
| Median | 139 | 98 | 44 | ||||||
| Range | 51-207 | 8-144 | 3-97 | ||||||
| Matched URD/CR2 HCT | |||||||||
| No. of patients | — | 107 | 300 | 380 | — | ||||
| Age, years | — | .02 | |||||||
| Median | 27 | 23 | 28 | ||||||
| Range | 2-49 | 1-49 | 1-50 | ||||||
| KPS ≥ 90 | — | 85 | 79 | 230 | 77 | 278 | 73 | .33 | |
| No comorbid condition | — | 93 | 87 | 238 | 79 | 251 | 66 | < .01 | |
| Time from diagnosis to transplantation < 12 months* | — | 20 | 19 | 47 | 16 | 70 | 18 | .70 | |
| Cytogenetics† | < .01 | ||||||||
| Good | — | 12 | 11 | 88 | 29 | 92 | 24 | ||
| Intermediate | — | 38 | 36 | 128 | 43 | 193 | 51 | ||
| Poor | — | 9 | 8 | 16 | 5 | 30 | 8 | ||
| Unknown | — | 48 | 45 | 68 | 23 | 65 | 17 | ||
| Bone marrow | — | 106 | 99 | 294 | 98 | 249 | 66 | < .01 | |
| BuCy | — | 47 | 44 | 133 | 44 | 170 | 45 | .96 | |
| HLA match status‡ | < .01 | ||||||||
| Well matched | — | 20 | 19 | 64 | 21 | 147 | 39 | ||
| Partially matched | — | 27 | 25 | 174 | 58 | 184 | 48 | ||
| Mismatched | — | 60 | 56 | 62 | 21 | 49 | 13 | ||
| Negative donor-recipient CMV | — | 32 | 30 | 103 | 34 | 114 | 30 | .50 | |
| GVHD prophylaxis | < .01 | ||||||||
| T-cell depletion | — | 40 | 37 | 76 | 25 | 48 | 13 | ||
| CSA + MTX | — | 49 | 46 | 184 | 61 | 194 | 51 | ||
| CSA ± other (not MTX) | — | 14 | 13 | 5 | 2 | 24 | 6 | ||
| Tacrolimus ± other | — | 2 | 2 | 33 | 11 | 109 | 29 | ||
| Other | — | 2 | 2 | 2 | 1 | 5 | 1 | ||
| Follow-up, months | — | ||||||||
| Median | 149 | 97 | 48 | ||||||
| Range | 23-204 | 12-151 | 3-89 | ||||||
Abbreviations: AML, acute myeloid leukemia; CR1, first complete response; CR2, second complete response; MRD, matched related donor; URD, unrelated donor; HCT, hematopoietic cell transplantation; KPS, Karnofsky performance score; CMV, cytomegalovirus; BuCy, busulfan/cyclophosphamide; GVHD, graft-versus-host disease; CSA, cyclosporine; MTX, methotrexate; FU, fluorouracil.
Comorbid conditions are reported by the transplantation centers as any pre-existing medical condition present at time of transplantation.
Cytogenetics are classified according to Slovak et al.26a Patients with normal cytogenetics are classified as having intermediate-risk disease.
Classification of HLA matching is based on Weisdorf et al26 on assessment of HLA matching for retrospective studies.
TRM
Univariate analysis demonstrated a steady drop in 3-year incidence of TRM over time in both MRD groups. For patients in CR1, it dropped from 29% (95% CI, 24% to 29%) in the 1985 to 1989 period to 15% (95% CI, 11% to 18%) in the 2000 to 2004 period (P < .001). For patients in CR2, the TRM rate fell from 37% (95% CI, 31% to 44%) to 13% (95% CI, 7% to 20%) over the same time period (P < .001). In the URD CR1 group, the incidences of TRM were 39% (95% CI, 33% to 54%), 46% (95% CI, 39% to 52%), and 31% (95% CI, 27% to 36%; P = .001) for the periods 1990 to 1994, 1995 to 1999, and 2000 to 2004, respectively. In the URD CR2 group, the incidences of TRM during the same period were 49% (95% CI, 40% to 59%), 44% (95% CI, 38% to 50%), and 36% (95% CI, 31% to 41%), respectively (P = .018; Fig 1). Older age was associated with higher TRM in all four groups across all four time periods. The probability of TRM according to age and at different time points is shown in Table 2.
