Abstract
Purpose
There is no consensus on the best regimen for the primary treatment of low-risk gestational trophoblastic neoplasia (GTN).
Patients and Methods
Two commonly used single-drug regimens were compared with respect to the proportion of patients meeting the criteria for a complete response (CR) in a randomized phase III trial conducted by the Gynecologic Oncology Group. Eligibility was purposefully broad to maximize the generalizability of the results and included patients with a WHO risk score of 0 to 6 and patients with metastatic disease (limited to lung lesions < 2 cm, adnexa, or vagina) or choriocarcinoma.
Results
Two hundred forty women were enrolled, and 216 were deemed eligible. Biweekly intravenous dactinomycin 1.25 mg/m2 was statistically superior to weekly intramuscular (IM) methotrexate 30 mg/m2 (CR: 70% v 53%; P = .01). Similarly, in patients with low-risk GTN as defined before the 2002 WHO risk score revisions (risk score of 0 to 4 and excluding choriocarcinoma), response was 58% and 73% in the methotrexate and dactinomycin arms, respectively (P = .03). Both regimens were less effective if the WHO risk score was 5 or 6 or if the diagnosis was choriocarcinoma (CR: 9% and 42%, respectively). There were two potential recurrences; one at 4 months (dactinomycin) and one at 22 months (methotrexate). Not all patients completed follow-up. Both regimens were well tolerated.
Conclusion
The biweekly dactinomycin regimen has a higher CR rate than the weekly IM methotrexate regimen in low-risk GTN, a generally curable disease.
INTRODUCTION
Low-risk gestational trophoblastic neoplasia is a highly curable disease. There are many effective single-drug chemotherapy regimens; however, the choice of both the drug and the regimen is highly institution specific. While the most important objective of treatment in these young reproductive-age women is effectiveness, other considerations, such as cost efficiency, ease of administration, toxicity, exposure to multidrug second-line regimens, patient preference, and compliance are also important outcomes.1–3
Both methotrexate and dactinomycin are effective first-line drugs for persistent, low risk trophoblastic disease. However, there are several dosing/cycling options for each drug.2–13 When the current trial was initiated (1999), the most commonly used regimen in North America was low-dose intramuscular (IM) methotrexate as described in a Gynecologic Oncology Group (GOG) study.12 In a follow-on phase II dosing study, the GOG demonstrated that escalating the methotrexate dose to 50 mg/m2 did not significantly alter response when compared with the fixed dose of 30 mg/m2 in the previous study.13 Furthermore, an earlier study of methotrexate 600 mg/m2 also did not demonstrate significant additional benefit over the study dosage.5 Single-agent dactinomycin was initially reported as a 5-day parenteral regimen, but it lost favor because of alopecia and nausea.9 More recently, biweekly single-injection dactinomycin was reported to be as effective a first-line treatment as weekly methotrexate.7,10,14
With a historic preference for methotrexate, a randomized phase III trial was initiated to test the hypothesis that biweekly dactinomycin might be superior or inferior to weekly methotrexate. This study was designed to compare the proportion of complete responders to weekly methotrexate 30 mg/m2 IM and biweekly dactinomycin 1.25 mg/m2 intravenously. The secondary objectives were to compare the toxicity of each regimen and to assess the accuracy of the definition of persistent disease using, as a surrogate measure, the frequency that the beta human chorionic gonadotropin (βhCG) level had spontaneously declined on day 0 (without treatment) compared with the preregistration levels.
PATIENTS AND METHODS
Eligible patients were initially defined by the existing International Federation of Gynecology and Obstetrics (FIGO) criteria for persistence (1982): one or more of a < 10% decrease in three consecutive weekly βhCG values (over 2 weeks); a > 20% rise in the βhCG value over any two consecutive weekly assays; an elevated βhCG level ≥ 4 months following initial uterine curettage; metastatic disease in the vagina, parametrium, or lung (if no single lesion exceeded 2 cm radiologically); and histologically proven nonmetastatic choriocarcinoma. In 2002, this definition and the WHO risk scoring criteria were both modified. Trial eligibility was amended shortly thereafter to include WHO intermediate-risk patients with scores of 5 or 6. However, the revised FIGO definition of persistent disease was not adopted for logistical reasons.15,16 If the risk score was between 0 and 4 (June 1999-June 2002) or 0 and 6 (July 2002-February 2007), the trial was offered to the patient.
