Abstract
Purpose
Adoptive immunotherapy using tumor-infiltrating lymphocytes represents an effective cancer treatment for patients with metastatic melanoma. The NY-ESO-1 cancer/testis antigen, which is expressed in 80% of patients with synovial cell sarcoma and approximately 25% of patients with melanoma and common epithelial tumors, represents an attractive target for immune-based therapies. The current trial was carried out to evaluate the ability of adoptively transferred autologous T cells transduced with a T-cell receptor (TCR) directed against NY-ESO-1 to mediate tumor regression in patients with metastatic melanoma and synovial cell sarcoma.
Patients and Methods
A clinical trial was performed in patients with metastatic melanoma or metastatic synovial cell sarcoma refractory to all standard treatments. Patients with NY-ESO-1–positive tumors were treated with autologous TCR-transduced T cells plus 720,000 iU/kg of interleukin-2 to tolerance after preparative chemotherapy. Objective clinical responses were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST).
Results
Objective clinical responses were observed in four of six patients with synovial cell sarcoma and five of 11 patients with melanoma bearing tumors expressing NY-ESO-1. Two of 11 patients with melanoma demonstrated complete regressions that persisted after 1 year. A partial response lasting 18 months was observed in one patient with synovial cell sarcoma.
Conclusion
These observations indicate that TCR-based gene therapies directed against NY-ESO-1 represent a new and effective therapeutic approach for patients with melanoma and synovial cell sarcoma. To our knowledge, this represents the first demonstration of the successful treatment of a nonmelanoma tumor using TCR-transduced T cells.
INTRODUCTION
The adoptive transfer of in vitro cultured melanoma-reactive T cells isolated from autologous tumor-infiltrating lymphocytes (TILs) after lymphodepleting chemotherapy has recently been shown to mediate objective tumor regression in 49% to 72% of patients with metastatic melanoma.1,2 The observation that melanoma-reactive TILs could be generated from only 50% of resected samples3 and the difficulty in generating tumor-reactive TILs from other cancer types have prompted cell transfer studies using autologous T cells that have been genetically engineered to express T-cell receptors (TCRs) directed against shared tumor antigens. In a recent trial targeting the MART-1 melanocyte differentiation antigen, an objective response rate of 30% was observed.4,5 This report details the results of, to our knowledge, the first clinical trial involving the adoptive transfer of autologous T cells transduced with a TCR directed against NY-ESO-1, a cancer/testis (CT) antigen expressed in 10% to 50% of metastatic melanomas, breast, prostate, thyroid, and ovarian cancers,6–9 as well as approximately 80% of synovial cell sarcomas,10 but not in any normal adult tissues except the testis, and represents the first successful immunotherapy for patients with synovial cell sarcoma.
PATIENTS AND METHODS
Patients
Patients 18 years of age or older with metastatic cancer refractory to standard treatments whose tumors expressed NY-ESO-1 as determined by immunohistochemical staining were eligible for the current trial. All patients' tumors stained strongly (2 to 4+, > 50%) for NY-ESO-1 antigen expression using the specific anti-NY-ESO-1 monoclonal antibody E97811 (Invitrogen, Carlsbad, CA).
Clinical Trial Design
This clinical trial (National Cancer Institute [NCI] 08-C-0121) was conducted in the Surgery Branch of the NCI and was reviewed and approved by the National Institutes of Health Institutional Biosafety Committee, the NCI Institutional Review Board, the National Institutes of Health Office of Biotechnology Activities, and the US Food and Drug Administration (all in Bethesda, MD). Genetically modified autologous T lymphocytes were adoptively transferred into patients after treatment with a lymphodepleting chemotherapy regimen consisting of cyclophosphamide (60 mg/kg/d for 2 days) and fludarabine (25 mg/m2/d for 5 days) as described in previous adoptive immunotherapy trials in patients with melanoma1,4,5 Greater than 108 T cells, which represented the minimum cell dose specified for treatment in the clinical protocol, were generated from 22 of the 22 cultures that were initiated from 17 patients' peripheral-blood mononuclear cells (PBMCs). HLA-A*0201–positive patients were enrolled onto two arms, one comprising patients with metastatic melanoma who were refractory to prior interleukin-2 (IL-2) therapy and a second including patients with metastatic synovial cell sarcoma refractory to multiple standard chemotherapy regimens.
