Abstract
Purpose
Downstaging (DS) of rectal cancers is achieved in approximately 45% of patients with neoadjuvant fluorouracil (FU) -based chemoradiotherapy (CRT). Polymorphisms in the thymidylate synthase gene (TYMS) had previously defined two risk groups associated with disparate tumor DS rates (60% v 22%). We conducted a prospective single-institution phase II study using TYMS genotyping to direct neoadjuvant CRT for patients with rectal cancer.
Patients and Methods
Patients with T3/T4, N0-2, M0-1 rectal adenocarcinoma were evaluated for germline TYMS genotyping. Patients with TYMS *2/*2, *2/*3, or *2/*4 (good risk) were treated with standard chemoradiotherapy using infusional FU at 225 mg/m2/d. Patients with TYMS *3/*3 or *3/*4 (poor risk) were treated with FU/RT plus weekly intravenous irinotecan at 50 mg/m2. The primary end point was pathologic DS. Secondary end points included complete tumor response (ypT0), toxicity, recurrence rates, and overall survival.
Results
Overall, 135 patients were enrolled, of whom 27.4% (37 of 135) were considered poor risk. The prespecified statistical goals were achieved, with DS and ypT0 rates reaching 64.4% and 20% for good-risk and 64.5% and 42% for poor-risk patients, respectively.
Conclusion
To our knowledge, this is the first study to prospectively use TYMS genotyping to direct neoadjuvant CRT in patients with rectal cancer. High rates of DS and ypT0 were achieved among both risk groups when personalized treatment was based on TYMS genotype. These results are encouraging, and further evaluation of this genotype-based strategy using a randomized study design for locally advanced rectal cancer is warranted.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer diagnosis among both sexes, with an estimated 142,570 new cases and approximately 51,370 deaths in the United States in 2010.1 Of these, 27.8% are rectal cancers. Neoadjuvant fluoropyrimidine-based chemoradiotherapy (CRT) is the standard therapy for patients with locally advanced rectal adenocarcinoma.2,3 Preoperative treatment was associated with lower risk of local recurrence and lower toxicities compared with radiotherapy (RT) alone4–6 or postoperative CRT.2,3 Preoperative chemoradiotherapy resulted in tumor T stage downstaging (DS) rates of approximately 45% (40% to 60%)7–12 and a pathologic complete response (pCR) rate of 15% to 30%.3,8–13 Pathologic DS or a pCR after preoperative CRT has been correlated with improved survival, decreased recurrence, and a higher rate of sphincter-preserving surgeries.9,14–17
Thymidylate synthase (TS) is critical in DNA synthesis and serves as the primary target of fluorouracil (FU). Its overexpression has been linked to resistance to fluoropyrimidine-based chemotherapy in numerous cancers.18–23 The TS gene (TYMS) contains a tandem repeat consisting of 28–base pair repeat units found in the 5′ untranslated region, which acts as an enhancer to the TYMS promoter (TS enhancer region [TSER]). In vitro and in vivo studies have shown that higher number of repeats (from TSER*2 to TSER*3 or higher) led to stepwise increases in TS expression24,25 and activity.26
TSER*3 homozygosity seems to be associated with a lower response to neoadjuvant FU-based CRT for patients with rectal cancer. Villafranca et al27 examined 65 patients with locally advanced rectal cancer treated with FU-based preoperative CRT. Patients with the TSER*3/*3 genotype achieved a DS rate of only 22% compared with 60% for those patients with either the TSER*2/*2 or TSER*2/*3 genotypes. Later, Spindler et al28 demonstrated that patients with the TSER*2/*2 genotype experienced a 53% pCR compared with 26% for those with TSER*2/*3 and only 17% for patients with the TSER*3/*3 variants. The negative effect of the TSER*3 allele was also observed on survival of patients with locally advanced gastric cancer treated with neoadjuvant FU- based chemotherapy.29
Thus we conducted a prospective nonrandomized single-institution tandem phase II study using TYMS genotyping to direct neoadjuvant CRT for patients with locally advanced and metastatic rectal cancer. Patients with germline TSER*2/*2 or TSER*2/*3, deemed good risk for a favorable response to FU, were treated with standard CRT. Poor-risk patients (TSER*3/*3 or TSER*3/*4 genotypes) who were unlikely to derive significant benefit from FU chemotherapy were treated with irinotecan in addition to standard FU/CRT. The primary end point of this study was to determine whether TYMS genotype-directed neoadjuvant CRT would result in greater rates of tumor DS compared with those predicted among historical controls. The secondary end points were to assess the complete pathologic response rates, toxicities, recurrence rates, and survival of both regimens.
PATIENTS AND METHODS
Eligibility
Patients 18 years or older, with biopsy-proven clinical T3/T4, N0-2, M0-1 adenocarcinoma of the rectum and a Karnofsky performance status of 60% or more were eligible. Inclusion and exclusion criteria are described in the Appendix (online only).
Study Design and Treatment
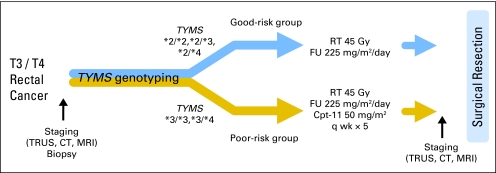
This is a single-institution, multidisciplinary, prospective, tandem, phase II nonrandomized study using TYMS genotyping to direct neoadjuvant CRT for patients with rectal cancer (Fig 1). Before treatment, clinical staging was performed, blood samples were obtained, and TSER polymorphisms were evaluated using a previously described polymerase chain reaction–based assay.30 Patients carrying at least one *2 allele (TSER*2/*2, *2/*3, or *2/*4) were assigned to the good-risk genotype group (study 1) and treated with standard preoperative CRT. Radiotherapy consisted of a total of 45 to 50.4 Gy delivered in 25 to 28 fractions (1.80 to 2.0 Gy per fraction) by a multiple-field technique using image-guided radiotherapy with radiotherapy target volume consistent with the Radiation Therapy Oncology Group consensus guidelines.31 The administration of additional boost radiation for a total of 50.4 Gy was done at the discretion of the treating radiation oncologist. Concurrent continuous intravenous infusion of FU at a dose of 225 mg/m2/d was administered throughout radiation with no weekend breaks. Patients with TSER*3/*3 or TSER*3/*4 were assigned to the poor-risk genotype group (study 2) and treated with weekly intravenous irinotecan at 50 mg/m2 for 5 weeks in addition to standard CRT identical to the treatment in the good-risk group. Clinical restaging and resection of the primary rectal lesion were performed 6 to 10 weeks after completion of preoperative CRT. Additional therapy, whether adjuvant or for metastatic disease, were administered at the discretion of the treating physician.