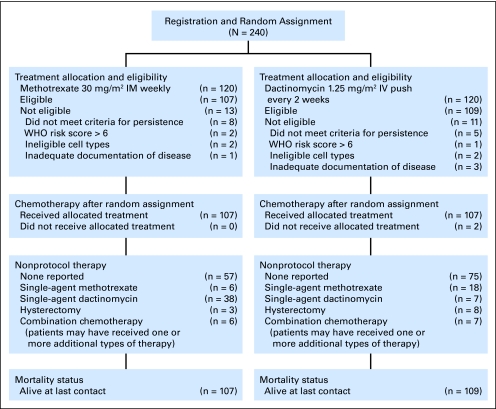
Pretreatment staging of eligible patients included a chest x-ray, pelvic ultrasound, and βhCG assay. Computed tomography (CT) scans of the lung, if performed, were not used to determine the WHO risk score. A quantitative βhCG assay was obtained at registration and again on the first day of treatment (day 0). The uterine curettage material was centrally reviewed on all patients by members of the GOG Pathology Committee. Written informed consent consistent with institutional, state, and federal regulations was obtained before entry onto the study and random assignment. Local institutional review board approval was obtained. Following central random assignment through the GOG Statistical and Data Center, protocol therapy was randomly allocated with equal probability, within the main member institution, between one of the two study regimens as primary treatment: either weekly IM methotrexate 30 mg/m2 or biweekly intravenous dactinomycin 1.25 mg/m2 (Fig 1). Neither the treating physician nor the patient was blinded to the allocated regimen; however, treatment assignments were concealed from the institutions and patients until registration.
Fig 1.
CONSORT diagram. IM, intramuscular; IV, intravenous.
Patients continued on treatment until the βhCG assay had reached the institutional normal, or until either a rise or plateau in the βhCG level was observed. Once normal, the βhCG level was determined biweekly for 1 month, and then monthly for a further 11 months (12-month follow-up). In the event of a rise or plateau, the patient was removed from the study treatment, and the patient's response was classified as a treatment failure. These patients were managed thereafter at the treating physician's discretion, but their subsequent treatments and eventual outcome were noted. Patients who had a complete response (CR) received one additional cycle of their assigned treatment regimen after the first normal βhCG level. Specific patient characteristics were prospectively collected at the centrally located Statistical and Data Center. The study chair reviewed the data to verify the WHO score, to assess protocol compliance, and to review adverse events and response.
Given concerns about patient compliance rather than cure, the proportion of eligible patients who experienced an objective CR, as determined by a normal βhCG sustained over four weekly measurements, was chosen as the primary study end point. A sample size of 216 eligible patients, equally allocated between assigned treatment regimens, was necessary to provide 80% power to detect an absolute difference in the proportion responding of 0.14 when setting the type I error at 0.05 for a two-tail test.17 The response proportion for the methotrexate regimen was assumed to be 0.78.12,13 A single planned interim analysis of response was reported to the Data Monitoring Committee in January 2004 when 131 eligible patients had been evaluated for response. An O'Brien-Fleming error spending function was used to determine stopping rules.18 A χ2 test was used to test the independence of assigned treatment and response. The significance level at the final analysis was set at 0.047 to adjust for the occurrence of a single interim analysis, keeping the cumulative type I error level at 0.05. In January 2007, the statistical trial design was reviewed by the Data Monitoring Committee because of the low overall proportion of patients responding to treatment, and the Committee decided that no action was necessary.
Adverse event data, regardless of attribution, were collected prospectively and graded according to standardized criteria (Common Terminology Criteria for Adverse Events [CTCAE] v2.0). The Kruskal-Wallis test with adjustment for massive ties was used to test the independence of assigned treatment and all reported adverse events among treated patients. Logistic regression was used to assess the individual prognostic effect of covariates on the odds of response.
RESULTS
The trial was opened in June 1999 with a planned accrual of 216 eligible women. Two hundred forty patients were enrolled onto the study over 7.5 years. Accrual was completed in February 2007. Patients were enrolled from the GOG (206 patients), GOG Japan (six patients), the National Cancer Institute of Canada (23 patients), and an Eastern Cooperative Oncology Group (ECOG) affiliate in South Africa (five patients).
Twenty-four patients were later deemed ineligible. Of these, thirteen did not meet the criteria for persistent disease, four did not have a gestational neoplasm after pathology review, four had inadequate documentation of disease, and three were determined to have a WHO risk score > 6 based on end-of-study central data review by the study chair. The records of 216 women were available for analysis.
The distributions of the patient characteristics were similar between the two treatment regimens although there were notable differences in some of the subcategories including age, race, and molar classification (Table 1). There were slightly more patients with choriocarcinoma in the methotrexate arm (seven v three) but there were an equal number of patients with a risk score of 5 or 6 (nine v eight). Eleven percent of the patients were younger than age 20 years and 11% were older than age 39 years. Eighty-one percent of the patients had a complete mole, 13% a partial mole, and 5% had choriocarcinoma. Twelve percent of the patients had a WHO risk score of 0 while 8% had a WHO risk score of 5 or 6. The registration βhCG level was < 10 mIU/mL in 1% of the patients in each arm and > 100,000 mIU/mL in 6% and 4% of patients, respectively (Table 2). Registration pelvic ultrasound was abnormal in 67% and 79% of the patients, and chest x-ray was abnormal in 8% and 11% of patients, respectively. CT scan of the lung was abnormal in 18 women whose chest x-ray was normal (six and 12 patients, respectively; Table 2).
Table 1.