Retroviral Vectors and T-Cell Transduction
A retroviral vector encoding a TCR that recognizes the peptide SLLMWITQC, corresponding to residues 157 to 165 of NY-ESO-1 (NY-ESO-1:157-165), in the context of the HLA-A*0201 class I restriction element, was generated in the MSGV1 retroviral vector backbone as previously described.12 This TCR, termed 1G4-α95:LY, contained two amino acid substitutions in the third complementarity determining region of the native 1G4 TCR α chain that conferred to CD8+ and CD4+ T cells an enhanced ability to recognize HLA-A*0201-positive target cells expressing the NY-ESO-1 antigen.12 Clinical grade good manufacturing practice retroviral supernatants were obtained from the National Gene Vector Laboratory at Indiana University. Patient pheresis samples were stimulated using 50 ng/mL of soluble anti-CD3 antibody (OKT3; Ortho-Biotech, Bridgewater, NJ) in the presence of 300 iU of recombinant IL-2, transduced with a retrovirus encoding the modified 1G4-α95:LY anti–NY-ESO-1 TCR using retronectin, kindly provided by Takara Bio (Otsu, Japan), and expanded further in vitro before adoptive transfer, as previously described.5
Fluorescence Activated Cell Sorting Analysis
Samples of in vitro cultured T cells and PBMCs obtained approximately 1 month after adoptive transfer were analyzed with an anti-Vβ13.1 antibody (Beckman Coulter, Miami, FL) that recognizes the β chain of the 1G4-α95:LY anti–NY-ESO-1 TCR, an isotype control antibody, an HLA-A*0201 tetramer prepared with the NY-ESO-1:157-165 peptide (Beckman Coulter), and a control HLA-A*0201 tetramer prepared with the gp100:154-162 peptide epitope (Beckman Coulter). Samples were incubated with 10 μL of normal mouse serum for 10 minutes at room temperature, followed by incubation with 10 μL of tetramer for 30 minutes at 4°C. Cells gated initially using forward and side-scatter and then gated for live (PI-) CD3+ cells were analyzed by fluorescence activated cell sorting using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA), and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Evaluation of Interferon Gamma (IFN-γ) Secretion, IFN-γ Enzyme-Linked Immunosorbent Spot Assay Responses, and Serum Anti–NY-ESO-1 Antibody Titers
Cocultures of in vitro cultured T cells and PBMCs were carried out with target cells for 18 hours, and soluble IFN-γ was detected as previously described.12 Enzyme-linked immunosorbent spot (ELISPOT) assays were carried out by incubating PBMCs overnight in the absence of exogenous cytokine, followed by culturing 105 PBMCs with 105 target cells for 18 hours and evaluating the number of cells secreting IFN-γ as previously described.5 Serum anti–NY-ESO-1 antibody titers were evaluated by analyzing serial dilutions of patient sera that were obtained before treatment with TCR-transduced T cells as previously described.13
RESULTS
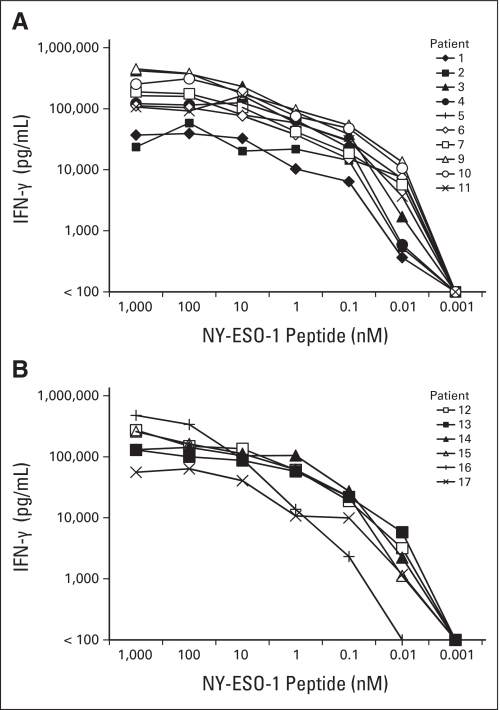