Fig 1.
Transplantation-related mortality by 5-year periods. (A) Recipients of HLA-matched related donors in first complete remission, (B) recipients of matched related donors in second complete remission, (C) recipients of unrelated donors in first complete remission, and (D) recipients of unrelated donors in second complete remission.
Table 2.
Univariate Probabilities of TRM by Age Among Patients With AML in CR1 Who Received HCT From an HLA-MRD and Overall TRM in Patients With AML in CR1 and CR2 Who Received MRD and URD HCT, Reported to the CIBMTR Between 1985 and 2004
| Univariate Outcome | 1985-1989 |
1990-1994 |
2000-2004 |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Evaluated | Probability | 95% CI | No. Evaluated | Probability | 95% CI | No. Evaluated | Probability | 95% CI | ||
| TRM MRD/CR1 at 3 years by age group | ||||||||||
| 0-10 | 122 | 13 | 7 to 19 | — | — | — | 58 | 9 | 2 to 19 | .229 |
| 11-20 | 218 | 22 | 17 to 28 | — | — | — | 83 | 7 | 2 to 13 | < .001 |
| 21-30 | 339 | 27 | 22 to 31 | — | — | — | 86 | 10 | 4 to 18 | < .001 |
| 31-40 | 316 | 32 | 27 to 37 | — | — | — | 101 | 16 | 10 to 24 | .001 |
| 41-50 | 119 | 34 | 26 to 42 | — | — | — | 130 | 24 | 16 to 32 | .199 |
| TRM MRD/CR1 at: | 1,114 | 458 | ||||||||
| 30 days | 4 | 3 to 5 | — | — | — | 1 | 0 to 2 | < .001 | ||
| 100 days | 15 | 13 to 17 | — | — | — | 6 | 4 to 8 | < .001 | ||
| 1 year | 23 | 20 to 25 | — | — | — | 11 | 9 to 14 | < .001 | ||
| 3 years | 26 | 24 to 29 | — | — | — | 15 | 11 to 18 | < .001 | ||
| TRM MRD/CR2 at: | 202 | 121 | ||||||||
| 30 days | 10 | 7 to 15 | — | — | — | 1 | 0 to 3 | < .001 | ||
| 100 days | 25 | 19 to 31 | — | — | — | 5 | 2 to 10 | < .001 | ||
| 1 year | 35 | 28 to 41 | — | — | — | 8 | 4 to 13 | < .001 | ||
| 3 years | 37 | 31 to 44 | — | — | — | 13 | 7 to 20 | < .001 | ||
| TRM URD/CR1 at: | — | 82 | 438 | |||||||
| 30 days | — | — | 7 | 3 to 14 | 5 | 3 to 8 | .248 | |||
| 100 days | — | — | 22 | 14 to 31 | 15 | 12 to 19 | < .001 | |||
| 1 year | — | — | 34 | 24 to 45 | 26 | 22 to 30 | < .001 | |||
| 3 years | — | — | 39 | 29 to 50 | 31 | 27 to 36 | .002 | |||
| TRM URD/CR2 at: | — | 106 | 377 | |||||||
| 30 days | — | — | 8 | 3 to 13 | 7 | 4 to 9 | .504 | |||
| 100 days | — | — | 28 | 20 to 37 | 19 | 15 to 23 | .028 | |||
| 1 year | — | — | 44 | 35 to 54 | 31 | 27 to 36 | .016 | |||
| 3 years | — | — | 49 | 40 to 59 | 36 | 31 to 41 | .019 | |||
Abbreviations: TRM, transplantation-related mortality; AML, acute myeloid leukemia; CR1, first complete remission; HCT, hematopoietic cell transplantation; MRD, matched related donor; CR2, second complete remission; URD, unrelated donor; CIBMTR, Center for International Blood and Marrow Transplant Research.