Fig 1.
TYMS genotype-directed neoadjuvant chemoradiotherapy study outline. RT, radiotherapy; FU, fluorouracil; Cpt-11, irinotecan; TRUS, transrectal ultrasound; CT, computed tomography; MRI, magnetic resonance imaging.
Assessment of Efficacy and Toxicity
Baseline clinical tumor staging using rigid proctoscopy, transrectal ultrasound (TRUS), spiral computed tomography (CT), or magnetic resonance imaging (MRI) were performed within 28 days of enrollment. During CRT, weekly physical examination, toxicity assessment, CBC, and comprehensive metabolic panel were done. Clinical restaging with TRUS, CT, or MRI was repeated before resection. The surgical procedure performed was at the discretion of the treating surgical oncologist. Standardized institutional pathology examinations based on Westra et al32 were done, and the pathologic staging, as well as extent of residual tumor in the resected specimen, was classified using the American Joint Committee on Cancer version 6 criteria. Tumor DS was defined as a decrease in the T stage of the primary tumor by at least 1. Complete tumor response was defined as the absence of any viable tumor in the rectum (ypT0). pCR was defined as the absence of any viable tumor in the rectum or in the perirectal lymph nodes (ypT0N0). Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 2.0.
Dose adjustments (described in the Appendix, online only) were made as per a study-defined dose modification table depending on the type and severity of toxicities associated with study treatment.
Statistical Analyses
The primary end point was the rates of tumor DS among good-risk and poor-risk groups. Secondary end points include ypT0 rate, toxicity, overall survival (OS, and relapse-free survival (RFS). The two-stage study design proposed by Simon33 was used for sample size calculations for both groups: good-risk genotype (study 1) and poor-risk genotype (study 2). On the basis of both local and literature data, the DS rate for the general T3/T4 population was set at 45% with conventional therapy,7,8,10,12 and the predicted DS rate for good-risk TYMS was 60%.27 Study 1 required a sample size of 77 good-risk patients to have an 80% power at a significance level of .05 to reject a DS rate of less than 45% in favor of a DS rate of ≥ 60%. Study 2 assumed that the DS rate is 22% for poor-risk genotype patients with conventional therapy.27 A sample size of 31 patients was necessary to reject a DS rate of 22% in favor of a DS rate of ≥ 45% with a power of 80% power at a significance level of .05. Baseline characteristics between good- and poor-risk groups have been compared with a t test for age and with Fisher's exact tests for all other characteristics. The proportion of patients with DS within each risk group was compared with historical rates by using one sample binomial tests. OS and RFS were analyzed using Kaplan-Meier models. OS or RFS by presence/absence of downstaging were compared with log-rank tests generated by Kaplan-Meier models.
RESULTS
Patient Characteristics
Patient baseline characteristics are listed in Table 1. Between February 2003 and July 2008, 135 patients with rectal cancer were enrolled onto the trial; 98 patients (72.6%) had good-risk genotypes (TSER *2/*2, *2/*3, or *2/*4), whereas 37 patients (27.4%) had poor-risk genotypes (TSER *3/*3, *3/*4). Clinical staging was performed using physical findings plus TRUS (60%), CT (20%), and MRI (20%) of patients. There was no difference in baseline characteristics between the good-risk and poor-risk groups. Fourteen percent of patients had metastatic disease on enrollment. Four patients, two from each group, withdrew consent before any study treatment and were excluded from analysis of the primary and secondary end points.
Table 1.
