Abstract
Purpose
Approximately 35% of HER2-amplified breast cancers have coamplification of the topoisomerase II-alpha (TOP2A) gene encoding an enzyme that is a major target of anthracyclines. This study was designed to evaluate whether TOP2A gene alterations may predict incremental responsiveness to anthracyclines in some breast cancers.
Methods
A total of 4,943 breast cancers were analyzed for alterations in TOP2A and HER2. Primary tumor tissues from patients with metastatic breast cancer treated in a trial of chemotherapy plus/minus trastuzumab were studied for amplification/deletion of TOP2A and HER2 as a test set followed by evaluation of malignancies from two separate, large trials for changes in these same genes as a validation set. Association between these alterations and clinical outcomes was determined.
Results
Test set cases containing HER2 amplification treated with doxorubicin and cyclophosphamide (AC) plus trastuzumab, demonstrated longer progression-free survival compared to those treated with AC alone (P = .0002). However, patients treated with AC alone whose tumors contain HER2/TOP2A coamplification experienced a similar improvement in survival (P = .004). Conversely, for patients treated with paclitaxel, HER2/TOP2A coamplification was not associated with improved outcomes. These observations were confirmed in a larger validation set, where HER2/TOP2A coamplification was again associated with longer survival when only anthracycline-containing chemotherapy was used for treatment compared with outcome in HER2-positive cancers lacking TOP2A coamplification.
Conclusion
In a study involving nearly 5,000 breast malignancies, both test set and validation set demonstrate that TOP2A coamplification, not HER2 amplification, is the clinically useful predictive marker of an incremental response to anthracycline-based chemotherapy. Absence of HER2/TOP2A coamplification may indicate a more restricted efficacy advantage for breast cancers than previously thought.
INTRODUCTION
Anthracycline-based chemotherapy is the mainstay of current adjuvant treatments for early-stage breast cancer. This is supported by a meta-analysis of several randomized studies showing slightly higher (approximately 4%) disease-free survival (DFS) and overall survival (OS) rates achieved with anthracycline-based versus nonanthracycline chemotherapies.1 However, anthracyclines have significant long-term toxicities including cardiac dysfunction and/or induction of myelodysplasia and acute leukemias.2–4 Several studies have reported an association between HER2 amplification/overexpression and increased responsiveness to anthracycline-based chemotherapy5–9; however, underlying biologic mechanism(s) are unclear. Indeed, in vitro and in vivo studies indicate that HER2 overexpression alone does not alter anthracycline sensitivity.10 HER2 is located on the long arm of chromosome 17 (17q11.2-12) in close proximity to topoisomerase II-α (TOP2A) at 17q21-22. Although HER2 is considered the target of the amplification event, HER2 amplicon size is variable and contains other genes11–14 occasionally including TOP2A.12,15–17 Because TOP2A is a target of anthracyclines, it is possible that this gene, not HER2, is the link between HER2-positive disease and anthracycline responsiveness.18 The objectives of this study were three-fold: determine the nature and frequency of TOP2A copy-number alterations in clinically annotated breast cancers using molecularly validated cutoffs; determine how often these alterations are found in both HER2-positive and -negative breast cancers; and evaluate any association between such alterations and response to anthracycline-based chemotherapy. We addressed these questions using a retrospective evaluation of 4,943 breast cancers. The first group was a hypothesis generating test set of 339 cancers from women enrolled in a trial of HER2-positive metastatic disease in which patients were treated with anthracycline-based or nonanthracycline chemotherapy plus/minus trastuzumab. Clinical response data was then correlated with the presence or absence of HER2 and TOP2A alterations. To validate any observed associations from the test set, we next evaluated 4,604 samples from two larger studies, Breast Cancer International Research Group (BCIRG) -006 (2,990 patients) and BCIRG-005 (1,614 patients). This validation set was used to define the frequency of TOP2A copy-number changes in HER2-amplifed and HER2-normal patients and determine whether TOP2A or HER2 alterations were correlated with anthracycline response.
METHODS
Patients
Test set patients (Figs 1, 2) consisted of patients enrolled in the original randomized phase III trastuzumab registration study (H0648g) designed to evaluate chemotherapy plus/minus trastuzumab in patients with HER2-positive metastatic breast cancer.19 Validation set patients consisted of participants in the BCIRG-005 and BCIRG-006 adjuvant breast cancer trials which accrued 3,298 and 3,222 patients, respectively, between August 2000 and March 2004. BCIRG-005 evaluated combination versus sequential chemotherapy in HER2-normal, node-positive, early-stage breast cancers20 and BCIRG-006 studied node-positive and high-risk, node-negative, HER2-amplified breast cancer21,22 comparing two different adjuvant trastuzumab/chemotherapy regimens (one with and one without anthracyclines) to anthracycline-based chemotherapy alone. Details of patient tissue samples and clinical study designs are described separately (Appendix, online only).
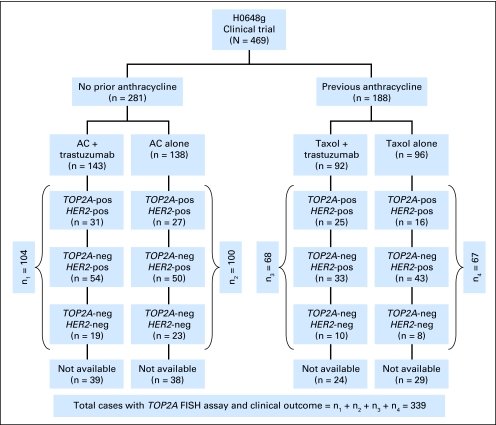
Fig 1.
Specimen accountability in the H0648 test set clinical trial. This schematic diagram summarizes the number of women entered in each treatment arm of the H0648g pivotal clinical trial and the breast cancer specimens analyzed by fluorescent in situ hybridization (FISH) in each treatment arm. A, anthracycline (doxorubicin or epirubicin); C, cyclophosphamide; HER2-pos, HER2 gene amplification; HER2- neg, lacking HER2 gene amplification; TOP2A-pos, TOP2A gene amplification; TOP2A-neg, lacking TOP2A gene amplification including both TOP2A normals and TOP2A gene deletions.
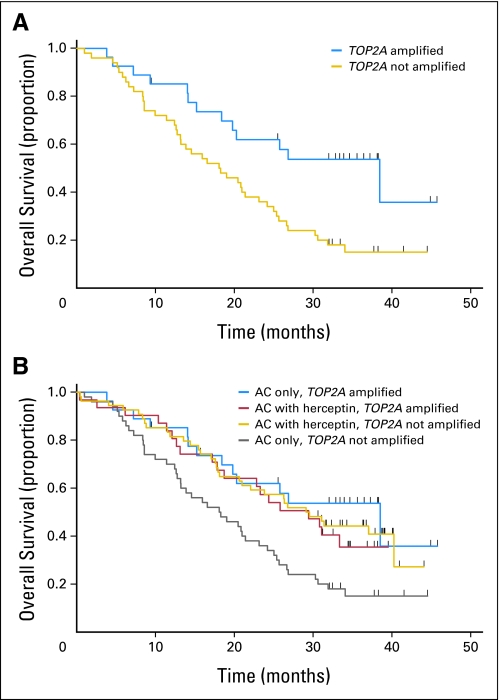
Fig 2.
Overall survival of women with HER2-gene amplified metastatic breast cancer treated in the test set H0648 clinical trial with anthracycline-containing chemotherapy. (A) Women treated with doxorubicin and cyclophosphamide (AC) alone (n = 77) comparing those with TOP2A-amplified tumors (TOP2A fluorescent in situ hybridization [FISH] ratio ≥ 2.00; n = 27) with those whose tumors are not TOP2A amplified (TOP2A FISH ratio < 2.00; n = 50; log-rank test P = .004). (B) Overall survival of women with HER2-amplified metastatic breast cancer treated with AC alone chemotherapy compared with AC plus trastuzumab by TOP2A status. Three of the subsets have similar overall survival (women whose cancers were HER2/TOP2A coamplified and were treated with AC alone; women whose cancers were HER2/TOP2A coamplified and were treated with AC plus trastuzumab; women whose tumors were HER2 amplified but not TOP2A amplified and were treated with AC plus trastuzumab), which was significantly longer than the subset of women whose cancers were HER2 amplified but not TOP2A amplified and were treated with AC alone.
Tissue Analyses: Validation of Probes and Cutoffs Used to Determine HER2/TOP2 Amplification/Deletion
TOP2A and HER2 amplification/deletion status was determined by fluorescent in situ hybridization (FISH) using commercial probes (Abbott-Vysis, Inc; Downers Grove, IL). 22,23 Analysis of both genes was performed simultaneously using SpectrumGreen-labeled HER2 and SpectrumOrange-labeled TOP2A, respectively. Chromosome-17 centromere numbers were determined using a SpectrumAqua-labeled chromosome enumeration probe (CEP17). TOP2A and HER2 copy numbers were determined in a minimum of 20 interphase, nonoverlapping, tumor cell nuclei and compared with chromosome 17 centromeres in those same nuclei. To insure that probes and cutoffs used to generate the amplification/deletion status of TOP2A and HER2 in the test and validation sets were correct, the status of these two genes was first determined in a molecularly characterized panel of known material using amplicon mapping techniques (Appendix).