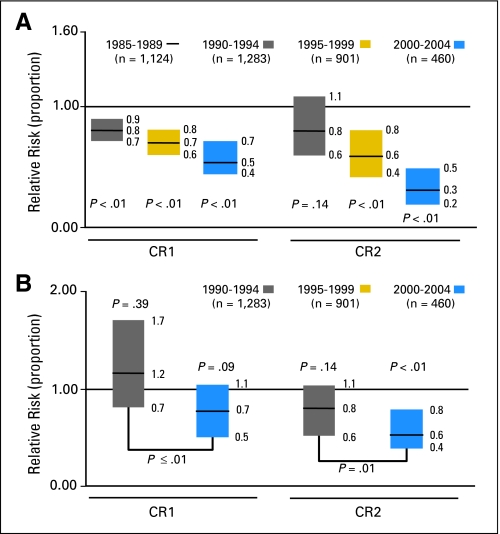
Adjusting for changes in patient and disease characteristics over time, the multivariate analyses demonstrated significant reductions in TRM over time in three of the four groups (Fig 2). In MRD HCT recipients, the relative risks (RRs) for TRM in 2000 to 2004 (compared with those in 1985 to 1989) were 0.5 (95% CI, 0.37 to 0.66; P < .001) and 0.25 (95% CI, 0.15 to 0.44; P < .001) for the CR1 group (adjusted for age, KPS, comorbid conditions, and cytogenetics) and CR2 group (adjusted for age), respectively. For URD HCT recipients, the RRs for TRM in 2000 to 2004 (compared with those in 1990 to 1994) were 0.73 (95% CI, 0.5 to 1.06; P = .095) and 0.58 (95% CI, 0.42 to 0.79; P < .001) for the CR1 group (adjusted for age) and CR2 group (adjusted for age and comorbid conditions), respectively. In the latter group, an interaction was observed between recipient age and year of transplantation; significant reductions in TRM occurred only in patients who were older than age 30 years.
Fig 2.
Transplantation-related mortality adjusted for patient and disease characteristics. (A) Recipients of HLA-matched related donor grafts and (B) recipients of unrelated donor grafts.
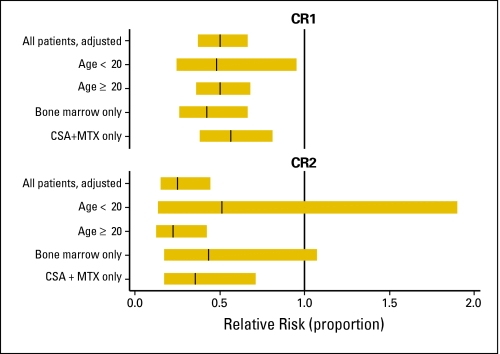
When we examined the potential influences of specific changes in practice on the decrease in RR for TRM over time in the MRD/CR1, MRD/CR2, and URD/CR1 groups, adjustment for the effects of conditioning regimen and CMV serologic status had no significant impact (data not shown). In multivariate analyses restricted to BM recipients, the RRs for TRM were 0.6 (95% CI, 0.48 to 0.75; P < .001) and 0.43 (95% CI, 0.17 to 1.07; P = .069) in the 2000 to 2004 period compared with those in 1985 to 1990 in patients in the MRD/CR1 and MRD/CR2 groups, respectively. In BM recipients in the URD/CR1 group, the RR of TRM in 2000 to 2004 was 0.66 (95% CI, 0.47 to 0.92; P = .016) compared with 0.35 (95% CI, 0.22 to 0.55; P < .001) in 1990 to 1994. The RRs of TRM for patients who received CSA/MTX were 0.56 (95% CI, 0.38 to 0.81; P = .002), 0.35 (95% CI, 0.17 to 0.71; P = .003), and 0.35 (95% CI, 0.22 to 0.55; P < .001) in the MRD/CR1, MRD/CR2, and URD/CR1 groups in 2000 to 2004. For the URD/CR1 patients who received partially matched grafts, the RR of TRM in 2000 to 2004 was 0.64 (95% CI, 0.36 to 1.12; P = .118). The adjusted RR of TRM after MRD transplantation for selected subgroups is shown in Figure 3.