Patients with progressive metastatic melanoma (n = 11) or synovial cell sarcoma (n = 6) expressing high levels of NY-ESO-1 received nonmyeloablative chemotherapy followed by a median of 5 × 1010 T cells transduced with an anti–NY-ESO-1 TCR (range, 1.6 to 130 × 109) plus systemic IL-2 (Table 1). Patients presented with metastases in a wide variety of anatomic sites and were refractory to extensive prior treatment. All of the patients with melanoma had received prior treatment with high-dose IL-2, and all of the patients with synovial cell sarcoma experienced progression after extensive treatment with multiple courses of chemotherapy, all of which included doxorubicin and alkylating agents such as ifosfamide or cyclophosphamide. Many patients had also received second-line agents such as epirubicin, etoposide, or irinotecan and experimental monoclonal antibodies (Table 2). With the exception of patient 15, CD8+ T cells composed more than two thirds of the administered T cells (Table 1). A median of 78% of the transferred CD8+ T cells (range, 63% to 87%) and 65% of CD4+ T cells (range, 57% to 79%) bound the NY-ESO-1 tetramer, and a median of 92% of total CD3+ cells (range, 85% to 96%) bound an anti-Vβ13.1 antibody, which was reactive with the β chain of the transduced TCR (Table 1). The transduced T cells were highly reactive with an HLA-A*0201–positive, NY-ESO-1–positive tumor cell line (Table 1), and peptide titrations revealed that T cells from each of the 16 treatment samples that were tested recognized HLA-A*0201–positive target cells pulsed with a minimum concentration of between 0.1 and 0.01 nmol/L of the NY-ESO-1:157-165 peptide (Appendix Fig. A1, online only). In response to the NY-ESO-1–positive and HLA-A*0201–positive tumor target 624 mel, between 9,400 and 20,600 IFN-γ ELISPOTs/105 T cells were detected in the five samples of infused T cells that were tested, and responses against peptide pulsed targets ranged between 20,300 and 30,700 IFN-γ ELISPOTs/105 T cells in the four samples of infused T cells that were tested.
Table 1.
Characteristics of Patients and Administered T Cells
| Patient No. | Age(years) | Sex | Sites of Disease | Prior Treatment | No. of Cells(×109) | No. of IL-2 Doses | % of CD3 |
NY-ESO-1 Tetramer Positive |
Vβ13.1 Positive(% of CD3) | Tumor Cell Targets (pg/mL IFN-γ)* |
Response† | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD8 | CD4 | % of CD8 | % of CD4 | NY-ESO-1 Positive | NY-ESO-1 Negative | |||||||||
| Melanoma | ||||||||||||||
| 1 | 52 | M | ln | R, S, I | 130 | 6 | 97 | 2 | 86 | 64 | 94 | 515 | < 30 | PR (8) |
| 2 | 60 | F | sc, lu | S, I | 71 | 6 | 82 | 17 | 76 | 53 | 90 | 3,890 | < 30 | PD |
| 3 | 30 | F | bo, ln, panc, sb | R, S, I | 47 | 1 | 98 | 1 | 80 | 65 | 91 | 11,978 | 130 | PD |
| 4 | 56 | M | lu, ki | R, S, I | 50 | 7 | 91 | 9 | 80 | 74 | 94 | 11,230 | < 30 | CR (22+) |
| 5 | 32 | M | ln | S, C, I | 64 | 4 | 98 | 2 | 85 | 76 | 94 | 26,019 | 288 | CR (20+) |
| 6 | 38 | M | ln | S, I | 51 | 7 | 93 | 7 | 87 | 79 | 94 | 28,907 | 536 | PR (3) |
| 7 | 47 | M | ln, lu | R, S, I | 23 | 7 | 96 | 4 | 70 | 58 | 90 | 9,577 | 178 | PD |
| 8 | 39 | F | ln, br, lu | R, S, C, I | 38 | 8 | 68 | 32 | 78 | 70 | 94 | ND | ND | PD |
| 9 | 51 | F | lu, ln, li | S, C, I | 31 | 10 | 94 | 6 | 83 | 69 | 96 | 11,952 | 35 | PD |