HER2 amplification was defined as a HER2 gene-to-CEP17 ratio ≥ 2.0, which is the US Food and Drug Administration–approved ratio, rather than the American Society of Clinical Oncology-College of American Pathologists guideline ratio for reasons published elsewhere.24 The identical ratio was used to define TOP2A gene amplification25,26 since both genes are part of the same amplification event. All specimens from the three trials were retested by FISH for this study, blinded to the original results and categorized as either amplified/deleted or nonamplified for TOP2A and HER2. Concordance between original and current FISH results for HER2 status in H0648g test set patients was 97%.23 HER2 status was also repeated for the validation set to determine if comparable FISH results were seen in a tissue microarray format resulting in a 99.8% and 99.6% concordance between the original and current analyses for BCIRG-005 and BCIRG-006, respectively.22
Statistical Methods
Clinical outcomes (defined as overall response rates, progression-free survival [PFS], and OS in H0648 and DFS and OS in BCIRG-005 and BCIRG-006) were compared in TOP2A-amplified and nonamplified subgroups using χ2, Mantel-Haenszel, and log-rank tests (Appendix).27,28 In addition, in trial BCIRG-006, used as the validation set for the effect of TOP2A amplification in the adjuvant setting, a Cox regression model was fitted with an indicator for TOP2A amplification, an indicator for randomized treatment, and an interaction term between these two indicators.
RESULTS
Association Between Clinical Outcomes and TOP2A Alterations in Test Set Patients
Of 469 participants in the HER2-positive metastatic study H0648g,19 339 specimens (72%) were available for this analysis. Clinical characteristics did not differ between the original study population and current test set patients (Table 1). FISH analyses confirmed that 279 (82%) of 339 were HER2 amplified, while 60 (18%) of 339 were not (Table 1). Using molecularly validated cutoff ratios for TOP2A (Appendix), a total of 99 (29.2%) of 339 test set patients showed TOP2A coamplification, while 47 (13.9%) of 339 had TOP2A deletion (Table 2). The remaining 193 (56.9%) of 339 did not contain TOP2A alterations (Table 2). Of note, TOP2A amplification was not detected in any HER2-normal cancers (Table 2) while two (3%) of 60 had evidence of TOP2A deletion.
Table 1.
Comparison of Characteristics for Women in H0648g Test Set Clinical Trial Whose Breast Cancers Were Analyzed for TOP2A Gene Amplification
| Characteristic | All Women in H0648g Clinical Trial19 (n = 469) |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| With Analysis of TOP2A Gene Amplification(n = 339) |
Not Analyzed for TOP2A Gene Amplification(n = 130) |
|||||||
| Yes |
No |
|||||||
| No. | % | No. | % | No. | % | No. | % | |
| HER2 gene amplification by FISH | 279 of 339 | 82 | 60 of 339 | 18 | Not applicable | |||
| Yes | 344 of 451 | 76 | ||||||
| No | 107 of 451 | 24 | ||||||
| Mean age, years | 52 | 52 | 53 | 53 | ||||
| SD | 10.7 | 10.4 | 11.2 | 11.1 | ||||
| Range | 25-77 | 25-77 | 27-76 | 26-73 | ||||
| Karnofsky score | ||||||||
| 90-100 | 309 of 457 | 67.6 | 187 of 271 | 69.0 | 39 of 57 | 68.4 | 83 of 129 | 64.3 |
| < 90 | 148 of 457 | 32.4 | 84 of 271 | 31.0 | 18 of 57 | 31.6 | 46 of 129 | 35.7 |
| Median No. of involved nodes at diagnosis | 2 | 2 | 3 | 2 | ||||
| Range | 0-42 | 0-30 | 0-32 | 0-42 | ||||
| Missing | 67 | 39 | 11 | 17 | ||||
| Prior therapy | ||||||||
| Chemotherapy* | 314 of 464 | 67.7 | 193 of 276 | 69.9 | 33 of 58 | 56.9 | 88 of 130 | 67.7 |
| Hormone† | 265 of 461 | 57.5 | 153 of 275 | 55.6 | 35 of 57 | 61.4 | 77 of 129 | 59.7 |
| Radiation† | 279 of 463 | 60.3 | 170 of 276 | 61.6 | 32 of 58 | 55.2 | 77 of 129 | 59.7 |
| Metastatic sites at enrollment | ||||||||
| ≤ 1 | 154 of 465 | 33.1 | 98 of 277 | 35.4 | 19 of 58 | 32.8 | 37 of 130 | 28.5 |
| 2 | 154 of 465 | 33.1 | 93 of 277 | 33.6 | 20 of 58 | 34.5 | 41 of 130 | 31.5 |
| ≥ 3 | 157 of 465 | 33.8 | 86 of 277 | 31.0 | 19 of 58 | 32.8 | 52 of 130 | 40.0 |
| Median disease-free interval, months | 22.2 | 21.1 | 26.6 | 22.0 | ||||
| Range | 0-225 | 0-225 | 0-152 | 0-223 | ||||
| Missing | 5 | 2 | 2 | 1 | ||||
| HER2 status (by IHC) | ||||||||
| 2+ | 120 of 469 | 25.6 | 30 of 279 | 10.8 | 49 of 60 | 81.7 | 41 of 130 | 31.5 |
| 3+ | 349 of 469 | 74.4 | 249 of 279 | 89.2 | 11 of 60 | 18.3 | 89 of 130 | 68.5 |
Abbreviations: FISH, fluorescent in situ hybridization; SD, standard deviation; IHC, immunohistochemistry.
Adjuvant chemotherapy only.
As adjuvant, for metastasis or both.
Table 2.
HER2 and TOP2A Gene Amplification in the Test Set Clinical Trial by Fluorescent In Situ Hybridization Assay and Received Chemotherapy
| Patients |
HER2 Gene |
|||||
|---|---|---|---|---|---|---|
| Not Amplified |
Amplified |
Totals |
||||
| No. | % | No. | % | No. | % | |
| All patients with TOP2A results | ||||||
| TOP2A deleted | 2 | 3 | 45 | 16 | 47 | 14 |
| TOP2A normal | 58 | 97 | 135 | 48 | 193 | 57 |
| TOP2A amplified | 0 | 0 | 99 | 36 | 99 | 29 |
| Total | 60 | 100 | 279 | 100 | 339 | 100 |
| Patients with TOP2A results receiving AC regimens | ||||||
| TOP2A deleted | 1 | 2 | 27 | 17 | 28 | 14 |
| TOP2A normal | 41 | 98 | 77 | 47 | 118 | 58 |
| TOP2A amplified | 0 | 0 | 58 | 36 | 58 | 28 |
| Total | 42 | 100 | 162 | 100 | 204 | 100 |
| Patients with TOP2A results receiving paclitaxel regimens | ||||||
| TOP2A deleted | 1 | 6 | 18 | 15 | 19 | 14 |
| TOP2A normal | 17 | 94 | 58 | 50 | 75 | 56 |
| TOP2A amplified | 0 | 0 | 41 | 35 | 41 | 30 |
| Total | 18 | 100 | 117 | 100 | 135 | 100 |
NOTE. The H0648g pivotal clinical trial of trastuzumab in metastatic breast cancer was sponsored by Genentech.
Abbreviation: AC, doxorubicin and cyclophosphamide.
Clinical outcomes from 204 patients treated with the doxorubicin and cyclophosphamide (AC) regimen and 135 treated with the paclitaxel regimen (each alone or in combination with trastuzumab) were reviewed. A total of 281 (60%) of 469 patients in the original trial were randomly assigned to AC therapy of which 143 received trastuzumab while 138 did not (Fig 1). TOP2A results were available for 204 (73%) of these malignancies. Among 162 patients treated with anthracycline-based therapy, either alone or in combination with trastuzumab, those with HER2/TOP2A coamplification showed trends toward longer median PFS (7.6 v 6.7 months; P = .064) and OS (30.8 v 21.7 months; P = .069) compared to those without coamplification (Appendix Tables A2, A3, online only). Trastuzumab treatment was associated with significantly improved PFS for both HER2/TOP2A-coamplified cancers (8.6 v 7.1 months; P = .034) as well as those lacking TOP2A coamplification (7.3 v 5.6 months; P = .0026, Appendix Table A2). However, for patients treated with AC alone, there was a distinct difference in outcomes between HER2/TOP2A-coamplified cancers and those with only HER2 amplification. In this group, HER2/TOP2A coamplified patients demonstrated a trend toward longer PFS (7.1 v 5.6 months; P = .11) and a statistically significant increase in OS (38.5 v 18.2 months; P = .004; Figs 2A, 2B; Appendix Tables A2, A3) despite the fact that these patients had not received trastuzumab. Women whose breast cancers had TOP2A deletions experienced clinical outcomes that were not significantly different from women who had TOP2A-normal cancers.
To determine whether the association was specifically related to anthracycline-based therapy as opposed to other chemotherapy, the same analysis was performed for the 135 patients treated with a nonanthracycline regimen (ie, paclitaxel plus/minus trastuzumab). Unlike patients receiving anthracycline-based chemotherapy, patients treated with paclitaxel alone showed no differences in PFS or OS related to presence or absence of TOP2A coamplification (PFS, 4.3 v 2.8 months; P = .20; OS, 18.4 v 20.6 months; P = .96, Appendix Tables A4, A5, online only). Overall data from the test set indicated that HER2-positive patients receiving chemotherapy without trastuzumab have an incremental improvement in clinical outcome associated with anthracyclines only if their cancers contain TOP2A coamplification.