Fig 3.
Transplantation-related mortality adjusted for patient and disease characteristics from 2000 to 2004 compared with that for 1985 to 1989 (baseline), among selected subgroups of HLA-identical sibling transplantation recipients with acute myeloid leukemia in first complete remission (CR1) and second complete remission (CR2). CSA, cyclosporine; MTX, methotrexate.
Leukemia Relapse, LFS, and OS
In the multivariate analysis, there were no significant differences in RR for relapse over time. Compared with the 1985 to 1989 baseline, the RRs of relapse in 2000 to 2004 were 1.09 (95% CI, 0.85 to 1.4; P = .509) for the MRD/CR1 patients, 1.25 (95% CI, 0.79 to 1.98; P = .0.34) for the MRD/CR2 patients, 1.3 (95% CI, 0.73 to 2.3; P = .38) for the URD/CR1 patients, and 1.21 (95% CI, 0.71 to 2.06; P = .492) for the URD/CR2 patients.
In the multivariate analyses for LFS, after adjustment for changes in patient and disease characteristics over time, RRs of treatment failure in the 2000 to 2004 period (compared with those in 1985 to 1989) were 0.75 (95% CI, 0.63 to 0.91; P < .01) in the MRD/CR1 group and 0.64 (95% CI, 0.45 to 0.90; P = .01) in the MRD/CR2 group. In the URD/CR1 group, the hazards for treatment failure were nonproportional. Adjusted probabilities of LFS at 1 year were 53% (95% CI, 42% to 64%) for the 1990 to 1994 period and 57% (95% CI, 52% to 62%; P < .01) for the 1990 to 1994 and 2000 to 2004 periods. Three-year probabilities of LFS were 46% (95% CI, 35% to 57%) and 45% (95% CI, 40% to 49%; P = .36), respectively. In the URD/CR2 group, the RR for treatment failure in 2000 to 2004 was 0.78 (95% CI, 0.59 to 1.03; P = .077) compared with that for 1990 to 1994. An interaction between transplantation period and age was noted; the RR was significant for the 41 to 50 years age group (RR, 0.46; 95% CI, 0.26 to 0.82; P < .01) but not the other groups (data not shown).
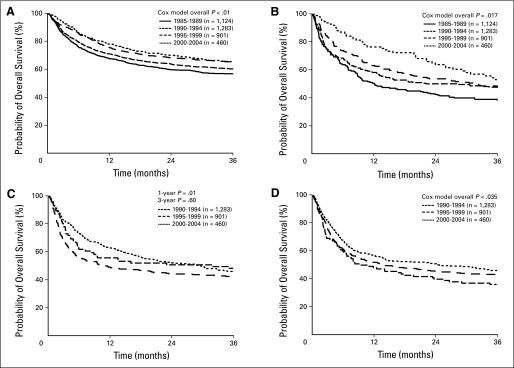
In the multivariate analyses for OS, after adjusting for changes in patient and disease characteristics over time, the RRs for all mortality causes for MRD HCT recipients were 0.73 (95% CI, 0.61 to 0.89; P = .001) for the CR1 group and 0.60 (95% CI, 0.42 to 0.86; P = .005) for the CR2 group in 2000 to 2004 compared with those for 1985 to 1989. In the URD/CR1 group, the hazards for all mortality causes were nonproportional (Fig 4). Adjusted probabilities of OS at 1 year were 56% (95% CI, 45% to 66%) for the 1990 to 1994 period and 63% (95% CI, 58% to 67%; P = .02) for the 2000 to 2004 period. Three-year probabilities of OS were 48% (95% CI, 37% to 59%) and 46% (95% CI, 41% to 51%; P = .47), respectively. In the URD/CR2 group, the RR for all mortality causes was 0.74 (95% CI, 0.56 to 0.97; P = .031) in 2000 to 2004 compared with that for 1990 to 1994. An interaction between year of transplantation and age was detected in the model for this group. The drop in mortality was greatest in patients older than age 40 years (RR, 0.46; 95% CI, 0.26 to 0.82). The impact of center on outcome was assessed and did not significantly influence the results (data not shown).