Patient Characteristics
| Characteristic | Methotrexate |
Dactinomycin |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age group, years | ||||
| 10-19 | 14 | 13.1 | 10 | 9.2 |
| 20-29 | 52 | 48.6 | 45 | 41.3 |
| 30-39 | 31 | 29.0 | 41 | 37.6 |
| 40-49 | 7 | 6.5 | 11 | 10.1 |
| 50-59 | 3 | 2.8 | 2 | 1.8 |
| Ethnicity | ||||
| Hispanic or Latino | 12 | 11.2 | 14 | 12.8 |
| Non-Hispanic | 85 | 79.4 | 88 | 80.7 |
| Unknown/not specified | 10 | 9.3 | 7 | 6.4 |
| Race | ||||
| Unknown | 11 | 10.3 | 7 | 6.4 |
| Asian | 9 | 8.4 | 16 | 14.7 |
| American Indian/Alaskan native | 1 | 0.9 | 3 | 2.8 |
| Black/African American | 17 | 15.9 | 16 | 14.7 |
| White | 69 | 64.5 | 67 | 61.5 |
| Molar class | ||||
| Complete mole | 81 | 75.7 | 94 | 86.2 |
| Partial mole | 17 | 15.9 | 12 | 11.0 |
| Choriocarcinoma | 7 | 6.5 | 3 | 2.8 |
| Indeterminate/not specified | 1 | 1.9 | 0 | 0.0 |
| WHO score | ||||
| 0 | 14 | 13.1 | 13 | 11.9 |
| 1 | 40 | 37.4 | 38 | 34.9 |
| 2 | 20 | 18.7 | 30 | 27.5 |
| 3 | 15 | 14.0 | 14 | 12.8 |
| 4 | 10 | 9.3 | 5 | 4.6 |
| 5 | 4 | 3.7 | 5 | 4.6 |
| 6 | 4 | 3.7 | 4 | 3.7 |
Table 2.
Clinical Findings From Staging Tests
| Clinical Test Category | Methotrexate |
Dactinomycin |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Pelvic ultrasound | ||||
| Abnormal | 72 | 67.3 | 86 | 78.9 |
| Normal | 35 | 32.7 | 21 | 19.3 |
| Unknown or negative posthysterectomy | 0 | 0.0 | 2 | 1.8 |
| Chest x-ray | ||||
| Abnormal | 9 | 8.4 | 12 | 11.0 |
| Normal | 92 | 86.0 | 91 | 83.5 |
| Not done or unknown | 6 | 5.6 | 6 | 5.5 |
| Registration βhCG, mIU/mL | ||||
| < 10.1 | 1 | 0.9 | 1 | 0.9 |
| 10.1-100.0 | 5 | 4.7 | 7 | 6.4 |
| 100.1-1,500.0 | 21 | 19.6 | 17 | 15.6 |
| 1,500.1-5,000.0 | 27 | 25.2 | 27 | 24.8 |
| 5,000.1-10,000.0 | 12 | 11.2 | 14 | 12.8 |
| 10,000.1-100,000.0 | 35 | 32.7 | 39 | 35.8 |
| 100,000.1-1,000,000.0 | 6 | 5.6 | 4 | 3.7 |
Abbreviation: βhCG, beta human chorionic gonadotropin.
One hundred thirty-three of the 216 patients met the criteria for a CR to their allocated treatment: 57 of 107 (53%) in the methotrexate arm and 76 of 109 (70%) in the dactinomycin arm. This difference was significant, favoring dactinomycin (P = .013; Table 3).
Table 3.
Response by Allocated Treatment
| Response Category | Methotrexate |
Dactinomycin |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete response | 57 | 53.3 | 76 | 69.7 |
| No response | 48 | 44.9 | 29 | 26.6 |
| Not evaluable for response | 2 | 1.9 | 4 | 3.7 |
| Total | 107 | 100.0 | 109 | 100.0 |
Seventy-seven women were designated as nonresponders: 48 (45%) of 107 in the methotrexate arm and 29 (27%) of 109 in the dactinomycin arm (Table 3). A further six patients were considered not evaluable, two of whom were never treated because their βhCG level normalized spontaneously.
The average number of cycles given to patients with a CR was eight (range, two to 26) with methotrexate and four (range, two to 14) with dactinomycin. Among those in whom treatment failed, the criteria for treatment failure were met later on methotrexate (median, seven treatments; range, one to 22 treatments) than on dactinomycin (median, two treatments; range, zero to 11 treatments).
Five patients on methotrexate and six patients on dactinomycin did achieve a CR with continued protocol therapy (median, three additional cycles) despite, on retrospective central data review, having arithmetically met the strict protocol criteria of nonresponse earlier in their treatment (any set of three consecutive assay results that declined by < 10%). A further 13 patients, 11 on methotrexate and two on dactinomycin, met the criteria for treatment failure but still received further cycles of the assigned treatment because the failure was not immediately recognized (median of three and two additional treatments, respectively). All patients who received additional treatment cycles after meeting the criteria for treatment failure, whether ultimately responding or not, were counted as nonresponders in the outcome analysis.