Baseline Patient and Tumor Characteristics (n = 135)
| Characteristic | All Patients |
Good Risk |
Poor Risk |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| No. of patients | 135 | 100 | 98 | 72.6 | 37 | 27.4 | |
| TSERgenotype | |||||||
| *2/ *2 | 26 | 26.5 | |||||
| *2/ *3 | 71 | 72.5 | |||||
| *2/ *4 | 1 | 1.0 | |||||
| *3/ *3 | 35 | 94.6 | |||||
| *3/ *4 | 2 | 5.4 | |||||
| Age, years | |||||||
| Median | 56 | 55 | 59 | .64 | |||
| Range | 26-85 | 32-85 | 26-77 | ||||
| Race/ethnicity | |||||||
| White | 115 | 85.2 | 86 | 87.8 | 29 | 78.4 | |
| African American | 18 | 13.3 | 10 | 10.2 | 8 | 21.6 | |
| Hispanic | 1 | 0.7 | 1 | 1.0 | — | ||
| Asian | 1 | 0.7 | 1 | 1.0 | — | .18* | |
| Sex | |||||||
| Male | 93 | 68.9 | 69 | 70.4 | 24 | 64.9 | |
| Female | 42 | 31.1 | 29 | 29.6 | 13 | 35.1 | .54 |
| ECOG performance status | |||||||
| 0 | 89 | 65.9 | 63 | 64.3 | 26 | 70.3 | |
| 1 | 45 | 33.3 | 34 | 34.7 | 11 | 29.7 | |
| 2 | 1 | 0.7 | 1 | 1.0 | 0 | .55† | |
| Baseline stage | |||||||
| Stage IIA (T3, N0, M0) | 34 | 25.2 | 24 | 24.5 | 10 | 27.0 | |
| Stage IIB (T4, N0, M0) | 3 | 2.2 | 3 | 3.1 | 0 | ||
| Stage IIIA (T1-2, N1, M0) | 1 | 0.7 | 1 | 1.0 | 0 | ||
| Stage IIIB (T3-4, N1, M0) | 72 | 53.3 | 53 | 54.1 | 19 | 51.4 | |
| Stage IIIC (T-any, N2, M0) | 6 | 4.4 | 3 | 3.1 | 3 | 8.1 | |
| Stage IV (T-any, N-any, M1) | 19 | 14.1 | 14 | 14.3 | 5 | 13.5 | .66‡ |
| Baseline clinical T stage | |||||||
| T1-2 | 2 | 1.5 | 2 | 2.0 | 0 | ||
| T3 | 109 | 80.7 | 77 | 78.6 | 32 | 86.5 | |
| T4 | 24 | 17.8 | 19 | 19.4 | 5 | 13.5 | .64 |
| Baseline clinical N stage | |||||||
| N0 | 41 | 30.4 | 30 | 30.6 | 11 | 29.8 | |
| N1 | 86 | 63.7 | 64 | 65.3 | 22 | 59.5 | |
| N2 | 8 | 5.9 | 4 | 4.1 | 4 | 10.8 | .36 |
| Baseline clinical M stage | |||||||
| M0 | 116 | 85.9 | 84 | 85.7 | 32 | 86.5 | |
| M1 | 19 | 14.1 | 14 | 14.3 | 5 | 13.5 | .99 |
| Clinical staging modality | |||||||
| EUS | 81 | 60 | 60 | 61.2 | 21 | 56.8 | |
| CT ± PET | 28 | 20.7 | 22 | 22.5 | 6 | 16.2 | |
| MRI | 26 | 19.3 | 16 | 16.3 | 10 | 27.1 | .35 |
| Tumor distance from anal verge, cm | |||||||
| < 5 | 45 | 33.3 | 33 | 33.7 | 12 | 32.4 | |
| 5-10 | 78 | 57.8 | 57 | 58.2 | 21 | 56.8 | |
| > 10 | 12 | 8.9 | 8 | 8.2 | 4 | 10.8 | .91 |
| Tumor grade | |||||||
| Well differentiated | 12 | 8.9 | 8 | 8.2 | 4 | 10.8 | |
| Moderately differentiated | 93 | 68.9 | 58 | 69.4 | 25 | 67.6 | |
| Poorly differentiated | 24 | 17.8 | 17 | 17.3 | 7 | 19.9 | |
| Not reported | 6 | 4.4 | 5 | 5.1 | 1 | 2.7 | .95 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EUS, endoscopic ultrasound; CT, computed tomography; PET, positron emission tomography; MRI, magnetic resonance imaging.
Race is compared in two categories, white versus other.
ECOG performance status is compared in two categories, 0 versus 1 or 2.
Baseline stage is compared in four categories: IIA or IIB versus IIIA or IIIB versus IIIC versus IV.
Treatment
Ninety-six of the 98 good-risk patients received standard CRT using infusional FU (225 mg/m2 per day) throughout radiation. Two patients withdrew consent and were not treated.
Dose delays and dose reduction of FU occurred in 19 (20%) of 96 patients treated, mostly secondary to mucositis or enteritis. Thirty-four of the 37 poor-risk patients received weekly irinotecan with standard FU and radiation. Two patients were not treated because of consent withdrawal. One patient received only standard FU chemoradiotherapy without irinotecan because of physician error. A total of 149 doses of irinotecan were given (mean, four doses/patient; range, zero to six doses). Chemotherapy dose delays and dose reduction secondary to toxicities occurred in 19 (51.4%) of 37 patients treated. Four patients (two in each group) did not receive the full intended course of radiation.
Toxicities
Two deaths occurred on protocol: one patient in the good-risk group died as a result of a myocardial infarction, and another patient in the poor-risk group died as a result of an aneurysmal bleed. As shown in Table 2, 131 patients were evaluable for toxicity. Among the 96 evaluable patients in the good-risk arm, hospitalization rates were 16% as compared with 34% among the 35 evaluable poor-risk patients. In the poor-risk group, 19 (54.3%) of the 35 patients experienced grade 3 or 4 toxicities compared with 30.2% in the good-risk group. The incidence of grade 3 or greater diarrhea was higher in the poor-risk group (45.7%) treated with irinotecan-based chemoradiotherapy as compared with good-risk patients treated with FU and radiation alone (17.7%). Regarding the immediate postoperative toxicities, nine (9.9%) of the 91 patients who underwent resection in the good-risk group developed the following complications: abscess (n = 4), anastomotic leak (n = 3), and fistula formation (n = 2). Among the 31 patients treated with irinotecan-based chemoradiotherapy in the poor-risk group, four (12.9%) had abscess formation.
Table 2.