Association Between Clinical Outcomes and TOP2A Alteration in Validation Set Patients
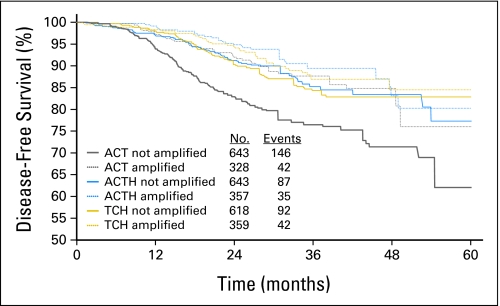
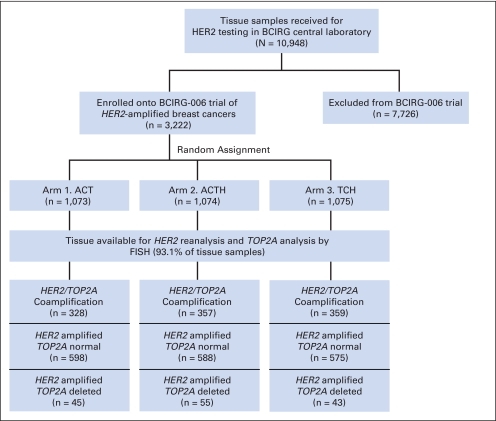
TOP2A status was next determined for 2,990 (93.1%) of 3,222 of the HER2-amplified tumors from BCIRG-006 (Appendix Figs A1, A2, online only). A total of 1,057 (35.4%) of 2,990 showed HER2/TOP2A coamplification while 1,788 (59.8%) of 2,990 were TOP2A-normal and 145 (4.8%) of 2,990 TOP2A deleted. Clinical characteristics of patients with TOP2A alterations did not differ in age, Karnofsky performance status, axillary node status, stage, or treatment arm (data not shown). Like test set patients, validation set patients were analyzed for clinical outcomes according to their TOP2A status. Overall, these data demonstrate that regardless of treatment arm, HER2/TOP2A coamplification is associated with a significantly longer DFS and OS (P < .001 and P < .001, respectively) when compared to women whose cancers do not contain TOP2A amplification. In TOP2A-normal patients who constitute 60% to 65% of HER2-positive cancers, trastuzumab significantly improves clinical outcomes whether used as doxorubicin, cyclophosphamide, docetaxel, and trastuzumab (ACTH) and docetaxel, carboplatin, and trastuzumab (TCH; DFS, P < .001; OS, P = .024; Fig 3; Table 3) with no difference in DFS or OS between the two regimens (P = .32 and P = .67, respectively). In these patients, trastuzumab resulted in significantly improved outcome for all comparisons (ie, AC→T v AC→TH, AC→T v TCH, or AC→T v AC→TH+TCH; Table 3). However, consistent with test set data, validation set patients demonstrated that for the 35% of HER2-positive breast cancers with TOP2A coamplification receiving anthracycline-based chemotherapy alone (ie, AC→T), there were significant improvements in both DFS and OS (P < .001 and P = .019, respectively); similar to that seen with trastuzumab-containing regimens (Fig 3). The test for interaction for an association between TOP2A coamplification and incremental anthracycline benefit was significant (P = .045, Table 3).
Fig 3.
Clinical outcome of women stratified by TOP2A status and by treatment arm in the Breast Cancer International Research Group (BCIRG) 006 clinical trial: comparison of disease-free survival (DFS) of women whose breast cancers lacked TOP2A gene coamplification and were treated on the doxorubicin, cyclophosphamide, and docetaxel (ACT) control treatment arm versus DFS of women whose breast cancers had TOP2A gene coamplification and were treated on the ACT control treatment arm and versus DFS of women whose breast cancers lacked TOP2A gene coamplification but were treated on either the doxorubicin, cyclophosphamide, docetaxel, and trastuzumab (ACTH) or the docetaxel, carboplatin, and trastuzumab (TCH) experimental treatment arms and with DFS of women whose breast cancers had TOP2A gene coamplification and were treated on either the ACTH or TCH experimental treatment arms. The number of patients and events in each treatment arm is listed for each arm. Comparisons of clinical outcome by TOP2A status (coamplified v not coamplified) and treatment arm are illustrated elsewhere.21 Table 3 provides the corresponding tests for treatment effects and interaction terms. The BCIRG-006 study compared two different experimental trastuzumab plus chemotherapy regimens with chemotherapy alone. The control arm (AC→T) consisted of four cycles of doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2 every 3 weeks followed by four cycles of docetaxel 100 mg/m2 every 3 weeks. Patients in the first experimental arm (ACTH) received the same chemotherapy with the addition of trastuzumab beginning with the first docetaxel dose followed by 2 mg/kg/week for 1 year. A second, nonanthracycline experimental arm (TCH) consisted of docetaxel 75 mg/m2 plus carboplatin at an area under the curve of 6 every 2 weeks for six cycles concurrently with trastuzumab. Trastuzumab was administered at 4 mg/kg for the first dose followed by 2 mg/kg/week until completion of chemotherapy, then at 6 mg/kg every 3 weeks to complete 1 year of treatment.
Table 3.
Treatment Effect and Interaction Between Treatment Effect and TOP2A Amplification Based on the BCIRG-006 Validation Set Trial
| Regimen |
TOP2A |
Interaction Test P | |||||
|---|---|---|---|---|---|---|---|
| Nonamplified (n = 1,904) |
Amplified (n = 1,044) |
||||||
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | ||
| ACT v ACTH | 0.53 | 0.40 to 0.69 | < .001 | 0.80 | 0.51 to 1.25 | .34 | .117 |
| ACT v TCH | 0.57 | 0.44 to 0.74 | < .001 | 0.92 | 0.60 to 1.42 | .65 | .063 |
| ACT v ACTH + TCH | 0.55 | 0.44 to 0.68 | < .001 | 0.85 | 0.59 to 1.25 | .41 | .045 |
NOTE. The BCIRG-006 study compared two different experimental trastuzumab plus chemotherapy regimens with chemotherapy alone. The control chemotherapy alone arm (ACT) consisted of doxorubicin and cyclophosphamide followed by docetaxel. Patients in the first experimental arm of anthracycline-containing chemotherapy (ACTH) received the same chemotherapy with the addition of trastuzumab beginning with the first docetaxel. A second, nonanthracycline experimental arm (TCH) consisted of docetaxel plus carboplatin concurrently with trastuzumab.
Abbreviations: BCIRG, Breast Cancer International Research Group; ACT, doxorubicin, cyclophosphamide, docetaxel; ACTH, doxorubicin, cyclophosphamide, docetaxel, and trastuzumab; TCH, docetaxel, carboplatin, and trastuzumab.
Frequency of TOP2A Alterations in HER2-Normal Breast Cancers and Association With Anthracycline Benefit
To determine the frequency of genomic alterations of TOP2A in breast cancers without HER2 amplification, we analyzed samples from the BCIRG-005 adjuvant study, a trial that accrued only HER2-negative patients.20 All patients in this trial received anthracycline-based therapy as part of a combination or sequential regimen (ie, TAC [docetaxel plus doxorubicin plus cyclophosphamide] or ACT). At the time of this analysis, 611 disease-related events had occurred demonstrating no difference between the two treatment arms. Analysis for TOP2A in this portion of the validation set allowed assessment of the frequency and nature of TOP2A alterations in the absence of HER2 amplification and whether such changes were associated with different DFS/OS event rates. In 1,614 of these HER2-normal cases, no TOP2A amplification was observed while 42 (2.6%) were TOP2A deleted. These deletions were not differentially associated with either DFS or OS.
DISCUSSION
Anthracyclines are among the most frequently prescribed cytotoxics in the treatment of breast cancer; however, not all patients benefit equally and these agents have significant potential long-term toxicities.2–4,29–31 Attempts to identify patients most likely to benefit from their use, have resulted in a remarkably consistent observation. Data from numerous large clinical studies, performed by multiple groups, conducted over three decades demonstrate that only those breast cancers containing HER2 amplification/overexpression appear to incrementally benefit from anthracycline- versus nonanthracycline-based regimens.5–9,32–34 Conversely, the remaining 75% to 80% of breast cancers that are HER2 normal do not.5–9,32–34 A recently published meta-analysis of composite data from more than 5,000 breast cancers from these and other studies clearly confirms this association.35 One of the more recent of these studies is National Cancer Institute of Canada Mammary-5 trial comparing cyclophosphamide, high-dose epirubicin, and fluorouracil (CEF) to cyclophosphamide, methotrexate, and fluorouracil. It again confirmed that only cancers with HER2 amplification had superior relapse-free survival (RFS; P = .003) and OS (P = .06) when treated with CEF while those lacking HER2 amplification received no incremental benefit in either RFS or OS from this regimen.8 An important subsequent analysis showed that incremental CEF outcome benefits were found in those patients whose cancers also overexpressed the TOP2A protein36 or had TOP2A gene amplification/deletion.37 The Danish Breast Cancer Cooperative Group also compared cyclophosphamide, methotrexate, and fluorouracil to CEF and recently reported that incremental anthracycline benefits were restricted to TOP2A or TOP2A/TIMP–altered subgroups of breast cancer.38,39 While the exact biologic mechanisms underlying an association between HER2 amplification and increased anthracycline response remain unclear, these data as well as other studies40 implicate TOP2A alterations as a potential molecular basis for superior anthracycline sensitivity. This hypothesis gains added credence from studies demonstrating that HER2 overexpression alone does not enhance sensitivity to anthracyclines.10 Together, these observations provide insight into a possible mechanism(s) regarding why HER2-amplified breast cancers are uniquely associated with increased anthracycline sensitivity and implicate a potential biomarker for increased response to anthracyclines.41
Anthracyclines inhibit TOP2A protein activity, a key enzyme in DNA replication and RNA transcription.42 Moreover, in vitro studies indicate that sensitivity to TOP2A inhibitors is dependent on TOP2A expression levels in cancer cells.42–44 The TOP2A gene is located at chromosome 17q21-22 in close proximity to HER2 resulting in a proportion of HER2-amplified breast cancers also containing coamplification of TOP2A.12,45 This in turn is associated with overexpression of TOP2A protein and potential increased sensitivity to TOP2A inhibitors.12,42,45,46 Initial smaller studies of TOP2A alterations in breast cancer suggested they were frequently found in HER2-amplified tumors.47,48 Subsequent larger studies showed that between 33% to 60% of HER2-positive cancers contain concurrent TOP2A amplification25,26,45 while 20% to 42% are TOP2A deleted.26,45,48–50 Some published reports of HER2-normal breast cancers47,48 find TOP2A infrequently amplified or deleted while others, based on relatively few patients, report a 10% to 20% TOP2A amplification/deletion rate25,26 causing confusion regarding this matter. This wide variability presents a challenge for assessing any true association of this alteration with clinical outcomes and is likely related to different FISH ratio cut points used to define TOP2A amplification (≥ 1.5 or ≥ 2.0) and/or TOP2A deletions (≤ 0.67, ≤ 0.7, ≤ 0.8, or < 1.0).25,26,45,48,51,52
In this study of almost 5,000 new breast cancers from three separate trials, the following questions were addressed: what is the prevalence of TOP2A alterations? What is its concordance with HER2 amplification? And what is the association (if any) with incremental anthracycline sensitivity? Utilizing methods and probes validated from physical mapping of the 17q12-q21 amplicon, we find that 35% of HER2-amplified cancers contain TOP2A coamplification, 5% have deletions, and 60% are TOP2A normal. In addition, analysis of 1,614 HER2-normal cases revealed no TOP2A amplification but a 3% deletion rate. These data are consistent with other reports that TOP2A amplification, when present, is seen with HER2 coamplification41,45,48 and contrast with smaller studies reporting TOP2A amplification in HER2-normal breast cancers.26,53 Because of differences in methods and cutoffs to assess HER2 and TOP2A status,54 we again believe this published variability in TOP2A alterations is largely due to technical rather than biologic factors.