| 10 | 61 | M | ln, li, spl, lu, bo | R, S, C, I | 16 | 8 | 84 | 16 | 79 | 56 | 92 | 16,063 | 49 | PD |
| 11 | 46 | M | lu, li | R, S, I | 37 | 6 | 93 | 7 | 63 | 58 | 85 | 5,795 | < 30 | PR (9+) |
| Synovial cell sarcoma | ||||||||||||||
| 12‡ | 20 | M | lu, bo | R, S, C, I | 83 | 5 | 82 | 8 | 77 | 64 | 91 | 10,065 | 117 | PR (10) |
| 13‡ | 37 | F | lu | R, S, C | 50 | 8 | 90 | 5 | 78 | 78 | 93 | 11,656 | 94 | PR (18) |
| 14‡ | 47 | F | lu, ln | R, S, C | 56 | 8 | 89 | 11 | 81 | 76 | 91 | 10,836 | 50 | PR (5) |
| 15‡ | 19 | M | lu | R, S, C, I | 16 | 5 | 46 | 40 | 67 | 63 | 89 | 5,371 | < 30 | PD |
| 16 | 30 | M | pl, hi | S, C | 59 | 5 | 92 | 8 | 74 | 57 | 88 | 6,512 | 199 | PR (8) |
| 17 | 40 | M | pl, hi | R, S, C | 52 | 5 | 81 | 18 | 78 | 69 | 92 | 8,098 | < 30 | PD |
Abbreviations: IL-2, interleukin-2; IFN-γ, interferon gamma; M, male; ln, lymph node; R, radiation; S, surgery; I, immunotherapy; PR, partial response; F, female; sc, subcutaneous; lu, lung; PD, progressive disease; bo, bone; panc, pancreas; sb, small bowel; ki, kidney; CR, complete response; C, chemotherapy; br, brain; ND, not done; spl, spleen; pl, pleura; hi, hilum.
Overnight cocultures of infusion samples were carried out with HLA-A*0201–positive tumor cell lines that either expressed (624 mel) or did not express (526 mel) NY-ESO-1.
In parentheses are the durations of response in months from the day of cell infusion.
Patients 12, 14, and 15 received one (patients 14 and 15) or two (patient 12) additional infusions of 1G4-α95LY–transduced T cells but did not respond to the treatments. Patient 13 received a second infusion of transduced T cells 9 months after the initial treatment and demonstrated a partial response lasting 18 months from the time of the initial treatment with transduced T cells.
Table 2.
Prior Systemic Therapies in Patients Treated With Anti–NY-ESO-1TCR Transduced T Cells
| Diagnosis and Patient No. | Prior Therapies |
|---|---|
| Melanoma | |
| 1 | High-dose bolus IL-2 |
| 2 | Granulocyte-macrophage colony-stimulating factor; high-dose bolus IL-2 |
| 3 | High-dose bolus IL-2 |
| 4 | High-dose bolus IL-2 |
| 5 | Temozolomide; high-dose bolus IL-2 |
| 6 | High-dose bolus IL-2 |
| 7 | High-dose bolus IL-2 |
| 8 | High-dose bolus IL-2; denileukin diftitox |
| 9 | Cisplatin, vinblastine, temozolomide; high-dose bolus IL-2 |
| 10 | Taxotere, temozolomide, vinblastine; high-dose bolus IL-2 |
| 11 | Interferon gamma; granulocyte-macrophage colony stimulating factor; high-dose bolus IL-2 |
| Synovial cell sarcoma | |
| 12 | 12 cycles of vincristine, doxorubicin, and cyclophosphamide alternating with etoposide and ifosfamide; 4 cycles of lexatumumab; 4 cycles of ecteinascidin |
| 13 | 2 cycles of ifosfamide and doxorubicin; 3 cycles of ifosfamide and epirubicin; 4 cycles of ifosfamide |
| 14 | 4 cycles of ifosfamide and doxorubicin; 3 cycles of gemcitabine and docetaxel |
| 15 | Multiple cycles of doxorubicin and ifosfamide; 3 cycles of cediranib |
| 16 | 6 cycles of doxorubicin and ifosfamide; 6 cycles of ifosfamide |
| 17 | 6 cycles of doxorubicin and ifosfamide; 3 cycles of ifosfamide |
Abbreviations: TCR, T-cell receptor; IL-2, interleukin-2.