Grade 3 to 4 Toxicities and Hospitalizations Related to Treatment and Surgery (occurring within 30 days of surgery)
| Toxicity | All Patients |
Good Risk |
Poor Risk |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 135 | 98 | 37 | |||
| Evaluable patients | 131 | 96 | 35 | |||
| Death on protocol | 2 | 1 (myocardial infarction) | 1 (aneurysmal bleed) | |||
| Hospitalization | ||||||
| Diarrhea/RT enteritis | 14 | 10.7 | 7 | 7 | ||
| Perforation/abscess/leak fistula | 4 | 3.1 | 2 | 2 | ||
| Pneumonia | 1 | 0.8 | 0 | 1 | ||
| Febrile neutropenia | 1 | 0.8 | 0 | 1 | ||
| Aneurysmal bleed | 1 | 0.8 | 0 | 1 | ||
| Atrial fibrillation | 1 | 0.8 | 1 | 0 | ||
| Myocardial infarction | 1 | 0.8 | 1 | 0 | ||
| Gastrointestinal bleed | 1 | 0.8 | 1 | 0 | ||
| Anemia-blood transfusion | 1 | 0.8 | 1 | 0 | ||
| Hypoglycemia | 1 | 0.8 | 1 | 0 | ||
| Hernia repair | 1 | 0.8 | 1 | 0 | ||
| Crohn's flare | 1 | 0.8 | 1 | 0 | ||
| Total | 28 | 21.4 | 16/96 | 16.7 | 12/35 | 34.3 |
| Grade 3-4 toxicities | ||||||
| Nausea | 1 | 1.0 | 1 | 2.9 | ||
| Vomiting | 1 | 1.0 | 1 | 2.9 | ||
| Diarrhea | 33 | 25.2 | 17 | 17.7 | 16 | 45.7 |
| Dehydration | 10 | 7.0 | 3 | 3.2 | 7 | 20.0 |
| Mucositis | 5 | 3.9 | 4 | 4.2 | 1 | 2.9 |
| GI bleed | 2 | 1.6 | 2 | 2.1 | ||
| Ileitis | 1 | 1.0 | 1 | 2.9 | ||
| Enteritis | 1 | 1.0 | 1 | 1.0 | ||
| Dyspnea | 1 | 0.8 | 1 | 1.0 | ||
| Neutropenia | 1 | 1.0 | 1 | 2.9 | ||
| Anemia | 6 | 4.6 | 3 | 3.1 | 3 | 8.8 |
| Pain | 7 | 5.3 | 3 | 3.1 | 4 | 11.4 |
| Perforation | 3 | 2.3 | 2 | 2.1 | 1 | 2.9 |
| Pelvic abscess | 2 | 1.6 | 2 | 5.7 | ||
| PPE | 1 | 1.0 | 1 | 2.9 | ||
| Crohn's flare | 1 | 1.0 | 1 | 1.0 | ||
| Syncope | 2 | 1.6 | 2 | 2.1 | ||
| Rash | 2 | 1.6 | 2 | 2.1 | ||
| Fatigue | 1 | 1.0 | 1 | 1.0 | ||
| Atrial fibrillation | 1 | 1.0 | 1 | 1.0 | ||
| Infection | 2 | 1.6 | 1 | 1.0 | 1 | 2.9 |
| Headache | 1 | 1.0 | 1 | 1.0 | ||
| Small bowel obstruction | 1 | 1.0 | 1 | 1.0 | ||
Abbreviations: RT, radiotherapy; PPE, palmar-plantar erythrodysesthesia.
Surgery
Ninety-one (93%) of the 98 patients in the good-risk group and 32 (86%) of the 37 patients in the poor-risk group underwent surgery, with a mean resection rate of 91% for the whole group. Surgery procedures are presented in Table 3. The median time from completion of chemoradiotherapy to surgery was 57 days (range, 9 to 187 days), with no difference between the good-risk group (57 days) and the poor-risk group (59 days). One patient had a delayed surgery (187 days after the end of chemotherapy) because of morbid obesity. Among patients who underwent resection, sphincter-saving surgery was performed in 71 (78%) of 91 patients in the good-risk group and in 22 (69%) of 32 patients in the poor-risk group.
Table 3.
Surgery Procedures and Tumor Downstaging
| Surgery and Stage | Good Risk |
Poor Risk |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 98 | 37 | ||
| Type of surgery | ||||
| Lower anterior resection | 71* | 72.5 | 22† | 59.5 |
| Abdominoperineal resection | 15 | 15.3 | 9 | 24.3 |
| Total proctocolectomy | 4 | 4.1 | 1 | 2.7 |
| Pelvic exenteration | 1 | 1.0 | 0 | |
| Treated but no surgery | 5‡ | 5.1 | 3§ | 8.1 |
| Consent withdrawal or insurance denial | 2 | 2.0 | 2 | 5.4 |
| No. of patients with positive margins | 4 of 91 | 4.4 | 4 of 32 | 12.5 |
| Evaluable patients for downstaging | 90 | 31 | ||
| Nonevaluable patients for downstaging | 8 | 6 | ||
| Death prior to surgery | 1 | 1 | ||
| Clinical CR, no surgery | 1 | 1 | ||
| Refused surgery | 1 | 1 | ||
| Not resectable | 2 | 0 | ||
| Consent withdrawal or insurance denial | 2 | 2 | ||
| Delayed surgery and given FOLFOX before surgery | 1 (pCR) | 0 | ||
| Not given irinotecan by treating physician | NA | 1 | ||
| Post-treatment stage for evaluable patients | 90 | 31 | ||
| Stage 0 | 16 | 18.7 | 9 | 29.0 |
| Stage I (T1-T2 N0 M0) | 27 | 30.0 | 7 | 22.6 |
| Stage II (T3-T4 N0M0) | 12 | 13.3 | 5 | 16.1 |
| Stage III (T-any N1-2 M0) | 22 | 24.4 | 6 | 19.4 |
| Stage IV (T-any N-any M1) | 13 | 14.4 | 4 | 12.9 |
| T stage | ||||
| T0 | 18 | 20.0 | 13 | 41.9 |
| T1-2 | 34 | 37.8 | 7 | 22.6 |
| T3-4 | 38 | 42.2 | 11 | 35.5 |
| N stage | ||||
| N0 | 61 | 67.8 | 24 | 77.4 |
| N1 | 20 | 22.2 | 5 | 16.1 |
| N2 | 9 | 10.0 | 2 | 6.5 |
| T-stage downstaging | 58 | 64.4 | 20 | 64.5 |
| ypT0 | 18 | 20.0 | 13 | 41.9 |
| Pathologic complete response (ypT0N0) | 17 | 18.9 | 11 | 35.5 |
Abbreviations: CR, complete response; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; pCR, pathologic complete response; NA, not applicable.