There are also conflicting data regarding an association between TOP2A alterations and anthracycline sensitivity. In this study, we find such an association. Conversely, an analysis of almost 2,000 patients reported previously that HER2 and TOP2A have “only a clinically modest and statistically borderline predictive value.”55 However, these investigators noted difficulty in reproducing FISH results between and within laboratories involved in this study, demonstrating a concordance rate of only 69.2%. In this analysis of approximately 5,000 patients, there was no similiar difficulty, with a more than 97% concordance between prior and current analyses. A small study of 41 patients with TOP2A gene amplification also recently reported no association between TOP2A status and anthracycline dose-response56; however, all patients received the drug and no information was provided regarding distribution of patients across three anthracycline dose strata. Assuming an even distribution, only 14 patients would exist in each treatment arm resulting in a lack of sufficient statistical power to demonstrate any association. In a separate study of 2,123 patients, all of whom received identical anthracycline doses either in combination or sequence with other drugs, no association was found between TOP2A alterations and anthracycline sensitivity; however, there was an association with HER2 amplification.57 As noted by the authors57 and in the accompanying editorial,58 “the predictive information we need most in this area cannot be augmented from any analyses of the trial” given that all patients received an anthracycline. Finally, a recent study of pooled data from two trials (National Epirubicin Adjuvant Trial/BR9601)59,60 found no association between TOP2A status and response to anthracycline-based chemotherapy. However, this study inexplicably used different gene-to-centromere FISH ratios (ie, HER2/CEP17 ≥ 2.0 and TOP2A/CEP17 ≥ 1.5) for two genes in the same amplicon.60 No scientific rationale is offered for use of different ratios to assess loci within the same 17q12-q21 amplicon, especially since both are compared to the identical control probe (CEP17). Given that ≥ 2.0 is the established, US Food and Drug Administration–approved FISH ratio defining HER2 amplification, as well the ratio used for this study, we would estimate that approximately 55% of the TOP2A-amplified patients from the National Epirubicin Adjuvant Trial/BR9601 report are actually TOP2A normal with TOP2A/CEP17 ratios between 1.5 to 2.0. It is not surprising that inclusion of so many potential false-positive patients results in failure to demonstrate any association between TOP2A and anthracycline response.
Conversely, our test set of 339 patients indicates that patients with HER2-positive breast cancers containing TOP2A coamplification have longer PFS and improved OS when compared to TOP2-normal patients if they receive anthracyclines. Outcome data within the test set treatment arms show that HER2-positive patients receiving only AC have similar PFS/OS improvements to those receiving AC plus trastuzumab if their malignancies contain TOP2A coamplification (eg, Fig 2). HER2-positive cancers lacking TOP2A coamplification show no such incremental benefit from anthracycline compared to nonanthracycline treatment. The much larger validation set consisted of 4,604 patients from the BCIRG-005/-006 adjuvant trials and confirms a significant association between TOP2A coamplification and improved DFS/RFS as well as OS in women treated with anthracyclines. No association between TOP2A coamplification and outcome was observed in nonanthracycline-based treatment arms (taxol in the H0648 study and TCH in BCIRG-006) underscoring the biologic and therapeutic significance of an association between TOP2A amplification and incremental anthracycline sensitivity and indicating that TOP2A amplification is a predictive biomarker for anthracycline-based chemotherapies. It has also been suggested that TOP2A deletions are associated with increased anthracycline response, however, there are only two such studies, both showing nonsignificant trends between TOP2A deletions and anthracycline response.38,53 We are unaware of any other study reporting such an association and our current data, do not support this hypothesis. Moreover, compelling preclinical studies indicate that TOP2A deletions are associated with anthracycline resistance rather than improved responsiveness.42,45
Results from this study demonstrate that women whose cancers have HER2/TOP2A coamplification (approximately 8% of breast cancers) experience equivalent DFS, RFS, or OS outcomes whether treated with a trastuzumab-containing regimen or an anthracycline-based regimen without trastuzumab. Breast cancers containing both alterations (HER2/TOP2A coamplification) benefit equally when treated with either trastuzumab or anthracyclines; however, they appear to receive no additional benefit from combining trastuzumab with anthracyclines. The use of anthracyclines, particularly in combination with trastuzumab, is associated with significant additional long-term toxicities.29,31,61 The findings from multiple studies6,8,33,51,52,62–68 as well as a published meta-analysis35 indicate that the incremental benefit from anthracyclines reported in breast cancer is restricted to HER2-positive patients. This study of 4,943 patients confirms these findings and demonstrates that this differential anthracycline benefit is associated with TOP2A coamplification. Taken together, these data indicate that anthracycline-based adjuvant therapies, with their attendant short and long-term risks, should only be considered for the approximately 8% of human breast cancers that have HER2/TOP2A coamplification and, then, only in patients who do not receive a HER2-targeted therapy like trastuzumab.
Acknowledgment
We thank Genentech and Robert Mass, MD, for permission to analyze TOP2A in the H0648 trials samples; Grazyna Lieberman, PhD, for review of the statistical analyses of the test set cases; Josina C. Reddy, MD, and Pamela M. Klein, MD, for review of the description of the test set results; Jian-Yuan Zhou, MD, and Yanling Ma, MD, for technical assistance (USC); Jane Sullivan-Halley for statistical assistance (City of Hope); Marek Pawlicki, MD (City Oncology Dispensary, St Petersburg, Russia), Arlene Chan, MD (Mount Breast Group, Mount Hospital, Perth, Australia), and John Mackey, MD (Department of Oncology, University of Alberta, Canada) for high levels of accrual to the clinical trials; Toufik Bendahmane, MD, for advice; and Steven Seelig, MD, PhD, for provision of FISH probes and reagents. We would also like to thank the other physician investigators who accrued patients to these clinical trials and the women who participated as patients.
Appendix
Patients
Test set patients consisted of women with HER2-positive metastatic breast cancer enrolled on the original H0648g trastuzumab registration study. This randomized phase III trial evaluated chemotherapy plus or minus trastuzumab in patients with HER2-positive metastatic breast cancer.19 Between June 1995 and March 1997, this study enrolled 469 patients; 235 received trastuzumab plus chemotherapy and 234 received chemotherapy alone. Women with no prior adjuvant anthracycline treatment (n = 281) received an anthracycline and cyclophosphamide (AC) either with (n = 143) or without (n = 138) trastuzumab.19 Women with prior adjuvant anthracycline exposure (n = 188) were randomly assigned to receive paclitaxel with (n = 92) or without (n = 96) trastuzumab (Figs 1 and 2; Table 1).19 Validation set patients consisted of women who participated in the BCIRG-005 and BCIRG-006 adjuvant breast cancer trials which accrued 3,298 and 3,222 patients, respectively, between August 2000 and March 2004. Participants in BCIRG-005 had HER2-normal, node-positive, early-stage breast cancer and were randomly assigned to receive adjuvant treatment with one of two anthracycline-containing regimens; either four cycles of doxorubicin plus cyclophosphamide every 3 weeks followed by four cycles of docetaxel every 3 weeks (ACT) or six cycles of docetaxel plus doxorubicin plus cyclophosphamide every 3 weeks (TAC).20 BCIRG-006 participants had node-positive or high-risk, node-negative, invasive HER2-amplified breast cancers.21,22 This study compared two different experimental trastuzumab plus chemotherapy regimens (one with and one without anthracyclines) to anthracycline-based chemotherapy alone (Fig 3). All three studies followed patients for either progression-free survival (PFS), or disease-free survival (DFS) as well as overall survival (OS) and all provided consent for centralized molecular analysis of their primary tumors. This study was approved by the University of Southern California institutional research board.
Tissue Specimen Analyses
Validation of probes and cutoffs used to determine HER2/TOP2 amplification or deletion status by fluorescence in situ hybridization.