Fig 4.
Adjusted overall survival by 5-year periods. (A) Recipients of HLA-matched related donors in first complete remission, (B) recipients of matched related donors in second complete remission, (C) recipients of unrelated donors in first complete remission, and (D) recipients of unrelated donors in second complete remission.
DISCUSSION
We observed a decline over time in the unadjusted probability of TRM after allogeneic transplantation using myeloablative conditioning for patients with AML who were younger than age 50 years. Since our primary objective was to estimate the collective impact of changes in transplantation practice on the risk for TRM, we calculated rates that were adjusted for changes in relevant patient and disease characteristics. Reductions in TRM remained significant in three of the four groups (MRD/CR1, MRD/CR2, URD/CR2), suggesting that changes in practice rather than patient characteristics were the primary factors driving the decrease in risk for TRM.
An alternative explanation for the decrease in the incidence of TRM is that improvements in the pretransplantation health of HCT recipients occurred over time, making them less susceptible to complications. Such an improvement could have arisen either through advances in supportive care during chemotherapy or perhaps through more discriminating selection of patients for transplantation. Although such an improvement could have contributed to the reduction in TRM, it is unlikely to be the sole cause. First, the proportions of patients with poor performance status or a comorbid condition in each group either increased over time or remained stable. Second, we adjusted for changes in patient and disease characteristics over time to isolate the effect of changes in practice. Finally, recognizing the potential selection bias that the increase in the use of reduced-intensity conditioning regimens for patients who are marginal candidates for myeloablative conditioning might engender, we chose to study younger patients for whom myeloablative conditioning remains the norm.
An important finding in our study is that for the three groups in which the adjusted risk for TRM decreased over time, there was an accompanying improvement in survival. Although the reduction in TRM and improvement in survival are encouraging, our results also draw attention to the fact that the risk for TRM after allogeneic HCT remains high, especially after URD transplantation.
Since the 1980s, there has been a steady succession of innovations designed to reduce the risk of TRM. More effective cyclosporine-based GVHD prophylaxis was adopted in the 1980s.27 In the 1990s, another calcineurin inhibitor, tacrolimus, was introduced,28 and other innovations occurred, including the introduction of fluconazole prophylaxis to prevent invasive fungal infections,29,30 leukocyte reduction of blood products, new screening assays to prevent CMV disease,16,19 and busulfan pharmacokinetic testing.17 Since 2000 there have been other advances, including the adoption of broader, molecularly defined HLA matching for the selection of URDs.18 In addition, in the last decade, PBPCs have largely supplanted BM for adults undergoing MRD HCT for hematologic malignancies, although its overall impact on TRM has been ambiguous.31,32 A limitation of our study, which relied on data from the CIBMTR, was the inability to directly gauge the impact of these and other individual innovations. We were able to indirectly estimate the effect of a limited set of changes by subgroup analysis and other means and did not identify any specific advance or advances that were primarily responsible for the reduction in TRM.
We believe that our results in AML can be generalized to other diseases in which HCT with myeloablative conditioning is performed since the causes of TRM are largely the same regardless of indication for transplantation. This is substantiated by the results of a large Italian single-center trial that demonstrated reductions in TRM over time in patients with a variety of hematologic malignancies.