One patient had delayed surgery as a result of morbid obesity and received FOLFOX and had a complete pathologic response.
One patient who underwent lower anterior resection was not evaluable as a result of not receiving irinotecan.
One patient died as a result of myocardial infarction before surgery, one patient had a clinical complete response and did not undergo surgery, one patient refused surgery, and two patients were found to have unresectable disease during exploratory laparotomy.
One patient died before surgery, one patient had clinical complete response and did not undergo surgery, and one patient refused surgery.
Response to Neoadjuvant Chemoradiotherapy
Response to neoadjuvant CRT, including DS results, are presented in Table 3. Among the 98 patients with good-risk genotype enrolled, eight were nonevaluable for response (Table 3). Of the 90 evaluable patients, 58 (64.4%) had T-stage DS (95% CI, 54.1% to 74.6%; P = .0001). Rates for ypT0 and pCR were 20% and 18.9%. Within the good-risk group, patients with TSER *2/*2 experienced similar DS and ypT0 rates (64% and 20%) compared with those with TSER *2/*3 or *2/*4 (64% and 20%). The nonevaluable good-risk patient who had four doses of oxaliplatin-based chemotherapy before surgery had a pCR (ypT0N0).
Among the 37 patients with poor-risk genotype, six were nonevaluable for response (Table 3). For the 31 evaluable patients, T-stage DS was achieved in 20 patients (64.5%; 95% CI, 43.7% to 78.9%; P < .0001). Rates for ypT0 and pCR were 41.9% and 35.5%. The nonevaluable poor-risk patient who did not receive irinotecan did not have tumor DS. Post-treatment pathologic stage is shown in Table A1 (Appendix, online only).
Recurrence and Survival
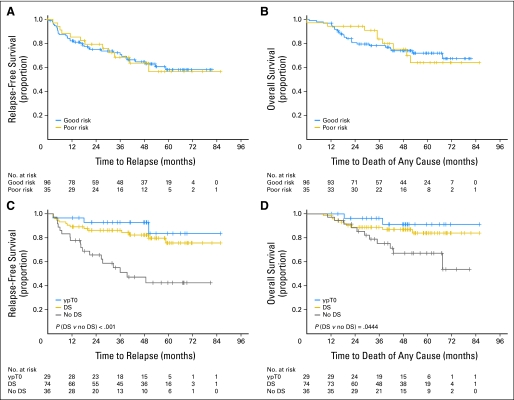
RFS and OS data were monitored as secondary objectives. After a median follow-up period of 45 months, 97 (74%) of the evaluable 131 patients remain alive. RFS and OS plots for both good- and poor-risk groups are shown in Figures 2A and 2B.
Fig 2.
Kaplan-Meier curves showing (A, C) relapse-free survival and (B, D) overall survival by groups (A, B) and according to the existence of a pathologic complete response (ypT0), a tumor downstaging (DS; including patients with ypT0), or no DS (C, D). A and B include 131 patients; C and D correspond to patients with nonmetastatic disease only (n = 110).
Among the 96 evaluable good-risk patients, 14 had metastatic rectal cancer at the time of enrollment. Median survival for all 96 patients has not yet been reached. One-year, 2-year, and 3-year OS were 96.9%, 80.6%, and 78.2%, respectively. Among the 82 initially nonmetastatic patients (stages II and III), 17 patients have died and 22 patients have experienced recurrence. One-year, 2-year, and 3-year OS rates were 97.6%, 84.8%, and 82%, respectively. One-year, 2-year, and 3-year RFS rates were 85.2%, 78.3%, and 73.4%, respectively. Among the patients who experienced recurrence, only one patient (4.5%) had a local recurrence, and 21 patients (95.5%) had distant recurrence (mainly lung and liver metastasis) in the good-risk group.
Among the 35 evaluable poor-risk patients, three patients had stage IV disease before surgery. Median survival for all poor-risk patients has not been reached. One-year, 2-year, and 3-year OS rates were 94.3%, 94.3%, and 83.6%, respectively. For the 32 patients with no metastatic disease at the time of enrollment, 1-, 2- and 3-year RFS and OS are as follows: 87% and 93.8%, 80.5% and 93.8%, and 72.4% and 81.8%, respectively. Regarding the recurrences, one patient (10%) had a local recurrence, and nine patients (90%) had distant recurrence (mainly lung and liver metastasis) in the poor-risk group.
Regardless of genotype risk group, patients who achieved any downstaging, including those with ypT0, had significantly improved RFS and OS as compared with patients with no DS. Those who achieved ypT0 have the best outcomes. The differences in RFS and OS observed between patients with or without DS were statistically significant in all patients (P = .0003 and P = .0185 respectively, data not shown) and in nonmetastatic patients (P = .0005 and P = .0444; Figs 2C and 2D).
DISCUSSION
This genotype-driven study demonstrated that the prospective use of pharmacogenetic information to individualize cancer therapy is feasible. Prior studies demonstrated that the DS rates for unselected patients with rectal cancer treated with CRT was 45% (range, 40% to 60%), with a pCR rate of 8% to 14%.2,7,8 By selecting a population likely to respond to standard CRT using TYMS genotyping, DS and ypT0 rates among patients with germline TSER *2/*2 or TSER *2/*3 were 64.4% and 20%, respectively. The DS rate was significantly better than the predicted DS rate of 45% (P = .0001) and also higher than the 60% rate observed by Villafranca et al27 in that particular subset of patients. The 18.9% pCR rate is higher than that reported by Sauer et al2 (8%) and the National Surgical Adjuvant Breast and Bowel Project R-03 study (15%)3 using only fluoropyrimidine with RT (Table 4). Thirty percent of patients experienced grade 3 to 4 toxicities, also comparable to that reported by Sauer et al2 for preoperative FU-based CRT.2
Table 4.