Five of the 11 patients with metastatic melanoma experienced an objective response by Response Evaluation Criteria in Solid Tumors (RECIST), including two complete responses, ongoing at 22 and 20 months, and one partial response, ongoing at 9 months. Four of the six patients with synovial cell sarcoma exhibited objective partial responses, with one lasting 18 months. Examples of these responses are shown in Figure 1
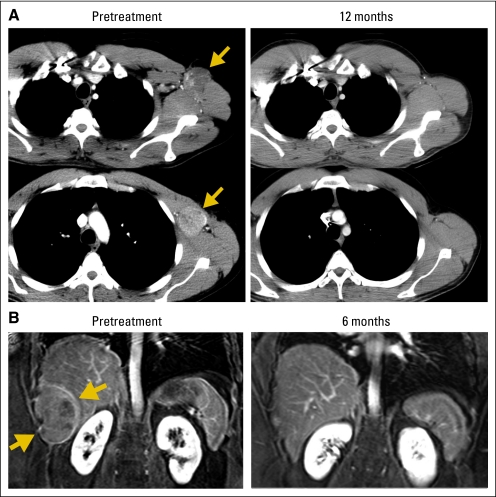
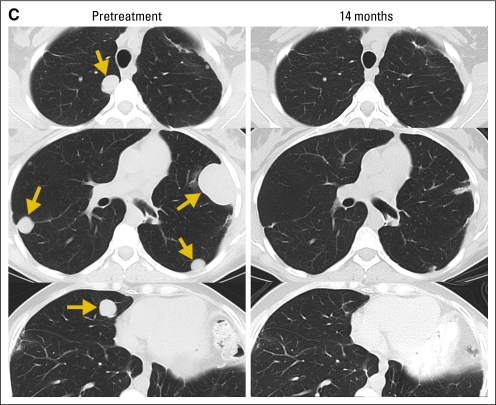
Fig 1.
Computed tomography scans demonstrating tumor regression. Radiologic studies were obtained before therapy and after adoptive transfer of NY-ESO-1 T-cell receptor (TCR) –transduced T cells. Tumors indicated by arrows. (A) Regression of multiple recurrent axillary lymph nodes from metastatic melanoma in patient 5 with a complete response now ongoing at 20 months. (B) Regression of a perihepatic chest wall lesion of synovial cell sarcoma in patient 16, who demonstrated a partial response lasting 8 months. (C) Regression of multiple lung metastases of synovial cell sarcoma in patient 13, who received two treatments with NY-ESO-1 TCR-transduced T cells and demonstrated a partial response lasting 18 months. No toxicities attributable to the administered cells were observed in these patients.
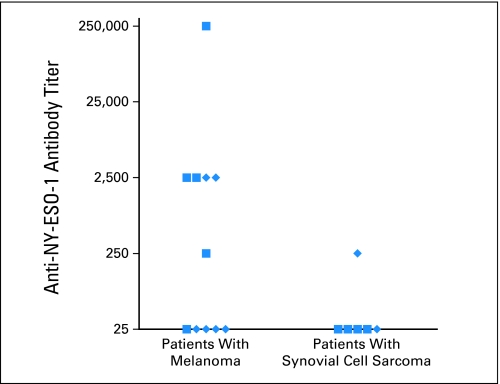
Significant anti–NY-ESO-1 antibody titers were observed in pretreatment serum samples from six of 11 patients with melanoma and one of six patients with synovial cell sarcoma (Fig 2). In all patients, there was no association between anti–NY-ESO-1 antibody titers in serum obtained from responders and nonresponders before treatment (P = .70) when analyzed using the nonparametric Mann-Whitney U test; however, it is difficult to draw any firm conclusions regarding the association between antibody titers and clinical response, given the relatively small number of patients treated in this trial.
Fig 2.
Serum anti–NY-ESO-1 antibody titers. The titers of anti–NY-ESO-1 antibodies in pretreatment sera were evaluated by enzyme-linked immunosorbent assay. Clinically responding patients are designated with square symbols, and nonresponding patients are designated with diamonds. Clinical responses were not correlated positively or negatively with anti–NY-ESO-1 antibody titers.