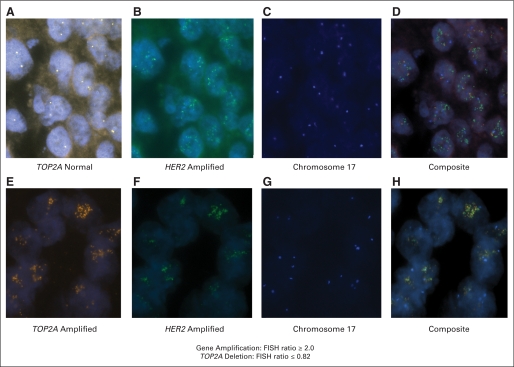
TOP2A and HER2 amplification/deletion status was determined by fluorescence in situ hybridization (FISH) using probes22,23 provided by Abbott-Vysis, Inc (Downers Grove, IL). Analysis of both genes was performed simultaneously using SpectrumGreen-labeled HER2 and SpectrumOrange-labeled TOP2A probes, respectively. The number of chromosome 17 centromeres was determined using a SpectrumAqua-labeled chromosome enumeration 17 (CEP) probe. TOP2A and HER2 gene copy number was determined in a minimum of at least 20 interphase, nonoverlapping tumor cell nuclei and compared with chromosome 17 centromeres in the same nuclei. To ensure that the probes and cutoffs used to generate the amplification/deletion status of TOP2A and HER2 in the test and validation sets were correct, the status of these two genes was first determined in a molecularly characterized panel of known material. This was accomplished using a series of 36 human breast cancer cell lines and two normal breast epithelial lines with known HER2 and TOP2A status. All 38 lines had HER2 and TOP2A gene copy numbers determined by physical mapping of the 17q12-q21 amplicon using overlapping bacterial artificial chromosome (BAC) probes in both metaphase spreads and intact interphase nuclei (Pauletti et al, unpublished). This approach provided the actual size and boundaries of each 17q12-q21 amplicon as well as the correct amplification/deletion status of all genes centromeric and telomeric to HER2. This analysis yielded the exact copy number of each normal, coamplified, or deleted gene including TOP2A for all 38 cell lines. To replicate the conditions and procedures used for the test and validation specimens, pellets of each of these fully characterized cell lines were then prepared and processed as formalin-fixed, paraffin-embedded (FFPE) specimens and sections were cut for FISH analyses as described below for clinical samples. Scoring these FFPE sections was performed blinded to their individual identity to ensure an unbiased result. These HER2 and TOP2A results were then directly compared to the results determined by direct chromosome 17q12-q21 mapping. These analyses yielded complete concordance between FISH copy numbers for both HER2 and TOP2A as determined in FFPE sections and using overlapping BAC array analyses or hybridization of metaphase spreads for each of the 38 unique cell lines. We subsequently utilized these validated probes and cutoff ratios for analysis of all clinical specimens (Appendix Table A1, online only).
HER2 amplification was defined as a HER2 gene-to-CEP17 ratio ≥ 2.0, using the ratio approved by the US Food and Drug Administration, rather than the American Society of Clinical Oncology-College of American Pathologists guideline ratio for reasons described elsewhere in detail.24 The identical ratio was used to define TOP2A gene amplification,25,26 with the rationale that the established cutoff used for HER2-gene amplification with Southern hybridization is an index gene-to-control gene ratio of ≥ 2.0 (Slamon D, Clark G, Wong S, et al: Science. 235:177-182, 1987; Slamon D, Godolphin W, Jones L et al: Science 244:707-712, 1989); the accepted US Food and Drug Administration–approved FISH ratio for HER2 amplification is ≥ 2.0 and the same cutoff for TOP2A, which, when amplified in the same amplicon, provides internal consistency; a portion of the cell population is dividing at any one time and a ratio of ≥ 2.0 is unlikely to lead to confusion with nonamplified, actively dividing cells; and these ratios had all been validated in a molecularly characterized cell line panel as outlined above. A TOP2A-to-chromosome 17 FISH ratio of ≤ 0.82 was identified to indicate TOP2A gene deletion identifying those breast cancers that lose a single copy of the gene. This approach separates true TOP2A deletants from the 75% of breast cancers that are tetraploid or near-tetraploid/aneuploid (Bacus S, Bacus J, Slamon D, et al: Arch Pathol Lab Med 114:164-169, 1990). A similar cut point has been used by other investigators to identify cases with TOP2A gene deletions26,51,52; while others have used different ratios including lower than 1.0,25 lower than 0.9, ≤ 0.745 and ≤ 0.67.48 While these latter ratios may not be arbitrary, no scientific or other rationale has been delineated for these alternative cutoffs.
Test set specimens consisted of 5-μm sections cut from 339 unique breast cancer specimens derived from the H0648 study which were originally tested for HER2 by immunohistochemistry and subsequently by FISH to evaluate HER2 copy number23 then for TOP2A status using FISH as described above. Validation set specimens were available as tissue microarrays (Kononen J, Bubendorf L, Kallioniemi A, et al: Nat Med 4:844-847, 1998) or individual slides, for 1,614 (49%) of 3,298 of BCIRG-005 and 2,990 (93%) of 3,222 of BCIRG-006 participants, respectively. Breast cancers from all three clinical trials were retested by FISH for this study, blinded to the original results and categorized as either amplified/deleted or nonamplified/deleted for TOP2A and HER2. The concordance between the original scoring and current retesting for HER2 status in the H0648g test set patients was 97%. In the validation set, HER2 status was also repeated to determine if comparable FISH results could be obtained in a tissue microarray format. This resulted in a 99.8% and 99.6% concordance between the original and current analyses for BCIRG-005 and BCIRG-006, respectively.22,23 The high concordance rates effectively exclude issues related to the age of stored tissue blocks or related to the use of TMAs instead of individual individual tissue sections.
Statistical Methods
Clinical outcomes (overall response rates, PFS, and OS in trial H0648, DFS in trials BCIRG-005 and BCIRG-006 and OS in all trials) were compared in the TOP2A-amplified and -nonamplified subgroups. Data from patients whose tumors had TOP2A gene deletions were grouped with data from patients who had a normal TOP2A score to form the TOP2A nonamplified subgroup as well as analyzed as separate groups. Differences in response rates were compared using a χ2 test. To control for trastuzumab therapy, the Mantel-Haenszel test was used for overall comparisons. Differences in PFS, DFS, and OS were compared using the log-rank test.27,28 All P values reported are two sided; no adjustments were made for multiple comparisons. PFS, DFS, and OS curves were plotted using Kaplan-Meier product limit estimates. All analyses were performed using the SAS statistical package (version 9.0, SAS Institute, Cary, NC). The predictive value of TOP2A was tested through interaction tests in Cox models containing indicator variables for TOP2A gene amplification and treatment.
Results
Validation of TOP2A FISH cutoffs for gene amplification and deletion with human breast cancer cell lines.
Use of accurate methods to correctly determine the TOP2A and HER2 gene status in clinical material are critical to all analyses performed in and results derived from this study and this necessitated extensive validation of the probes and procedures utilized. To ensure that the probes and cutoffs used to generate the amplification/deletion rates of TOP2A and HER2 in both the test and validation sets were correct, the exact status of these two genes was first determined in a molecularly characterized panel of known material. This was accomplished using a series of 36 human breast cancer cell lines and two normal breast epithelial cell lines with known HER2 and TOP2A gene status (Appendix Table A1). All 38 lines had their HER2 and TOP2A gene copy numbers determined by actual physical mapping of the 17q12-q21 amplicon using overlapping, fluorescently labeled BAC probes in both metaphase spreads and intact interphase nuclei (Pauletti et al, unpublished). This approach provided the actual size of each amplicon as well as the correct amplification/deletion status of the various genes proximal or distal to HER2 yielding the exact copy number of each coamplified/deleted gene including TOP2A. To replicate the conditions and procedures used for the test and validation clinical specimens, pellets of each of these cell lines were prepared and processed as FFPE specimens and sections were cut for FISH analyses as described below for the clinical samples. These sections were then used in FISH hybridization studies to ascertain copy number of both HER2 and TOP2A. Scoring the FFPE sections of the cell line pellets was performed blinded to their individual identity to ensure an unbiased result. These HER2 and TOP2A results were then directly compared to the molecularly validated HER2 and TOP2A results determined by direct chromosome 17q12-q21 mapping. These analyses yielded complete concordance between FISH copy numbers for both HER2 and TOP2A determined in FFPE sections and the data using overlapping BAC array analyses and hybridization of metaphase spreads in the same 38 unique cell lines. We subsequently used these validated probes and cutoff ratios for analysis of all clinical specimens used in this study (Appendix Table A1).
In order to confirm that the TOP2A-to-CEP17 ratio used for TOP2A deletion did not overlap with ratios observed in normal cells, we analyzed these ratios in normal cells found in the breast cancer tissue specimens. TOP2A-to-CEP17 ratios from 20 different patients were used to confirm that our 0.82 cutoff determined from breast cancer cell lines was sufficiently remote from ratios in normal cells. The ratios in normal cells ranged from 1.03 to 1.19. The mean TOP2A-to-CEP17 ratio is 1.10 with a standard deviation of 0.058. Three standard deviations on either side of the mean would include TOP2A-to-CEP17 ratios between 0.926 and 1.274. Clearly, the 95% CI around the mean of normal cell TOP2A ratios did not include our 0.82 cutoff for TOP2A deletion.
Discussion
In biomarker studies a hypothesis is often established in a test or training set of patients, and then validated in a similar, but independent set of patients. In this study patients and clinical outcomes in the test set are different than those in the validation set, although the underlying biologic hypothesis is similar. Both our test set and validation set examine women who have (predominantly) HER2-amplified breast cancers. Women in the test set were treated for advanced breast cancer, while women in the validation set had nonmetastatic disease treated in the adjuvant setting. Treatment of both sets involved anthracyclines with or without trastuzumab. Although the extent of disease and anthracycline-containing chemotherapy regimens were not the same, we do not believe this detracts from the generality of the findings. The fact that the observations and results were so similar between the test set and the validation set, despite these differences, strengthens, not weakens, the validity and generality of our conclusions.