Overview of Preoperative Chemoradiation Studies Using FU and Irinotecan
| First Author/ Study | No. of Patients | RT (Gy) | FU | Irinotecan | pCR (%) | DS (%) | Grade 3 to 4 Overall Toxicity |
|---|---|---|---|---|---|---|---|
| FU-based neoadjuvant CRT | |||||||
| Sauer2 | 421 | 50.4 | PVI 1,000 mg/m2/d 5 days a week × 5 | 8 | NA | 27 | |
| Bosset4,34 | 506 | 45 | 350 mg/m2 + LV 20 mg/m2, days 1-5 and 29-33 | 14 | 57 | 13 | |
| Gerard6 | 375 | 45 | 350 mg/m2 + LV 20 mg/m2, days 1-5 and 29-33 | 11 | NA | 15 | |
| Brændengen5 | 98 | 50 | 400 mg/m2 bolus + LV 100 mg, days 1-2, 21-22, 35-36 | 16 | NA | 29 | |
| Aschele35 | 379 | 50.4 | PVI 225 mg/m2/d 5 d a wk | 16 | NA | 8 | |
| Roh3 NSABP R-03 | 130 | 50.4 | 500 mg/m2 + LV 20 mg/m2 once per week × 6 | 17 | NA | 23 | |
| Good-risk group of present study | 90 | 45 | PVI 225 mg/m2/d 5 days a week | 19 | 64 | 30 | |
| FU + irinotecan-based neoadjuvant CRT | |||||||
| Mehta36 | 32 | 50.4 | PVI 200 mg/m2/d , days 1-33 | 50 mg/m2 weekly × 4 | 37 | 71 | 28* |
| Klautke37 | 37 | 50.4 | PVI 250 mg/m2, days 1-43 | 40 mg/m2 weekly × 6 | 22 | 76 | 32* |
| Navarro38 | 74 | 45 | PVI 225 mg/m2/d, 5 days a week | 50 mg/m2 weekly × 5 | 14 | 49 | 14* |
| Mohiuddin39 | 106 | Arm 1: hyperfractionated RT 55.2 to 60 Gy at 1.2 Gy twice a day | Arm 1: PVI 225 mg/m2/d, 7 days a week | 28 | 78 | 28* | |
| Arm 2: radiation therapy 50.4 to 54 Gy at 1.8 Gy per day | Arm 2: PVI 225 mg/m2/d 5 days a week | Arm 2: 50 mg/m2 weekly × 4 | 28 | 78 | 37* | ||
| Glynne-Jones40 | 57 | 45 | 350 mg/m2 + LV 20 mg/m2, days 1-5 and 29-33 | Dose escalation: 6 to 20 mg/m2 | 21 | 41 | 12* |
| Iles41 | 31 | 45 | PVI: FU 200 mg/m2, daily over 5 weeks | 60 mg/m2 weekly × 4 | 29 | 79 | 13* |
| Poor-risk group of present study | 37 | 45 | PVI 225 mg/m2/d 5 days a week | 50 mg/m2 weekly × 5 | 36 | 65 | 46* |
Abbreviations: FU, fluorouracil; RT, radiotherapy; pCR, pathologic complete response; DS, downstaging; CRT, chemoradiotherapy; NA, not available; PVI, protracted venous infusion; LV, leucovorin; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Grade 3 to 4 diarrhea.
On the basis of previous published results,27 we hypothesized that patients homozygous for the TSER*3 allele would only have a tumor DS rate of 22% with standard neoadjuvant FU-based CRT. With chemotherapy intensification using weekly irinotecan added to standard CRT in this study, the downstaging rate for poor-risk patients was significantly better than expected at 64.5% (P < .0001). pCR and ypT0 were achieved in 35.5% and 42% of patients, respectively. However, higher rates of grade 3 to 4 toxicities (54.3%) were observed with the addition of irinotecan to CRT, with 34% of patients requiring hospitalization during treatment.
Preclinical and clinical evidence suggests that the relationship between TSER*3 allele and FU response is due to a transcriptional effect that leads to a higher amount of TS protein responsible for FU resistance.24–26 However, recent studies42,43 conducted in rectal cancer showed that high levels of TS in the tumor was associated with a better tumor response. The small number of patients included in these studies and the variety of drugs used in their treatment (FU/oxaliplatin and capecitabine/oxaliplatin) may contribute to the discrepancy with our results.
Several studies evaluating the addition of irinotecan at doses ranging from 40 to 60 mg/m2 with FU/CRT have also reported high rates of DS and pCR rates37–41(Table 4). The Radiation Therapy Oncology Group trial 001239 reported a DS rate of 78% and a ypCR rate of 28% among 53 patients treated with the chemotherapy intensification arm using weekly irinotecan plus infusional FU-based CRT. However, similar to our results, the addition of irinotecan also was associated with high-dose delay rates (45%), enhanced acute hematologic and nonhematologic grade 3 to 4 toxicities (12% and 45%, respectively), and low rates of late toxicities.
A rational strategy is to select patients who require chemotherapy intensification to achieve DS while sparing those who would otherwise achieve good responses to standard CRT from the greater toxicities associated with this approach. As a result of genotype-directed individualized treatment, poor-risk patients achieve the same DS, 3-year RFS, and OS as good-risk patients. Compared with the 41% 3-year DFS for poor-risk patients treated with standard CRT reported by Villafranca et al,27 poor-risk patients treated with irinotecan chemotherapy-intensified CRT achieved a 72.4% 3-year RFS. Moreover, consistent with other studies reporting better survival associated with DS,44–46 those with tumor DS had better OS and DFS as compared with those with no DS. Enrichment of the population predicted to respond well to standard CRT also explains the significantly better-than-predicted DS rates for good-risk patients. These favorable outcomes were achieved without exposing these good-risk patients to undue toxicities associated with chemotherapy intensification, which constituted more than 70% of our study patients. In this study, the chemotherapy intensification agent added to neoadjuvant therapy for poor-risk patients was irinotecan. The benefit of irinotecan-based CRT in good-risk patients was not assessed in our study. It is unclear whether there is an association between TYMS genotype and sensitivity or toxicity to irinotecan. Alternative neoadjuvant regimens using oxaliplatin in combination with FU or capecitabine did not seem to result in any significant benefit compared with FU-based CRT alone in genotype-unselected patients.35,47 Whether the addition of oxaliplatin or a biologic agent such as bevacizumab or cetuximab to standard CRT will improve responses and outcomes for either risk group is unclear. These strategies need to be evaluated in future studies.