Approximately 1 month after transfer, between 2% and 60% of the CD8+ T cells present in PBMCs obtained from 14 of the 17 treated patients bound the NY-ESO-1 tetramer (Table 3). In PBMCs obtained at the same time from patients 6, 10, and 17, as well PBMCs obtained from each of the patients before transfer, less than 1% of the CD8+ T cells bound to the NY-ESO-1 tetramer. Between 4% and 45% of peripheral CD4+ T cells from 11 of 17 patients stained with the NY-ESO-1 tetramer, whereas significant tetramer staining was not seen in samples obtained from the remaining six patients (Table 3). The percentage of T cells detected in PBMCs at 1 month that expressed Vβ13.1 ranged between 3% and 85% of CD8+ T cells and between 3% and 63% of CD4+ T cells. The expression of Vβ13.1 was highly correlated with NY-ESO-1 tetramer expression (Fig 3A). The disparity between NY-ESO-1 tetramer staining and Vβ13.1 staining (Tables 1 and 3) presumably reflects higher affinity of the anti-Vβ13.1 antibody for TCR-transduced T cells, the detection of the Vβ13.1-positive transgene paired with endogenous α chains that results in nonfunctional TCRs, and the population of native Vβ13.1-positive T cells in the recovering peripheral repertoire after adoptive transfer.
Table 3.
Characteristics of Patient PBMCs Analyzed Approximately 1 Month After Adoptive Transfer
| Patient No. | % of CD3 |
NY-ESO-1 Tetramer |
Vβ13.1 |
Target (IFN-γ ELISPOTs/105 PBMCs) |
||||
|---|---|---|---|---|---|---|---|---|
| CD8 | CD4 | % of CD8 | % of CD4 | % of CD8 | % of CD4 | Tumor Cell* | Peptide† | |
| Melanoma | ||||||||
| 1 | 91 | 9 | 13 | 14 | 48 | 43 | 120 | 1,220 |
| 2 | 61 | 39 | 60 | 38 | 85 | 63 | 2,380 | 15,000 |
| 3 | 76 | 24 | 2 | < 1 | 32 | 5 | 23 | 84 |
| 4 | 93 | 7 | 3 | < 1 | 23 | 29 | 35 | 1,440 |
| 5 | 70 | 28 | 11 | < 1 | 20 | 4 | 130 | 2,250 |
| 6 | 94 | 6 | < 1 | < 1 | 4 | 5 | 1 | 33 |
| 7 | 71 | 27 | 18 | 7 | 38 | 11 | 400 | 3,050 |
| 8 | 66 | 32 | 11 | 19 | 30 | 53 | 840 | 6,270 |
| 9 | 41 | 50 | 39 | 26 | 76 | 44 | 540 | 5,280 |
| 10 | 65 | 31 | < 1 | < 1 | 3 | 10 | 1 | 15 |
| 11 | 69 | 28 | 12 | 7 | 42 | 29 | 210 | 2,450 |
| Synovial cell sarcoma | ||||||||
| 12 | 28 | 50 | 32 | 45 | 50 | 59 | 125 | 1,750 |
| 13 | 42 | 53 | 7 | 6 | 12 | 13 | 130 | 1,840 |
| 14 | 76 | 23 | 38 | 24 | 71 | 56 | 6,600 | 13,400 |
| 15 | 69 | 26 | 6 | 12 | 26 | 42 | 274 | 2,780 |
| 16 | 68 | 28 | 7 | 4 | 24 | 18 | 570 | 1,480 |
| 17 | 57 | 39 | < 1 | < 1 | 7 | 3 | 0 | 1 |
Abbreviations: PBMCs, peripheral-blood mononuclear cells; IFN-γ, interferon gamma; ELISPOTs, enzyme-linked immunosorbent spots.
The HLA-A*0201–positive NY-ESO-1–positive cell line 624 mel was used to evaluate tumor-specific ELISPOT responses. Less than 10 ELISPOTs/105 PBMCs were observed in response to the HLA-A*0201–positive NY-ESO-1–negative tumor cell line 526 mel with the exception of patients 7, 14, and 15, for whom 41, 40, and 27 ELISPOTs/105 PBMCs were detected, respectively.
The response against the unpulsed C1RA2 target cells was subtracted from the response against the NY-ESO-1 peptide. Pretreatment ELISPOT responses to the NY-ESO-1 peptide were less than 10 ELISPOTs/105 PBMCs with the exception of patient 3, whose PBMCs contained 55 ELISPOTs/105 PBMCs.