In a secondary analysis, the outcome for patients with a risk score of 0 to 4 and excluding choriocarcinoma (FIGO definition of low-risk disease before the 2002 amendments) was evaluated. Primary response was achieved in 56 (58%) of 96 eligible patients in the methotrexate arm and in 71 (73%) of 97 patients in the dactinomycin arm. This result was also statistically significant (P = .03).
The βhCG level fell on the first day of treatment relative to the level reported at study entry in 72 of the 178 women tested. The proportion of responding patients with either a fall or rise in βhCG level on either regimen was not significantly different. The level declined in 19 (63%) of 30 patients given methotrexate and in 28 (52%) of 54 patients given dactinomycin. The level rose in 31 (74%) of 42 patients and 33 (63%) of 52 patients, respectively (Table 4).
Table 4.
Complete Response Within Subgroups
| Characteristic | Methotrexate |
Dactinomycin |
||
|---|---|---|---|---|
| No. of Responders/ Total | % | No. of Responders/ Total | % | |
| Age group, years | ||||
| 10-29 | 38/66 | 57.6 | 37/55 | 67.3 |
| 30-39 | 16/31 | 51.6 | 33/41 | 80.5 |
| 40-59 | 3/10 | 30.0 | 6/13 | 46.1 |
| Racial designation | ||||
| White | 40/69 | 58.0 | 50/67 | 74.6 |
| Black | 11/17 | 64.7 | 11/16 | 68.7 |
| All others | 6/21 | 28.6 | 15/26 | 57.7 |
| WHO score | ||||
| 0-1 | 38/54 | 70.4 | 41/51 | 80.4 |
| 2-4 | 18/45 | 40.0 | 31/49 | 63.3 |
| 5-6 | 1/8 | 12.5 | 4/9 | 44.4 |
| Registration βhCG, mIU/mL | ||||
| < 50 | 1/3 | 33.3 | 3/3 | 100.0 |
| 50-99.9 | 2/3 | 66.7 | 3/5 | 60.0 |
| ≥ 100 | 54/101 | 53.5 | 70/101 | 69.3 |
| Registration βhCG, mIU/mL | ||||
| < 5,000 | 37/54 | 68.5 | 42/52 | 80.8 |
| > 5,000 | 20/53 | 37.7 | 34/57 | 59.7 |
| βhCG level change between registration and initiation of treatment | ||||
| Fell | 19/30 | 63.3 | 31/42 | 73.8 |
| Did not fall | 28/54 | 51.8 | 33/52 | 63.5 |
| Registered and treated on the same day | 7/17 | 41.2 | 9/11 | 81.8 |
| Not evaluated on first day of treatment | 3/6 | 50.0 | 3/4 | 75.0 |
| GOG molar classification | ||||
| Partial | 9/17 | 52.9 | 9/12 | 75.0 |
| Complete | 47/81 | 58.0 | 66/94 | 70.2 |
| Choriocarcinoma | 0/7 | 0.0 | 1/3 | 33.3 |
| Indeterminate | 1/2 | 50.0 | 0 | |
| WHO score and histology | ||||
| 0-4 and mole | 56/96 | 58.3 | 71/97 | 73.2 |
| 5-6 or choriocarcinoma | 1/11 | 9.1 | 5/12 | 41.7 |
| Chest CT/chest x-ray results | ||||
| Abnormal/normal | 2/6 | 33.3 | 9/12 | 75.0 |
Abbreviations: βhCG, beta human chorionic gonadotropin; GOG, Gynecologic Oncology Group; CT, computed tomography.
Six women who were given methotrexate and twelve who were given dactinomycin had a normal chest x-ray and a positive CT lung scan at registration. The response rates were two (33%) of six and nine (75%) of 12, respectively, favoring dactinomycin (Table 4). Among patients with a risk score of 5 or 6 or choriocarcinoma, response was observed in only one (9%) of 11 patients receiving methotrexate and five (42%) of 12 receiving dactinomycin (Table 4). In multiple variable logistic regression models, age, race, WHO score, histologic classification, and registration βhCG levels had a statistically significant effect on prognosis as individual covariates.
There were two recurrences: one at 4 months from entry (dactinomycin) and a second at 22 months (methotrexate). The study was not designed to test whether they represented true late recurrences or whether either was the result of a second consecutive molar gestation. Both received the other study drug as initial second-line treatment. The patient who had initially experienced treatment failure with methotrexate subsequently required hysterectomy for cure.
Most patients who did not respond to their allocated therapy subsequently received the other study drug, either as a 1-day or a 5-day regimen. Eleven patients underwent hysterectomy. Six patients given methotrexate and seven patients given dactinomycin were treated with multiagent chemotherapy. Patients were followed longitudinally, and all who completed follow-up were cured.