There have been few practice-changing studies of biomarkers in oncology. Recently introduced predictive markers have relied on retrospective trials in which there is dramatic discernment of clinical outcome (ie, KRAS mutations). Although the positive results of the present study are intriguing, a prospective randomized trial in which patients in each genotype are treated with FU/RT or FU/RT plus irinotecan should be undertaken to validate the use of TYMS genotyping to direct treatment selection in the clinical setting.
Appendix
Inclusion and exclusion criteria.
Patients with metastatic disease whose primary tumors were deemed resectable were also eligible. Patients who qualified had adequate hematologic (absolute neutrophil count 1,500/μL, platelets count ≥100,000/μL), renal (creatinine ≤ 2.0 mg/dL), and hepatic functions with total bilirubin ≤ 2.0 mg/dL and AST and alkaline phosphatase ≤ 2× the upper limit of normal. Exclusion criteria included prior pelvic radiation, prior malignancies in the past 5 years except for skin cancer and in-situ cervical cancers, and known allergy to fluorouracil (FU) or irinotecan. This study was approved by the institutional review board at Washington University School of Medicine, and informed consent was obtained from all participants before enrollment.
Dose adjustments.
For patients who develop grade 3 or higher treatment-related toxicities during neoadjuvant chemoradiation, radiation was interrupted and infusional FU and irinotecan doses were held until toxicities resolve to grade 1 or less. On recovery, FU was reduced by 25%, and the irinotecan dose was reduced to 40 mg/m2 for patients who experienced grade 3 toxicities. FU and irinotecan doses were reduced by 50% for those who experienced grade 4 toxicities. Additional dose modifications were allowed for further grade 3 to 4 toxicities. However, should grade 3 or higher toxicities persist for more than 2 weeks, chemotherapy was discontinued. No dose escalation was allowed after dose modification. All supportive therapy for optimal medical care was given at the discretion of the treating physician; however, routine prophylactic use of granulocyte colony-stimulating growth factors was not allowed.
Table A1.
Post-CRT Pathologic TN Staging Versus Initial Pre-CRT Clinical TN Staging for Evaluable Patients Who Underwent Surgery, Including Those With Metastatic Disease (n = 121)
| Pre-CRT Clinical TN Stage | Post-CRT Pathologic TN stage (No. of patients) |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0N0 | T1-2, N0 | T3N0 | T4N0 | T0N1 | T1-2, N1-2 | T3, N1-2 | T4, N1-2 | ||
| Good risk | |||||||||
| T3N0 | 8 | 9 | 3 | 1 | 0 | 2 | 1 | 0 | |
| T4N0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | |
| T2N1-2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T3N1-2 | 8 | 12 | 5 | 1 | 1 | 3 | 17 | 1 | |
| T4N1-2 | 1 | 4 | 3 | 1 | 0 | 0 | 4 | 0 | |
| Total | 17 | 29 | 12 | 3 | 1 | 5 | 22 | 1 | 90 |
| Poor risk | |||||||||
| T3N0 | 3 | 3 | 2 | 0 | 1 | 0 | 0 | 0 | |
| T4N0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T2N1-2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T3N1-2 | 6 | 3 | 3 | 0 | 1 | 0 | 5 | 0 | |
| T4N1-2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Total | 11 | 7 | 5 | 1 | 2 | 0 | 5 | 0 | 31 |
Abbreviation: CRT, chemoradiation.
Footnotes
Supported in part by the National Institutes of Health Pharmacogenetics Research Network (Grant No. U01 GM63340), the Siteman Comprehensive Cancer Center, and National Cancer Institute Cancer Center Support Grant No. P30 CA091842. F.T. was supported by a grant from the Fondation de France.