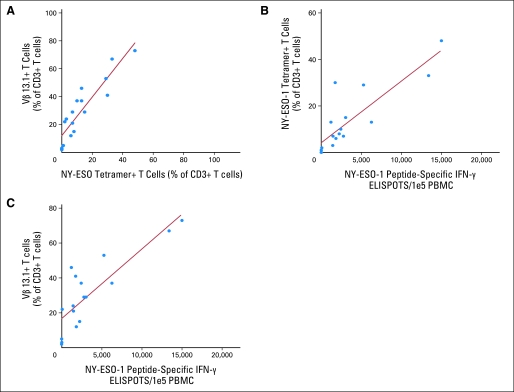
Fig 3.
Correlations between T-cell persistence and enzyme-linked immunosorbent spot (ELISPOT) responses. Highly significant correlations were found (A) between NY-ESO-1 tetramer and Vβ13.1 expression (r2 = 0.82; P < .001), (B) between NY-ESO-1 peptide–reactive interferon gamma (IFN-γ) ELISPOTs and NY-ESO-1 tetramer expression (r2 = 0.72; P < .001), and (C) between NY-ESO-1 peptide–reactive ELISPOTs and Vβ13.1 expression (r2 = 0.70; P < .001) in patient peripheral-blood mononuclear cells (PBMCs) obtained approximately 1 month after adoptive transfer.
Peptide-specific and tumor-specific IFN-γ ELISPOT responses were detected in PBMCs obtained from all of the patients 1 month after therapy with the exception of patients 6, 10, and 17, whose peripheral CD8+ T cells at 1 month also failed to demonstrate significant binding of the NY-ESO-1 tetramer (Table 3). Before transfer of 1G4-α95:LY TCR-transduced T cells, less than 10 IFN-γ ELISPOTs/105 PBMCs were detected in response to NY-ESO-1 peptide pulsed target cells in PBMC samples obtained from 16 of the 17 patients treated in this trial, whereas 55 ELISPOTs/105 PBMCs were detected in patient 3. After adoptive transfer, more than 1,000 ELISPOTs/105 PBMCs were detected in response to NY-ESO-1 peptide pulsed target cells in samples from 13 of 17 patients, whereas less than 100 ELISPOTs/105 PBMCs were detected in samples from patients 3, 6, 10, and 17. The number of NY-ESO-1 peptide–specific IFN-γ ELISPOTs detected in PBMCs 1 month after treatment correlated with NY-ESO-1 tetramer and anti-Vβ13.1 antibody binding (Figs 3B and 3C). Tumor regression was not correlated with persistence of the transferred T cells as measured by NY-ESO-1 tetramer staining, Vβ13.1 staining, or ELISPOT responses.
The levels of NY-ESO-1–specific T cells detected using IFN-γ ELISPOT assays were significantly lower than the number of NY-ESO-1 tetramer–positive T cells detected 1 month after transfer in the 14 patients who demonstrated significant T-cell persistence. Several factors may influence these findings, including the fact that only approximately 10% to 30% of the in vitro cultured T cells assayed before infusion were detected in the ELISPOT assay, as well as the fact that whole PBMCs rather than purified T cells were used to evaluate these responses. The analysis of clinical adoptive immunotherapy trials carried out with MART-1– and gp100-reactive TCRs revealed that the levels of expression of multiple genes in PBMCs containing persistent T cells 1 month after adoptive transfer were low relative to those observed in in vitro cultured T cells.14 The IFN-γ ELISPOT assay results also demonstrated that responses of PBMCs obtained 1 month after transfer to an HLA-A*0201–positive and NY-ESO-1–positive tumor target were lower than responses to peptide pulsed target cells. This may have resulted from the insensitivity of quiescent T cells present in peripheral blood at this time to the relatively low levels of endogenously presented antigen on the tumor cell surface.
No toxicities were attributed to the transferred cells, although all patients experienced the transient neutropenia and thrombocytopenia induced by the preparative regimen and the transient toxicities associated with IL-2. All patients recovered well after the completion of treatment.