Two patients did not receive any study therapy and were omitted from the evaluation of adverse events. Overall, grade 3 and 4 adverse events were infrequent. No patient had her allocated treatment discontinued because of toxicity. In general, there was more low-grade GI toxicity among patients receiving dactinomycin compared with those receiving methotrexate (P < .01). Additionally, there was more frequent and more severe (grade 2) nausea (P = .025), grades 1 and 2 vomiting (P < .001), dermatologic events (rash or alopecia; P = .027), and neutropenia (P = .045) associated with dactinomycin compared with methotrexate. Reported alopecia was not significant among patients receiving dactinomycin (27 women with grade 1 dermatologic toxicity, either rash or alopecia; Table 5).
Table 5.
Adverse Events Graded and Categorized According to Common Terminology Criteria for Adverse Events Version 2
| Adverse Effect | Methotrexate |
Dactinomycin |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Total | Grade |
Total | |||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | |||
| Leukopenia | 87 | 17 | 3 | 0 | 0 | 0 | 107 | 85 | 15 | 7 | 0 | 0 | 0 | 107 |
| Neutropenia | 91 | 10 | 6 | 0 | 0 | 0 | 107 | 81 | 6 | 16 | 3 | 1 | 0 | 107 |
| Thrombocytopenia | 103 | 2 | 1 | 1 | 0 | 0 | 107 | 96 | 10 | 1 | 0 | 0 | 0 | 107 |
| Anemia | 70 | 30 | 4 | 3 | 0 | 0 | 107 | 63 | 30 | 11 | 3 | 0 | 0 | 107 |
| Other hematologic | 105 | 2 | 0 | 0 | 0 | 0 | 107 | 103 | 3 | 0 | 0 | 1 | 0 | 107 |
| Allergy | 103 | 2 | 2 | 0 | 0 | 0 | 107 | 106 | 1 | 0 | 0 | 0 | 0 | 107 |
| Auditory | 106 | 0 | 1 | 0 | 0 | 0 | 107 | 106 | 0 | 1 | 0 | 0 | 0 | 107 |
| Cardiovascular | 106 | 1 | 0 | 0 | 0 | 0 | 107 | 104 | 1 | 2 | 0 | 0 | 0 | 107 |
| Coagulation | 106 | 0 | 1 | 0 | 0 | 0 | 107 | 107 | 0 | 0 | 0 | 0 | 0 | 107 |
| Constitutional | 49 | 46 | 10 | 2 | 0 | 0 | 107 | 49 | 43 | 14 | 1 | 0 | 0 | 107 |
| Dermatologic | 93 | 13 | 1 | 0 | 0 | 0 | 107 | 80 | 27 | 0 | 0 | 0 | 0 | 107 |
| Endocrine | 105 | 2 | 0 | 0 | 0 | 0 | 107 | 105 | 0 | 2 | 0 | 0 | 0 | 107 |
| Nausea | 57 | 42 | 8 | 0 | 0 | 0 | 107 | 45 | 41 | 19 | 2 | 0 | 0 | 107 |
| Vomiting | 93 | 11 | 3 | 0 | 0 | 0 | 107 | 72 | 21 | 12 | 2 | 0 | 0 | 107 |
| Diarrhea | 91 | 14 | 2 | 0 | 0 | 0 | 107 | 99 | 7 | 1 | 0 | 0 | 0 | 107 |
| Stomatitis | 98 | 4 | 5 | 0 | 0 | 0 | 107 | 97 | 9 | 1 | 0 | 0 | 0 | 107 |
| Other GI | 75 | 25 | 7 | 0 | 0 | 0 | 107 | 55 | 38 | 12 | 2 | 0 | 0 | 107 |
| Genitourinary/renal | 98 | 6 | 3 | 0 | 0 | 0 | 107 | 99 | 8 | 0 | 0 | 0 | 0 | 107 |
| Hemorrhage | 82 | 16 | 6 | 3 | 0 | 0 | 107 | 78 | 16 | 10 | 3 | 0 | 0 | 107 |
| Hepatic | 97 | 8 | 1 | 1 | 0 | 0 | 107 | 100 | 4 | 2 | 1 | 0 | 0 | 107 |
| Infection/fever | 96 | 4 | 7 | 0 | 0 | 0 | 107 | 100 | 3 | 3 | 1 | 0 | 0 | 107 |
| Lymphatic | 106 | 1 | 0 | 0 | 0 | 0 | 107 | 106 | 1 | 0 | 0 | 0 | 0 | 107 |
| Metabolic | 99 | 8 | 0 | 0 | 0 | 0 | 107 | 94 | 11 | 1 | 1 | 0 | 0 | 107 |
| Musculoskeletal | 102 | 4 | 1 | 0 | 0 | 0 | 107 | 103 | 4 | 0 | 0 | 0 | 0 | 107 |
| Neurologic | 77 | 24 | 5 | 1 | 0 | 0 | 107 | 87 | 15 | 4 | 1 | 0 | 0 | 107 |
| Ocular/visual | 91 | 11 | 4 | 1 | 0 | 0 | 107 | 102 | 1 | 4 | 0 | 0 | 0 | 107 |
| Pain | 61 | 33 | 8 | 5 | 0 | 0 | 107 | 70 | 23 | 10 | 4 | 0 | 0 | 107 |
| Pulmonary | 99 | 8 | 0 | 0 | 0 | 0 | 107 | 95 | 4 | 6 | 2 | 0 | 0 | 107 |
| Sexual | 102 | 4 | 1 | 0 | 0 | 0 | 107 | 104 | 2 | 1 | 0 | 0 | 0 | 107 |
| Second primary | 107 | 0 | 0 | 0 | 0 | 0 | 107 | 107 | 0 | 0 | 0 | 0 | 0 | 107 |
NOTE. Table summarizes the combined adverse events for patients considered evaluable for toxicity. Two patients who did not receive any study therapy are omitted from this tabulation.