Ordinarily, we would recommend confirmation of retrospective findings by a prospective clinical trial. However, a large, prospective biomarker study, such as this one, specifically designed to address TOP2A as a predictive marker is not likely to be performed. Such a trial would be too expensive for a biomarker company and no pharmaceutical company would support such a trial to evaluate associations of a biomarker with responsiveness to a generic drug (doxorubicin). We believe our study, a planned retrospective analysis of a prospective clinical trial for TOP2A status, is as close as we are likely to come to this desired goal in the foreseeable future. We analyzed TOP2A gene amplification data from as many patients as are available in the rest of the world's literature combined. The results that we reported were a planned retrospective assessment of TOP2A alterations in three prospective clinical trials, two large (3,222 and 3,298 patients) and one smaller (469 patients) trial. These trials cost their pharmaceutical sponsors between $40 and $80 million for each trial, depending on the trial. While large pharmaceutical companies have the resources to conduct such expensive trials, biomarker companies do not have similar resources.
There are no studies being conducted or (to our knowledge) in the planning that will prospectively randomly assign patients to anthracycline versus nonanthracycline treatment regimens based on TOP2A status. Moreover, there is not sufficient financial incentive to motivate a diagnostic company to fund a sufficiently sized and powered study to address this question and they cannot partner with a therapeutic company given that the drugs in question are all off patent. The only ongoing trial that is randomizing between an anthracycline regimen and a nonanthracycline regimen is the “Tic-Tac-Toe” study that is being conducted by US Oncology and the National Surgical Adjuvant Breast and Bowel Project. This study is evaluating an anthracycline-based regimen (docetaxel, doxorubicin, and cyclophosphamide [TAC]) versus a nonanthracycline based regimen (docetaxel and cyclophosphamide [TC]) in a two-by-two factorial design that will evaluate the addition of bevicizumab to both treatment regimens; hence it will have industry support. Of note, this trial is in HER2-negative patients and the patients are not randomly assigned based on the TOP2A status of the patient, therefore, it will not be testing this biomarker any differently than we have in this manuscript. In addition, this study is for approximately 3,900 patients and currently has 1,300 patients accrued after being opened for 2 years. Patient samples will be tested posthoc for TOP2A alterations. Given this fact and based on the data contained in the literature, there will be many fewer topoisomerase alterations in this HER2-negative group of patients. Since the study will likely take another 2 to 3 years to accrue and an additional 3 to 4 years to mature and, given the exclusion of HER2-positive patients in the cohort, the DFS event rate will be lower than a study that would include HER2-positive patients, the study will take several years to report. As a result, we will not have an answer regarding a possible association of the TOP2A marker with response from this study for another 5 to 6 years and then it will only be another association study with smaller numbers of TOP2A alterations than we are providing in this TOP2A study.
Table A1.
Comparison of HER-2 and TOP2AStatus by FISH in Human Breast Cancer Cell Lines
| Human Breast Cancer Cell Line | FISH Analysis of Breast Cancer Cell Lines |
|||
|---|---|---|---|---|
| Tissue Sections |
Intact Interphase Nuclei |
|||
| HER2 | TOP2A | HER2 | TOP2A | |
| SK-BR-3 | Amplified | (low) ampl | Amplified | (low) amplified |
| HCC1954 | Amplified | Normal | Amplified | Normal |
| SUM225 | Amplified | Deleted | Amplified | Deleted |
| MDA-MB-453 | Amplified | Deleted | Amplified | Deleted |
| BT474 | Amplified | Normal | Amplified | Normal |
| SUM190 | Amplified | Deleted | Amplified | Deleted |
| HCC202 | Amplified | Deleted | Amplified | Deleted |
| HCC1419 | Amplified | Normal | Amplified | Normal |
| UACC732 | Amplified | Deleted | Amplified | Deleted |
| EFM192A | Amplified | Amplified | Amplified | Amplified |
| HCC2218 | Amplified | Normal | Amplified | Normal |
| MDA-MB-361 | Amplified | Deleted | Amplified | Deleted |
| UACC812 | Amplified | Amplified | Amplified | Amplified |
| UACC893 | Amplified | Normal | Amplified | Normal |
| ZR75-30 | Amplified | Deleted | Amplified | Deleted |
| JIMT-1 | Amplified | Normal | Amplified | Normal |
| HS578-T | Amplified | Amplified | Amplified | Amplified |
| HCC1143 | Normal | Normal | ||
| MDA-MD-415 | Normal | Normal | ||
| CAMA-1 | Normal | Normal | ||
| COLO824 | Normal | Normal | ||
| BT20 | Normal | Normal | ||
| KPL1 | Normal | Normal | ||
| 184A1 | Normal | Normal | ||
| MCF7-10A | Normal | Normal | ||
| BT549 | Normal | Normal | ||
| MB157 | Normal | Normal | ||
| MCF7 12A | Normal | Normal | ||
| CAL51 | Normal | Normal | ||
| MDA231 | Normal | Normal | ||
| MDA468 | Normal | Normal | ||
| T47D | Normal | Normal | ||
| HCC1500 | Normal | Normal/deleted | ||
| HCC38PAR | Normal | Normal | ||
| HCC1937 | Normal | Normal | ||
| HCC1806 | Normal | Normal | ||
| HCC1187 | Normal | Normal | ||
| 184B5 | Normal | Normal | ||
Abbreviation: FISH, fluorescent in situ hybridization.
Table A2.
Progression-Free Survival by TOP2AGene Amplification Status for Test Set Patients Treated in the H0648 Trial
| Parameter |
TOP2AStatus by Regimen |
|||||
|---|---|---|---|---|---|---|
| AC Alone |
AC + H |
Any AC |
||||
| Not Amplified | Amplified | Not Amplified | Amplified | Not Amplified | Amplified | |
| No. | 50 | 27 | 54 | 31 | 104 | 58 |
| Censored | 3 | 3 | 14 | 11 | 17 | 14 |
| % | 6 | 11 | 24 | 35 | 16 | 24 |
| Median, months | 5.6* | 7.1† | 7.3* | 8.6† | 6.7 | 7.6 |
| 95% CI | 4.6 to 6.7 | 4.7 to 9.8 | 6.9 to 9.4 | 7.1 to 12.7 | 5.6 to 7.3 | 7.1 to 9.8 |
| Log-rank P | .11 | .31 | .064‡ | |||
Abbreviations: A, doxorubicin; C, cyclophosphamide; H, trastuzumab.
Differences have a P = .0026.
Differences have a P = .0344.
P is based on stratified log-rank; stratification variable is treatment with trastuzumab.
Table A3.
Survival by TOP2AGene Amplification Status for Test Set Patients Treated in the H0648 Trial
| Parameter |
TOP2AStatus by Regimen |
|||||
|---|---|---|---|---|---|---|
| AC Alone |
AC + H |
Any AC |
||||
| Not Amplified | Amplified | Not Amplified | Amplified | Not Amplified | Amplified | |
| No. | 50 | 27 | 54 | 31 | 104 | 58 |
| Censored | 8 | 14 | 22 | 12 | 30 | 26 |
| % | 16 | 52 | 41 | 39 | 29 | 45 |
| Median, months | 18.2 | 38.5 | 29.3 | 29.4 | 21.7 | 30.8 |
| 95% CI | 13.2 to 23.2 | 19.8 to NR | 21.0 to NR | 18.7 to NR | 18.1 to 26.7 | 22.8 to NR |
| Log-rank P | .0040 | .66 | .069* | |||
Abbreviations: A, doxorubicin; C, cyclophosphamide; H, trastuzumab; NR, not reported.
P is based on stratified log-rank; stratification variable is treatment with H.
Table A4.
Progression-Free Survival by TOP2AGene Amplification Status for Test Set Patients Treated in the H0648 Trial (nonanthracycline chemotherapy arms)
| Parameter |
TOP2AStatus by Regimen |
|||||
|---|---|---|---|---|---|---|
| Paclitaxel Alone |
Paclitaxel + H |
Any Paclitaxel |
||||
| Amplified | Not Amplified | Amplified | Not Amplified | Amplified | Not Amplified | |
| Total | 16 | 43 | 25 | 33 | 41 | 76 |
| Progression | ||||||
| No | 3 | 1 | 9 | 14 | 12 | 15 |
| Yes | 13 | 42 | 16 | 19 | 29 | 61 |
| Median, months | 4.3 | 2.8 | 6.4 | 12.0 | 5.5 | 4.6 |
| Log-rank P | .20 | .22 | .65 | |||
Abbreviation: H, trastuzumab.
Table A5.
Survival by TOP2AGene Amplification Status for Test Set Patients Treated in the H0648 Trial (nonanthracycline chemotherapy arms)
| Parameter |
TOP2AStatus by Regimen |
|||||
|---|---|---|---|---|---|---|
| Paclitaxel Alone |
Paclitaxel + H |
Any Paclitaxel Regimen |
||||
| Amplified | Not Amplified | Amplified | Not Amplified | Amplified | Not Amplified | |
| Total | 16 | 43 | 25 | 33 | 41 | 76 |
| Alive | 4 | 13 | 12 | 17 | 16 | 30 |
| Died | 12 | 30 | 13 | 16 | 25 | 46 |
| Median, months | 18.4 | 20.6 | 30.7 | 35.7 | 22.1 | 25.2 |
| Log-rank P | .96 | .66 | .99 | |||
Abbreviation: H, trastuzumab.
Table A6.
Treatment Continuation/Crossover to Trastuzumab After Progression on Study H0648g
| Crossover Status |
TOP2AStatus by Regimen |
|||||||
|---|---|---|---|---|---|---|---|---|
| AC Alone |
AC + Trastuzumab* |
|||||||
| Amplified |
Not Amplified |
Amplified |
Not Amplified |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Crossed over | 19 | 70 | 33 | 66 | 8 | 26 | 22 | 41 |
| No crossover | 8 | 30 | 17 | 34 | 23 | 74 | 32 | 59 |
| Total | 27 | 50 | 31 | 54 | ||||
Abbreviation: AC, doxorubicin and cyclophosphamide.