Advances that hold the potential to further reduce the risk of TRM in patients undergoing HCT continue to be made. The recent identification of risk factors based on comorbidity and serum levels of biomarkers of inflammation, for example, now permits more careful patient selection.33,34 Ongoing studies may yield further gains. For example, genome-wide testing for genetic susceptibilities to the various causes of TRM is being performed using URD-recipient pair samples and data from the CIBMTR (personal communication, Theresa Hahn, August 2010). Such research may make it possible to minimize TRM by tailoring the transplantation approach to individual patients.
Our results indicate that the risk of leukemic relapse, unlike TRM, has not improved over time. Therefore, continued research toward enhancing the antileukemic effect of HCT is needed.
In conclusion, the risk for TRM in patients receiving myeloablative conditioning and allogeneic transplantation for AML has decreased since the 1980s, and this reduction appears to be primarily attributable to changes in practice.
Appendix
The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement No. U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); Grant/Cooperative Agreement No. 5U01HL069294 from NHLBI and NCI; Contract No. HHSH234200637015C with Health Resources and Services Administration, Department of Health and Human Services; Grants No. N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from the American Association of Blood Banks, Aetna, American Society for Blood and Marrow Transplantation, Amgen, anonymous donation to the Medical College of Wisconsin, Astellas Pharma, Baxter International, Bayer HealthCare Pharmaceuticals, Be the Match Foundation, Biogen Idec, BioMarin Pharmaceutical, Biovitrum AB, BloodCenter of Wisconsin, Blue Cross and Blue Shield Association, Bone Marrow Foundation, Buchanan Family Foundation, Canadian Blood and Marrow Transplant Group, CaridianBCT, Celgene, CellGenix, Centers for Disease Control and Prevention, Children's Leukemia Research Association, ClinImmune Labs, CTI Clinical Trial and Consulting Services, Cubist Pharmaceuticals, Cylex, CytoTherm, DOR BioPharma, Dynal Biotech, Eisai, Enzon Pharmaceuticals, European Group for Blood and Marrow Transplantation, Gamida Cell, GE Health Care, Genentech, Genzymen, Histogenetics, HKS Medical Information Systems, Hospira, Infectious Diseases Society of America, Kiadis Pharma, Kirin Brewery, The Leukemia and Lymphoma Society, Merck, Medical College of Wisconsin, MGI Pharma, Michigan Community Blood Centers, Millennium Pharmaceuticals, Miller Pharmacal Group, Milliman USA, Miltenyi Biotec, National Marrow Donor Program, Nature Publishing Group, New York Blood Center, Novartis Oncology, Oncology Nursing Society, Osiris Therapeutics, Otsuka America Pharmaceutical, Pall Life Sciences, Pfizer, Saladax Biomedical, Schering, Society for Healthcare Epidemiology of America, Soligenix, StemCyte, StemSoft Software, Sysmex America, Therakos, Thermogenesis, Vidacare, Vion Pharmaceuticals, ViraCor Laboratories, ViroPharma, and Wellpoint.
Footnotes
Supported by grants and contracts listed in the Appendix (online only).