DISCUSSION
In this study, the effectiveness was 70% for biweekly dactinomycin and 53% for weekly methotrexate (73% and 58%, respectively, with a WHO risk score of 0 to 4 and excluding choriocarcinoma). This phase III randomized trial provides a more valid and generalizable estimate of the true difference between the regimens than was available from the previous nonrandomized case series and phase II studies (Table 6).7,9,12–14
Table 6.
Chemotherapy Regimens for Low-Risk Gestational Trophoblastic Neoplasia
| Chemotherapy Regimen | Primary Remission Rate (%) |
Reference | |
|---|---|---|---|
| Current Study | Literature | ||
| Methotrexate 0.4 mg/kg/d (max 25 mg/kg/d) IV or IM for 5 days; repeat every 2 weeks | — | 60-89 | Lurain et al,2 Roberts et al,3 Soper et al4 |
| Methotrexate 30-50 mg/m2 IM weekly | 53 | 74-81 | Homesley et al,12 Homesley et al13 |
| Methotrexate 1 mg/kg IM days 1, 3, 5, 7; folinic acid 0.1 mg/kg IM days 2, 4, 6, 8; repeat every 15-18 days | — | 73-84 | Elit et al,5 Smith et al11 |
| Dactinomycin 1.25 mg/m2 IV every 2 weeks | 70 | 94 | Petrilli et al7 |
Abbreviations: IV, intravenously; IM, intramuscularly.
The βhCG levels normalized in both the dactinomycin and methotrexate patients in the same time (8 weeks), but the patients who received dactinomycin required one half the number of treatment cycles. Treatment failures were clinically apparent significantly later in the methotrexate than dactinomycin patients (median of seven v two treatments) resulting in earlier discontinuation of ineffective therapy (by 3 weeks) for patients on dactinomycin.
The WHO risk score was zero in 12% of the study patients, which is similar to the most recent review of the Charing Cross data on low-risk disease (6%).19 In an interim review of the accuracy of risk scoring in this trial, the WHO score was found to have been incorrectly calculated by the registering center in 32% (68 of 215) of the patients.20 The most common error was incorrect scoring of the βhCG level (scientific notation error).
Both drugs were less effective when the risk score was 5 or 6 or in patients with choriocarcinoma (response rate, 9% and 42%). There were zero of seven and one of three responses, respectively, when the pathologic diagnosis was choriocarcinoma. At a dose of 30 mg/m2, methotrexate appears ineffective in both scenarios.
Among nonresponding patients, five women who received methotrexate and six who received dactinomycin were continued on their allocated regimen despite experiencing (arithmetic) treatment failure. These 11 patients all achieved a CR. These arithmetic failures were only identified retroactively because the magnitude of the failure was numerically small, suggesting that the FIGO 2000 criteria for response may be too rigid and difficult to apply with precision. If these 11 women were designated as responders, and if the pre-FIGO 2000 criteria for persistence were also used, the response rates would have been 63% and 79%, respectively.
The failure rate for patients whose βhCG level declined on day 0 was similar to those whose assay level rose as expected, suggesting that a decline in the assay on day 0 should not exclude the diagnosis of persistent disease.14,21 Twenty-seven women (13 on the dactinomycin arm and 14 on the methotrexate arm) in this study had a WHO score of 0 and would not be treated in some studies or institutions. Treating these women with their excellent prognosis may inflate the CR rate for both arms of this study.
If the chest x-ray was normal, micrometastatic pulmonary disease (positive CT lung scan) did not have a significant adverse effect on outcome on either regimen: two (33%) of six patients receiving methotrexate and nine (75%) of 12 patients receiving dactinomycin. CT scanning of the lung appears to offer no additional therapeutic advantage but does add additional cost and time to patient management.22
Adverse events were assessed prospectively and were relatively modest on either regimen. No patient in the current study had to have the allocated treatment terminated because of drug-related toxicity. In comparison, the commonly used 5-day and 8-day methotrexate regimens have reported toxicity sufficient to require a change in treatment in up to 10% of treated patients.2–4,23–25 There was an observed increase in grades 1 and 2 GI adverse effects in patients receiving dactinomycin and higher grade 1 neurologic sequelae and grade 2 stomatitis in patients receiving methotrexate. Alopecia was coded under dermatologic events and was not significant on either regimen; however, alopecia is often under-reported in cancer clinical trials.