For AC + trastuzumab, crossed over means that patient received trastuzumab postprogression in study H0659g.
Fig A1.
Fluorescent in situ hybridization (FISH) of HER2 and TOP2A gene in breast cancer cells. (A) FISH of TOP2A gene in breast cancer without coamplification (TOP2A normal). (B) FISH showing HER2 gene amplification in the breast cancer from (A). (C) FISH showing chromosome 17 centromere in the breast cancer from (A). (D) Composite of (A), (B), and (C). (E) FISH of TOP2A gene in breast cancer with TOP2A coamplification. (F) FISH showing HER2 gene amplification in the breast cancer from (E). (G) FISH showing chromosome 17 centromere in the breast cancer from (E). (H) Composite of (E), (F), and (G).
Fig A2.
Specimen accountability for Breast Cancer International Research Group (BCIRG) 006 clinical trial. Although a total of 2,990 patients had TOP2A FISH data (1,057 coamplified + 1,788 TOP2A normal + 145 TOP2A deleted), only 2,948 patients (ie, safety population) were included in the outcome analyses. The safety population includes patients classified by treatment actually received; hence, this population differs slightly from the intent-to-treat (ITT) population. Forty-two patients (2,990 − 2,948 patients) were not included in the curves of Figure 3 or calculations shown in Table 3. The rationale for using the safety population instead of the ITT population was that for biomarker analyses, it makes sense to use the treatment patients actually received rather than that allocated by randomization. Nevertheless, because the difference between the ITT and safety populations was quite small (1% of patients), it did not make any substantial difference which of these two populations we used for analyses of TOP2A status compared with outcome; the conclusions are the same. ACT, doxorubicin, cyclophosphamide, and docetaxel; ACTH, doxorubicin, cyclophosphamide, docetaxel, and trastuzumab; TCH, docetaxel, carboplatin, and trastuzumab.
Footnotes
Supported by sanofi-aventis (BCIRG-005 and -006 clinical trials) and Genentech (H0648 clinical trial); by Grants No. CA48780 from the National Cancer Institute, DAMD17-03-1-0626 from the US Army Medical Research and Development Command, and 12IB-0155 from the California Breast Cancer Research Program Expedition Inspiration and by the Breast Cancer Research Foundation (M.F.P.); by Grant No. CA77398 from the National Cancer Institute (L.B., J.S.-H.); and by grant support from the Department of Defense Breast Cancer Research Innovator Award and Revlon/University of California Los Angeles Women's Program (D.J.S.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marc Buyse, International Drug Development Institute (C); Isabelle Tabah-Fisch, sanofi-aventis (C) Consultant or Advisory Role: Michael F. Press, Genentech (C), GlaxoSmithKline (C); Wolfgang Eiermann, sanofi-aventis (C), Roche (C), Novartis (C), AstraZeneca (C), Merck (C), Genentech (C); Miguel Martin, Roche (C); Nicholas Robert, Roche (C), sanofi-aventis (C) Stock Ownership: Marc Buyse, International Drug Development Institute; Isabelle Tabah-Fisch, sanofi-aventis Honoraria: Michael F. Press, Genentech, GlaxoSmithKline; Wolfgang Eiermann, sanofi-aventis, Roche, Merck, Novartis, AstraZeneca; Miguel Martin, Roche; Nicholas Robert, Roche, sanofi-aventis; John Crown, sanofi-aventis Research Funding: Michael F. Press, Genentech, GlaxoSmithKline, Ventana Medical Systems; Nicholas Robert, Roche, sanofi-aventis; John Crown, sanofi-aventis; Mary-Ann Lindsay, sanofi-aventis, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michael F. Press, Dennis J. Slamon
Financial support: Michael F. Press
Administrative support: Michael F. Press, Ivonne E. Villalobos, Isabelle Tabah-Fisch, Mary-Ann Lindsay
Provision of study materials or patients: Michael F. Press, Wolfgang Eiermann, Tadeusz Pienkowski, Miguel Martin, Nicholas Robert, John Crown, Kerry J. Flom, Giovanni Pauletti, Dennis J. Slamon
Collection and assembly of data: Michael F. Press, Guido Sauter, Marc Buyse, Roberta Guzman, Angela Santiago, Ivonne E. Villalobos, Wolfgang Eiermann, Nicholas Robert, Valerie Bee, Henry Taupin, Giovanni Pauletti
Data analysis and interpretation: Michael F. Press, Marc Buyse, Leslie Bernstein, Roberta Guzman, Angela Santiago, Miguel Martin, Valerie Bee, Henry Taupin, Kerry J. Flom, Giovanni Pauletti, Dennis J. Slamon
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Clarke M, Collins R, Darby S, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Doyle J, Neugut A, Jacobson J, et al. Chemotherapy and Cardiotoxicity in older breast cancer patients: A population-based study. J Clin Oncol. 2005;23:8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 3.Hershman D, Neugut A, Jacobson J, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 4.Pinder M, Duan Z, Goodwin J, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 5.Muss H, Thor A, Berry D, et al. cerb B-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 6.Paik S, Bryant J, Park C, et al. ErbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst. 1998;90:1361–1370. doi: 10.1093/jnci/90.18.1361. [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart M. Anthracyclines and the tailoring of treatment for early breast cancer. N Engl J Med. 2006;354:2177–2179. doi: 10.1056/NEJMe068065. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard K, Shepherd L, O'Malley F, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 9.Thor A, Berry D, Budman D, et al. ErB-2, p53, and Efficacy of Adjuvant Therapy in Lymph Node-Positive Breast Cancer. J Natl Cancer Inst. 1998;90:1346–1360. doi: 10.1093/jnci/90.18.1346. [DOI] [PubMed] [Google Scholar]
- 10.Pegram M, Finn R, Arzoo K, et al. The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene. 1997;15:537–547. doi: 10.1038/sj.onc.1201222. [DOI] [PubMed] [Google Scholar]
- 11.Bièche I, Tomasetto C, Régnier C, et al. Two distinct amplified regions at 17q11-q21 involved in human primary breast cancer. Cancer Res. 1996;56:3886–3889. [PubMed] [Google Scholar]
- 12.Smith K, Houlbrook S, Greenall M, et al. Topoisomerase II co-amplification with erbB2 in human primary breast cancer, and breast cancer cell lines: Relationship to m-AMSA and mitoxantrone sensitivity. Oncogene. 1993;8:933–938. [PubMed] [Google Scholar]
- 13.Tomasetto C, Regnier C, Moog-Lutz C, et al. Identification of four novel human genes amplified and overexpressed in breast carcinoma and located to the q11-q21.3 region of chromosome 17. Genomics. 1995;28:367–376. doi: 10.1006/geno.1995.1163. [DOI] [PubMed] [Google Scholar]
- 14.van de Vijver M, van de Bersselaar R, Devilee P, et al. Amplification of the neu (c-erbB-2) oncogene in human mammary tumors is relative frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol. 1987;7:2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoare S, Freeman C, Coutts J, et al. Identification of genetic changes associated with drug resistance by reverse in situ hybridization. Br J Cancer. 1997;75:275–282. doi: 10.1038/bjc.1997.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keith W, Douglas F, Wishart G, et al. Co-amplification of erbB2, topoisomerase II and retinoid acid receptor genes in breast cancer and allelic loss at topoisomerase I on chromosome 20. Eur J Cancer. 1993;29A:1469–1475. doi: 10.1016/0959-8049(93)90022-8. [DOI] [PubMed] [Google Scholar]
- 17.Murphy D, McHardy P, Coutts J, et al. Interphase cytogenetic analysis of erbB2 and topoII co-amplification in invasive breast cancer and polysomy of chromosome 17 in ductal carcinoma in situ. Int J Cancer. 1996;64:18–26. doi: 10.1002/ijc.2910640106. [DOI] [PubMed] [Google Scholar]
- 18.Park K, Kim J, Lim S, et al. Topoisomerase II-alpha (topoII) and HER2 amplification in breast cancers and response to preoperative doxorubicin chemotherapy. Eur J Cancer. 2003;39:631–634. doi: 10.1016/s0959-8049(02)00745-1. [DOI] [PubMed] [Google Scholar]
- 19.Slamon D, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin / cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with HER2-normal, node-positive breast cancer: Breast Cancer International Research Group (BCIRG)-005 Trial. J Clin Oncol in review 2010. doi: 10.1200/JCO.2010.28.5437. [DOI] [PubMed] [Google Scholar]
- 21.Slamon D, Eiermann W, Robert N, et al. Efficacy and Safety of Adjuvant Trastuzumab in HER-2 Positive Breast Cancer. N Engl J Med. 2010 in press. [Google Scholar]
- 22.Press M, Sauter G, Bernstein L, et al. Diagnostic Evaluation of HER-2/neu as a Molecular Therapeutic Target: Local Testing versus Centralized FISH Testing. Clinical Cancer Res. 2005;11:6598–6607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 23.Mass R, Press M, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clinical Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 24.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 25.Bofin A, Ytterhus B, Hagmar B. Topo2A and HER-2 gene amplfication in fine needle aspirates of breast carcinomas. Cytopathology. 2003;14:314–321. doi: 10.1046/j.0956-5507.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 26.Olsen K, Knudsen H, Rasmussen B, et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol. 2004;43:35–42. doi: 10.1080/02841860310019007. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 28.Peto R, Pike M, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien KR. Herceptin and the heart–a molecular modifier of cardiac failure. N Engl J Med. 2006;354:789–790. doi: 10.1056/NEJMp058315. [DOI] [PubMed] [Google Scholar]