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00682786.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Howard L. McLeod, Myriad Genetics (C), Medco Health Solutions (C), Gentris (C) Stock Ownership: None Honoraria: None Research Funding: Howard L. McLeod, Myriad Genetics Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin R. Tan, Barbara Zehnbauer, Kathryn Trinkaus, James W. Fleshman, Howard L. McLeod
Financial support: James W. Fleshman, Howard L. McLeod
Administrative support: Howard L. McLeod
Provision of study materials or patients: Benjamin R. Tan, Robert J. Myerson, Barbara Zehnbauer, Robert S. Malyapa, Matthew G. Mutch, Elliot E. Abbey, James W. Fleshman
Collection and assembly of data: Benjamin R. Tan, Robert J. Myerson, Barbara Zehnbauer, Robert S. Malyapa, Matthew G. Mutch, Elliot E. Abbey, Amer Alyasiry
Data analysis and interpretation: Benjamin R. Tan, Fabienne Thomas, Kathryn Trinkaus, Howard L. McLeod
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–3694. doi: 10.1200/JCO.2007.15.3858. [DOI] [PubMed] [Google Scholar]
- 6.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 7.Crane CH, Skibber JM, Birnbaum EH, et al. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2003;57:84–89. doi: 10.1016/s0360-3016(03)00532-7. [DOI] [PubMed] [Google Scholar]
- 8.Read TE, McNevin MS, Gross EK, et al. Neoadjuvant therapy for adenocarcinoma of the rectum: Tumor response and acute toxicity. Dis Colon Rectum. 2001;44:513–522. doi: 10.1007/BF02234323. [DOI] [PubMed] [Google Scholar]
- 9.García-Aguilar J, Hernandez de Anda E, Sirivongs P, et al. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 10.Onaitis MW, Noone RB, Hartwig M, et al. Neoadjuvant chemoradiation for rectal cancer: Analysis of clinical outcomes from a 13-year institutional experience. Ann Surg. 2001;233:778–785. doi: 10.1097/00000658-200106000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitz FR, Giacco GG, Hess K, et al. p53 immunohistochemical staining predicts residual disease after chemoradiation in patients with high-risk rectal cancer. Clin Cancer Res. 1997;3:1685–1690. [PubMed] [Google Scholar]
- 12.Tjandra JJ, Reading DM, McLachlan SA, et al. Phase II clinical trial of preoperative combined chemoradiation for T3 and T4 resectable rectal cancer: Preliminary results. Dis Colon Rectum. 2001;44:1113–1122. doi: 10.1007/BF02234631. [DOI] [PubMed] [Google Scholar]
- 13.Mohiuddin M, Mohiuddin MM, Marks J, et al. Future directions in neoadjuvant therapy of rectal cancer: Maximizing pathological complete response rates. Cancer Treat Rev. 2009;35:547–552. doi: 10.1016/j.ctrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Crane CH, Skibber JM, Feig BW, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517–524. doi: 10.1002/cncr.11075. [DOI] [PubMed] [Google Scholar]
- 15.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 16.Theodoropoulos G, Wise WE, Padmanabhan A, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. doi: 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 17.Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 18.Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994;12:2640–2647. doi: 10.1200/JCO.1994.12.12.2640. [DOI] [PubMed] [Google Scholar]
- 19.Johnston PG, Lenz HJ, Leichman CG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 20.Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 21.Lenz HJ, Danenberg KD, Leichman CG, et al. p53 and thymidylate synthase expression in untreated stage II colon cancer: Associations with recurrence, survival, and site. Clin Cancer Res. 1998;4:1227–1234. [PubMed] [Google Scholar]
- 22.Lenz HJ, Leichman CG, Danenberg KD, et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: A predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–182. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 23.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 24.Horie N, Aiba H, Oguro K, et al. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191–197. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 25.Pullarkat ST, Stoehlmacher J, Ghaderi V, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001;1:65–70. doi: 10.1038/sj.tpj.6500012. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami K, Omura K, Kanehira E, et al. Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res. 1999;19:3249–3252. [PubMed] [Google Scholar]
- 27.Villafranca E, Okruzhnov Y, Dominguez MA, et al. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19:1779–1786. doi: 10.1200/JCO.2001.19.6.1779. [DOI] [PubMed] [Google Scholar]
- 28.Spindler KL, Nielsen JN, Lindebjerg J, et al. Germline polymorphisms may act as predictors of response to preoperative chemoradiation in locally advanced T3 rectal tumors. Dis Colon Rectum. 2007;50:1363–1369. doi: 10.1007/s10350-007-0264-z. [DOI] [PubMed] [Google Scholar]
- 29.Ott K, Vogelsang H, Marton N, et al. The thymidylate synthase tandem repeat promoter polymorphism: A predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer. 2006;119:2885–2894. doi: 10.1002/ijc.22235. [DOI] [PubMed] [Google Scholar]
- 30.Marsh S, Collie-Duguid ES, Li T, et al. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999;58:310–312. doi: 10.1006/geno.1999.5833. [DOI] [PubMed] [Google Scholar]
- 31.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A Radiation Therapy Oncology Group Consensus Panel Contouring Atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westra WH, Hruban RH, Phelps TH, et al. New York, NY: Springer Verlag; 2003. Surgical Pathology Dissection (ed 2) [Google Scholar]
- 33.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 34.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results–EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 35.Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU)-based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: Pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial. J Clin Oncol. 2009;27(suppl; abstr 4008):18s. [Google Scholar]
- 36.Mehta VK, Cho C, Ford JM, et al. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys. 2003;55:132–137. doi: 10.1016/s0360-3016(02)03863-4. [DOI] [PubMed] [Google Scholar]
- 37.Klautke G, Feyerherd P, Ludwig K, et al. Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br J Cancer. 2005;92:1215–1220. doi: 10.1038/sj.bjc.6602492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro M, Dotor E, Rivera F, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201–205. doi: 10.1016/j.ijrobp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 40.Glynne-Jones R, Falk S, Maughan TS, et al. A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: A Colorectal Clinical Oncology Group Study. Br J Cancer. 2007;96:551–558. doi: 10.1038/sj.bjc.6603570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iles S, Gollins S, Susnerwala S, et al. Irinotecan+5-fluorouracil with concomitant pre-operative radiotherapy in locally advanced non-resectable rectal cancer: A phase I/II study. Br J Cancer. 2008;98:1210–1216. doi: 10.1038/sj.bjc.6604292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlomagno C, Pepe S, D'Armiento FP, et al. Predictive factors of complete response to neoadjuvant chemoradiotherapy in patients with rectal cancer. Oncology. 2010;78:369–375. doi: 10.1159/000320464. [DOI] [PubMed] [Google Scholar]
- 43.Negri FV, Campanini N, Camisa R, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer. 2008;98:143–147. doi: 10.1038/sj.bjc.6604131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 45.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Downstaging without complete pathologic response after neoadjuvant treatment improves cancer outcomes for cIII but not cII rectal cancers. Ann Surg Oncol. 2010;17:1758–1766. doi: 10.1245/s10434-010-0924-4. [DOI] [PubMed] [Google Scholar]
- 46.Mohiuddin M, Hayne M, Regine WF, et al. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075–1080. doi: 10.1016/s0360-3016(00)00732-x. [DOI] [PubMed] [Google Scholar]
- 47.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]