While biweekly dactinomycin demonstrated a superior CR rate in this comparison to weekly methotrexate 30 mg/m2, several other methotrexate regimens have been in long-term use and have reported comparable response rates and toxicity. These studies have used a range of doses up to 1,000 mg and cycles up to 8 days long. These studies, none of which were randomized, often excluded choriocarcinoma and patients with pulmonary metastases. In addition, none used and so strictly applied the FIGO 2002 definitions of failure, as was the case in this trial.2,4–6,10,23–25
In conclusion, this cooperative group phase III study of low-risk gestational trophoblastic neoplasia, demonstrated a higher CR rate for the biweekly dactinomycin regimen than for the weekly IM methotrexate regimen. It has also demonstrated that methotrexate at this dose and schedule is not an effective drug for patients with a WHO risk score > 4 or with choriocarcinoma. Because dactinomycin is relatively easy to administer with an every 2-week treatment schedule and has a low toxicity profile, a phase III trial comparing this regimen with the other commonly used multiday methotrexate regimens is warranted.
Acknowledgment
We acknowledge Bette Stonebraker for her thorough and adept coordination of the clinical data.
Appendix
The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Mississippi Medical Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, State University of New York Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Cooper Hospital/University Medical Center, Columbus Cancer Council, M. D. Anderson Cancer Center, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, National Cancer Institute of Canada, University of Chicago, Eastern Collaborative Oncology Group, Case Western Reserve University, Gynecologic Oncology Group Japan-Saitama Medical University International Medical Center, University of Wisconsin Hospital, Odette Cancer Centre, University of Toronto, and Community Clinical Oncology Program.
Footnotes
See accompanying editorial on page 786
Supported by Grants No. CA 27469 from the National Cancer Institute to the Gynecologic Oncology Group Administrative Office and CA 37517 to the Gynecologic Oncology Group Statistical and Data Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003702.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Raymond J. Osborne, Virginia Filiaci, Julian C. Schink, David Scott Miller, Allan L. Covens
Administrative support: Julian C. Schink, David Scott Miller
Provision of study materials or patients: Raymond J. Osborne, Julian C. Schink, Angeles Alvarez Secord, Joseph L. Kelley, David Scott Miller, Allan L. Covens
Collection and assembly of data: Raymond J. Osborne, Virginia Filiaci, Julian C. Schink, Joseph L. Kelley, Janice M. Lage
Data analysis and interpretation: Raymond J. Osborne, Virginia Filiaci, Julian C. Schink, David Scott Miller, Allan L. Covens, Janice M. Lage
Manuscript writing: Raymond J. Osborne, Virginia Filiaci, Julian C. Schink, Robert S. Mannel, Angeles Alvarez Secord, Joseph L. Kelley, Diane Provencher, David Scott Miller, Allan L. Covens, Janice M. Lage
Final approval of manuscript: Raymond J. Osborne, Virginia Filiaci, Julian C. Schink, Robert S. Mannel, Angeles Alvarez Secord, Joseph L. Kelley, Diane Provencher, David Scott Miller, Allan L. Covens, Janice M. Lage
REFERENCES
- 1.Rustin GJ, Newlands ES, Lutz JM, et al. Combination but not single-agent methotrexate chemotherapy for gestational trophoblastic tumors increases the incidence of second tumors. J Clin Oncol. 1996;14:2769–2773. doi: 10.1200/JCO.1996.14.10.2769. [DOI] [PubMed] [Google Scholar]
- 2.Lurain JR, Elfstrand EP. Single-agent methotrexate chemotherapy for the treatment of nonmetastatic trophoblastic tumors. Am J Obstet Gynecol. 1995;172:574–579. doi: 10.1016/0002-9378(95)90575-8. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JP, Lurain JR. Treatment of low-risk metastatic gestational trophoblastic tumors with single-agent chemotherapy. Am J Obstet Gynecol. 1996;174:1917–1923. doi: 10.1016/s0002-9378(96)70229-6. discussion 1923–1924. [DOI] [PubMed] [Google Scholar]
- 4.Soper JT, Clarke-Pearson DL, Berchuck A, et al. 5-day methotrexate for women with metastatic gestational trophoblastic disease. Gynecol Oncol. 1994;54:76–79. doi: 10.1006/gyno.1994.1169. [DOI] [PubMed] [Google Scholar]