- 30.Levine M. Trastuzumab cardiac side effects: Only time will tell. J Clin Oncol. 2005;23:7775–7776. doi: 10.1200/JCO.2005.04.1558. [DOI] [PubMed] [Google Scholar]
- 31.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 32.Dressler L, Berry D, Broadwater G, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23:4287–4297. doi: 10.1200/JCO.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Paik S, Bryant J, Tan-Chiu E, et al. HER2 and Choice of Adjuvant Chemotherapy for Invasive Breast Cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-15. J Natl Cancer Inst. 2000;92:1991–1998. doi: 10.1093/jnci/92.24.1991. [DOI] [PubMed] [Google Scholar]
- 34.Ravdin P, Green S, Albain K, et al. Initial report of the SWOG biological correlative study of c-erB-2 expression as a predictor of outcome in a trial comparing adjuvant CAF T with tamoxifen (T) alone. Proc Am Soc Clin Oncol. 1998;17:A374–97a. [Google Scholar]
- 35.Gennari A, Sormani M, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 36.O'Malley F, Chia S, Tu D, et al. Prognostic and predictive value of topoisomerase II alpha in a randomized trial comparing CMF to CEF in premenopausal women with node positive breast cancer (NCIC CTG MA. 5) J Clin Oncol. 2006;Vol 24(No. 18S June 20 Supplement):533. [Google Scholar]
- 37.O'Malley F, Chia S, Tu D, et al. Topoisomerase II alpha and Responsiveness of Breast Cancer to Adjuvant Chemotherapy. J Natl Cancer Inst. 2009;101:644–650. doi: 10.1093/jnci/djp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen K, Ejlertsen B, Moller S, et al. The value of TOP2A gene copy number variation as a biomarker in breast cancer. Update of DBCG trial 89D Acta Oncologica. 2008;47:725–734. doi: 10.1080/02841860801995396. [DOI] [PubMed] [Google Scholar]
- 39.Ejlertsen B, Jensen MB, Nielsen KV, et al. HER2, TOP2A, and TIMP-1 and responsiveness to adjuvant anthracycline-containing chemotherapy in high-risk breast cancer patients. J Clin Oncol. 2010;28:984–990. doi: 10.1200/JCO.2009.24.1166. [DOI] [PubMed] [Google Scholar]
- 40.Pritchard K, Messersmith H, Elavathil L, et al. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–744. doi: 10.1200/JCO.2007.15.4716. [DOI] [PubMed] [Google Scholar]
- 41.Coon J, Marcus E, Gupta-Burt S, et al. Amplification and overexpression of topoisomerase IIalpha predict response to anthracycline-based therapy in locally advanced breast cancer. Clinical Cancer Res. 2002;8:1061–1067. [PubMed] [Google Scholar]
- 42.Withoff S, Keith W, Knol A, et al. Selection of a subpopulation with fewer DNA topoisomerase II alpha gene copies in a doxorubicin-resistant cell line panel. Br J Cancer. 1996;74:502–507. doi: 10.1038/bjc.1996.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gudkov A, Zelnick C, Kazarov A, et al. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci U S A. 1993;90:3231–3235. doi: 10.1073/pnas.90.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Zwelling L, Kawakami Y, et al. Adenovirus-mediated human topoisomerase IIalpha gene transfer increases the sensitivity of etoposide-resistant human breast cancer cells. Cancer Res. 1999;59:4618–4624. [PubMed] [Google Scholar]
- 45.Jarvinen T, Tanner M, Rantanen V, et al. Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. American J Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Withoff S, De Jong S, De Vries E, et al. Human DNA topoisomerase II: Biochemistry and role in chemotherapy resistance. Anticancer Res. 1996;16:1867–1880. [PubMed] [Google Scholar]
- 47.Durbecq V, Di Leo A, Cardoso F, et al. Comparison of topoisomerase-II alpha gene status between primary breast cancer and corresponding distant metastatic sites. Breast Cancer Res and Treatment. 2003;77:199–204. doi: 10.1023/a:1021874224490. [DOI] [PubMed] [Google Scholar]
- 48.Jarvinen T, Tanner M, Barlund M, et al. Characterization of topoisomerase II-alpha gene amplification and deletion in breast cancer. Genes Chromosomes Cancer. 1999;26:142–150. [PubMed] [Google Scholar]
- 49.Jarvinen T, Liu E. HER-2/neu and topoisomerase IIalpha in breast cancer. Breast Cancer Res Trt. 2003;78:299–311. doi: 10.1023/a:1023077507295. [DOI] [PubMed] [Google Scholar]
- 50.Jarvinen T, Liu E. Topoisomerase IIalpha gene (TOP2A) amplification and deletion in cancer–more common than anticipated. Cytopathology. 2003;14:309–313. doi: 10.1046/j.0956-5507.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 51.Di Leo A, Gancberg D, Larsimont D, et al. HER-2 amplification and topoisomerase IIalpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clinical Cancer Res. 2002;8:1107–1116. [PubMed] [Google Scholar]
- 52.Di Leo A, Larsimont D, Gancberg D, et al. HER-2 and topo-isomerase IIalpha as predictive markers in a population of node-positive breast cancer patients randomly treated with adjuvant CMF or epirubicin plus cyclophosphamide. Ann Oncol. 2001;12:1081–1089. doi: 10.1023/a:1011669223035. [DOI] [PubMed] [Google Scholar]
- 53.Knoop A, Knudsen H, Balslev E, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23:7483–7490. doi: 10.1200/JCO.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Press M, Slamon D, Flom K, et al. Evaluation of HER-2/neu Gene amplification and overexpression: Comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol. 2002;20:3095–3105. doi: 10.1200/JCO.2002.09.094. [DOI] [PubMed] [Google Scholar]
- 55.Di Leo A, Isola J, Piette F, et al. A meta-analysis of phase III trials evaluating the predictive value of HER2 and topoisomerase II alpha in early breast cancer patients treated with CMF or anthracycline-based adjuvant therapy. Cancer Res. 2009;69(suppl) abstr 705. [Google Scholar]
- 56.Harris LN, Broadwater G, Abu-Khalaf M, et al. Topoisomerase IIα amplification does not predict benefit from dose-intense cyclophosphamide, doxorubicin, and fluorouracil therapy in HER2-amplified early breast cancer: Results of CALGB 8541/150013. J Clin Oncol. 2009;27:3430–3436. doi: 10.1200/JCO.2008.18.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tubbs R, Barlow WE, Budd GT, et al. Outcome of patients with early-stage breast cancer treated with doxorubicin-based adjuvant chemotherapy as a function of HER2 and TOP2A status. J Clin Oncol. 2009;27:3881–3886. doi: 10.1200/JCO.2008.20.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pritchard KI. Are HER2 and TOP2A useful as prognostic or predictive biomarkers for anthracycline-based adjuvant chemotherapy for breast cancer? J Clin Oncol. 2009;27:3875–3876. doi: 10.1200/JCO.2009.22.8361. [DOI] [PubMed] [Google Scholar]
- 59.Bartlett J, Munro A, Cameron D, et al. Type 1 receptor tyrosine kinase profiles identify patients with enhanced benefit from anthracyclines in the BR9601 adjuvant breast cancer chemotherapy trial. J Clin Oncol. 2008;26:5027–5035. doi: 10.1200/JCO.2007.14.6597. [DOI] [PubMed] [Google Scholar]
- 60.Bartlett JMS, Munro AF, Dunn JA, et al. Predictive markers of anthracycline benefit: A prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601) Lancet Oncol. 2010;11:266–274. doi: 10.1016/S1470-2045(10)70006-1. [DOI] [PubMed] [Google Scholar]
- 61.Levine MN. Trastuzumab cardiac side effects: Only time will tell. J Clin Oncol. 2005;23:7775–7776. doi: 10.1200/JCO.2005.04.1558. [DOI] [PubMed] [Google Scholar]
- 62.Colozza M, Bisagni G, Mosconi A, et al. Epirubicin versus CMF as adjuvant therapy for stage I and II breast cancer: A prospective randomised study. Eur J Cancer. 2002;38:2279–2288. doi: 10.1016/s0959-8049(02)00452-5. [DOI] [PubMed] [Google Scholar]
- 63.Colozza M, Sidoni A, Mosconi A, et al. HER2 overexpression as a predictive marker in a randomized trial comparing adjuvant cyclophosphamide/methotrexate/5-fluorouracil with epirubicin in patients with stage I/II breast cancer: Long-term results. Clinical Breast Cancer. 2005;6:253–259. doi: 10.3816/cbc.2005.n.028. [DOI] [PubMed] [Google Scholar]
- 64.De Placido S, Perrone F, Carlomagno C, et al. CMF vs alternating CMF/EV in the adjuvant treatment of operable breast cancer. A single centre randomised clinical trial (Naples GUN-3 study) Br J Cancer. 1995;71:1283–1287. doi: 10.1038/bjc.1995.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher B, Brown A, Dimitrov N, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 66.Fisher B, Redmond C, Wickerham D, et al. Doxorubicin-containing regimens for the treatment of stage II breast cancer: The National Surgical Adjuvant Breast and Bowel Project experience. J Clin Oncol. 1989;7:572–582. doi: 10.1200/JCO.1989.7.5.572. [DOI] [PubMed] [Google Scholar]
- 67.Levine M, Bramwell V, Pritchard K, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. J Clin Oncol. 1998;16:2651–2658. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 68.Moliterni A, Menard S, Valagussa P, et al. HER2 overexpression and doxorubicin in adjuvant chemotherapy for resectable breast cancer. J Clin Oncol. 2003;21:458–462. doi: 10.1200/JCO.2003.04.021. [DOI] [PubMed] [Google Scholar]