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John T. Horan, Brent R. Logan, Andrea A. Bacigalupo, Karen K. Ballen, Matthew H. Carabasi, Hanna Jean Khoury, Mark B. Juckett, Mark R. Litzow, Franklin O. Smith, J. Douglas Rizzo, Marcelo C. Pasquini
Administrative support: Manza-A. Agovi-Johnson, Marcelo C. Pasquini
Provision of study materials or patients: Manza-A. Agovi-Johnson, Hillard M. Lazarus, Christopher N. Bredeson, Vikas Gupta, Mark B. Juckett, Mark R. Litzow, Rodrigo Martino, Philip L. McCarthy
Collection and assembly of data: Manza-A. Agovi-Johnson, J. Douglas Rizzo, Marcelo C. Pasquini
Data analysis and interpretation: John T. Horan, Brent R. Logan, Manza-A. Agovi-Johnson, Hillard M. Lazarus, Karen K. Ballen, Christopher N. Bredeson, Vikas Gupta, Gregory A. Hale, Hanna Jean Khoury, Mark B. Juckett, Mark R. Litzow, Rodrigo Martino, Philip L. McCarthy, Franklin O. Smith, J. Douglas Rizzo, Marcelo C. Pasquini
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhard D, Marks MI, Good RA. Infections in bone marrow transplant recipients. J Pediatr. 1986;108:335–346. doi: 10.1016/s0022-3476(86)80870-8. [DOI] [PubMed] [Google Scholar]
- 3.Upton A, Kirby KA, Carpenter P, et al. Invasive aspergillosis following hematopoietic cell transplantation: Outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 4.Yanik GA, Ho VT, Levine JE, et al. The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:3073–3081. doi: 10.1182/blood-2008-03-143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner RS, Bortin MM, Gale RP, et al. Interstitial pneumonitis after bone marrow transplantation: Assessment of risk factors. Ann Intern Med. 1986;104:168–175. doi: 10.7326/0003-4819-104-2-168. [DOI] [PubMed] [Google Scholar]
- 6.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Gale RP, Bortin MM, van Bekkum DW, et al. Risk factors for acute graft-versus-host disease. Br J Haematol. 1987;67:397–406. doi: 10.1111/j.1365-2141.1987.tb06160.x. [DOI] [PubMed] [Google Scholar]
- 9.Hahn T, McCarthy PL, Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miano M, Labopin M, Hartmann O, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: A survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89–99. doi: 10.1038/sj.bmt.1705550. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Storer BE, Scott BL, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: Similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–1387. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 13.Bunin NJ, Davies SM, Aplenc R, et al. Unrelated donor bone marrow transplantation for children with acute myeloid leukemia beyond first remission or refractory to chemotherapy. J Clin Oncol. 2008;26:4326–4332. doi: 10.1200/JCO.2008.16.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunin N, Carston M, Wall D, et al. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood. 2002;99:3151–3157. doi: 10.1182/blood.v99.9.3151. [DOI] [PubMed] [Google Scholar]
- 15.Ringdén O, Horowitz MM, Sondel P, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993;81:1094–1101. [PubMed] [Google Scholar]
- 16.Einsele H, Ehninger G, Hebart H, et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood. 1995;86:2815–2820. [PubMed] [Google Scholar]
- 17.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 18.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 19.Bowden RA, Slichter SJ, Sayers MH, et al. Use of leukocyte-depleted platelets and cytomegalovirus-seronegative red blood cells for prevention of primary cytomegalovirus infection after marrow transplant. Blood. 1991;78:246–250. [PubMed] [Google Scholar]
- 20.Macias WL, Mueller BA, Scarim SK, et al. Continuous venovenous hemofiltration: An alternative to continuous arteriovenous hemofiltration and hemodiafiltration in acute renal failure. Am J Kidney Dis. 1991;18:451–458. doi: 10.1016/s0272-6386(12)80113-2. [DOI] [PubMed] [Google Scholar]
- 21.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 22.Pasquini M, Wang Z. CIBMTR Summary Slides, CIBMTR Newsletter. 2009:6–11. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Klein J, Moeschberger M. New York, NY: Springer-Verlag; 2003. Survival Analysis: Techniques of Censored and Truncated Data (ed 2) [Google Scholar]
- 25.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 26.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 27.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 28.Nash RA, Piñeiro LA, Storb R, et al. FK506 in combination with methotrexate for the prevention of graft-versus-host disease after marrow transplantation from matched unrelated donors. Blood. 1996;88:3634–3641. [PubMed] [Google Scholar]
- 29.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation: A prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 30.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: Long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–2061. [PubMed] [Google Scholar]
- 31.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 32.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 33.Pavlù J, Kew AK, Taylor-Roberts B, et al. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010;115:4018–4020. doi: 10.1182/blood-2010-01-263624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]