- 5.Elit L, Covens A, Osborne R, et al. High-dose methotrexate for gestational trophoblastic disease. Gynecol Oncol. 1994;54:282–287. doi: 10.1006/gyno.1994.1211. [DOI] [PubMed] [Google Scholar]
- 6.Wong LC, Ngan HY, Cheng DK, et al. Methotrexate infusion in low-risk gestational trophoblastic disease. Am J Obstet Gynecol. 2000;183:1579–1582. doi: 10.1067/mob.2000.108077. [DOI] [PubMed] [Google Scholar]
- 7.Petrilli ES, Twiggs LB, Blessing JA, et al. Single-dose actinomycin-D treatment for nonmetastatic gestational trophoblastic disease: A prospective phase II trial of the Gynecologic Oncology Group. Cancer. 1987;60:2173–2176. doi: 10.1002/1097-0142(19871101)60:9<2173::aid-cncr2820600910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Kohorn EI. Is lack of response to single-agent chemotherapy in gestational trophoblastic disease associated with dose scheduling or chemotherapy resistance? Gynecol Oncol. 2002;85:36–39. doi: 10.1006/gyno.2001.6533. [DOI] [PubMed] [Google Scholar]
- 9.Osathanondh R, Goldstein DP, Pastorfide GB. Actinomycin D as the primary agent for gestational trophoblastic disease. Cancer. 1975;36:863–866. doi: 10.1002/1097-0142(197509)36:3<863::aid-cncr2820360306>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Twiggs LB. Pulse actinomycin D scheduling in non-metastatic gestational trophoblastic disease. Gynecol Oncol. 1983;16:190–195. doi: 10.1016/0090-8258(83)90093-8. [DOI] [PubMed] [Google Scholar]
- 11.Smith EB, Weed JC, Jr, Tyrey L, et al. Treatment of nonmetastatic gestational trophoblastic disease: Results of methotrexate alone versus methotrexate-folinic acid. Am J Obstet Gynecol. 1982;144:88–92. doi: 10.1016/0002-9378(82)90400-8. [DOI] [PubMed] [Google Scholar]
- 12.Homesley HD, Blessing JA, Rettenmaier M, et al. Weekly intramuscular methotrexate for nonmetastatic gestational trophoblastic disease. Obstet Gynecol. 1988;72:413–418. [PubMed] [Google Scholar]
- 13.Homesley HD, Blessing JA, Schlaerth J, et al. Rapid escalation of weekly intramuscular methotrexate for nonmetastatic gestational trophoblastic disease: A Gynecologic Oncology Group study. Gynecol Oncol. 1990;39:305–308. doi: 10.1016/0090-8258(90)90257-l. [DOI] [PubMed] [Google Scholar]
- 14.Schlaerth JB, Morrow CP, Nalick RH, et al. Single-dose actinomycin D in the treatment of postmolar trophoblastic disease. Gynecol Oncol. 1984;19:53–56. doi: 10.1016/0090-8258(84)90157-4. [DOI] [PubMed] [Google Scholar]
- 15.Kohorn EI. Negotiating a staging and risk factor scoring system for gestational trophoblastic neoplasia: A progress report. J Reprod Med. 2002;47:445–450. [PubMed] [Google Scholar]
- 16.Hancock B, Okines A. An evaluation of FIGO 2000: The first 5 years. Proceedings of the XIV World Congress of the ISSTD; November 2007; Fukuoka, Japan. [Google Scholar]
- 17.Casagrande JT, Pike MC, Smith PG. The power function of the “exact” test for comparing two binomial distributions. J R Stat Soc C. 1978;27:176–180. [Google Scholar]
- 18.DeMets DL, Lan G. The alpha spending function approach to interim data analyses. Cancer Treat Res. 1995;75:1–27. doi: 10.1007/978-1-4615-2009-2_1. [DOI] [PubMed] [Google Scholar]
- 19.McGrath S, Savage P, Seckl M. Management and outcome of women with post hydatidiform mole and low risk GTN with hCG levels in excess of 100,000 IU/L. Proceedings of the XV World Congress on GTD; November 2009; Cochin, India. [Google Scholar]
- 20.Osborne RJ, Filiaci V, Schink J, et al. Systematic risk score assignment errors in patients with gestational trophoblastic neoplasia. Proceedings of the XIII World Congress of the ISSTD; November 2005; Hong Kong. [Google Scholar]
- 21.Kohorn EI. Gestational trophoblastic neoplasia and evidence-based medicine. J Reprod Med. 2002;47:427–432. [PubMed] [Google Scholar]
- 22.Elit L, Gafni A, Levine MN. Better treatments that cost more: The dilemma. Gynecol Oncol. 2000;78:1–2. doi: 10.1006/gyno.2000.5885. [DOI] [PubMed] [Google Scholar]
- 23.Bagshawe KD, Dent J, Newlands ES, et al. The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumours (GTT) Br J Obstet Gynaecol. 1989;96:795–799. doi: 10.1111/j.1471-0528.1989.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 24.McNeish IA, Strickland S, Holden L, et al. Low-risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2002. J Clin Oncol. 2002;20:1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 25.Chapman-Davis E, Lurain J. Management of low-risk GTN. Proceedings of the XV World Congress on GTD; November 2009; Cochin, India. [Google Scholar]