DISCUSSION
Clinical trials in patients with metastatic melanoma have demonstrated the ability of adoptively transferred T cells to mediate durable complete cancer regression.1,15,16 These results have stimulated efforts to genetically modify lymphocytes to improve their antitumor efficacy and to extend the range of tumors that can be treated. In the first trial, to our knowledge, to examine the in vivo efficacy of TCR-transduced T cells in patients with cancer, tumor regression was observed in two of 13 patients receiving autologous T cells that were transduced with a MART-1–reactive TCR.4 In subsequent trials carried out with high-avidity TCRs, objective clinical responses were seen in six (30%) of 20 patients treated with autologous T cells that were transduced with a MART-1–reactive TCR4 and in three (19%) of 16 patients treated with a gp100-reactive TCR.5 Clinical trials have also used adoptive transfer of T cells transduced with chimeric antigen receptors (CARs) comprised of tumor-reactive antibody combining regions linked to T-cell signaling domains. Tumor regression was observed in two of 11 patients with neuroblastoma who received Epstein-Barr virus–specific T cells stimulated with anti-CD3 antibody and transduced with a CAR directed against the diasialoganglioside GD2.17 However, no clinical responses were seen in three patients with renal cancer who received autologous T cells transduced with a CAR directed against carbonic anhydrase-IX18 or in eight patients with ovarian cancer who received autologous PBMCs transduced with a CAR directed against the α-folate receptor.19
CT antigens represent promising targets for cancer immunotherapy because they are expressed in a wide variety of epithelial cancers but are restricted in their expression in normal adult tissues to cells in the testis that lack expression of class I major histocompatibility complex molecules and are thus not susceptible to damage by T cells that recognize these gene products. The NY-ESO-1 antigen is expressed in 15% to 50% of highly prevalent tumors that include breast, lung, prostate, and ovarian cancer,6–9 and effective therapies that target NY-ESO-1 could potentially be applied to the large population of patients with these malignancies. To test the effectiveness of adoptive immunotherapy with genetically engineered cells that target the NY-ESO-1 antigen, we treated patients with advanced synovial cell sarcoma because 80% of these patients express NY-ESO-1.10 Prior phase I clinical trials of cancer vaccines targeting NY-ESO-1 using peptides,20,21 recombinant vaccinia and fowlpox viruses encoding full-length NY-ESO-1,22 or recombinant NY-ESO-1 protein,23 as well as other cancer vaccine trials targeting additional CT antigens such as MAGE-A3, have thus far failed to demonstrate a clinical benefit in patients with advanced disease (reviewed in Caballero and Chen24). The adoptive transfer of an in vitro sensitized autologous CD4+ T-cell clone that recognized the HLA-DP*04–restricted NY-ESO-1:157-170 peptide epitope mediated regression of metastatic melanoma in one of nine patients.16,25 The results presented in this report indicate that the frequency of T cells reactive with epitopes of MART-1 and MAGE-A3 increased after injection of an NY-ESO-1–reactive CD4+ T-cell clone, which was administered in the absence of any conditioning regimen. In the current report, we have not examined the effects of adoptive immunotherapy on other endogenous antitumor responses.
To our knowledge, the current clinical trial reported here represents the first adoptive immunotherapy trial to treat cancer using genetic engineering of T cells to express a CT antigen-specific TCR. Response rates of 45% and 67% were observed in patients with melanoma and synovial cell sarcoma, respectively, all of whom had progressive disease after extensive prior treatment. No on-target toxicities were seen in the current trial because of the lack of NY-ESO-1 expression of normal tissues, which contrasts with our previous observation of vigorous on-target toxicity in patients with melanoma treated with T cells engineered to express MART-1– and gp100-reactive TCRs.5 In these trials, as well as the current trial, clinical responses to TCR-engineered T cells were highly variable, despite the similarity between the T-cell transduction levels and number of transduced T cells administered to individual patients. Overall levels of T-cell persistence also varied widely between patients and did not seem to be associated with clinical response to therapy, although a correlation was noted in our MART-1 and gp100 TCR trials between clinical response and the frequency of IFN-γ and IL-2 ELISPOTs detected in PBMCs obtained 1 month after treatment.5 Given the small number of patients treated in these trials, it is difficult to evaluate the significance of these results. Nevertheless, differences between patient reconstitution of lymphocyte subsets such as regulatory CD4+ T cells and differences in the characteristics of individual tumors represent at least some of the potential factors that might influence T-cell persistence and response to therapy. Potential strategies that may enhance responses include immunization with recombinant vaccines encoding the NY-ESO-1 antigen, elimination of host CD4+ regulatory T cells, and cotransduction of TCR constructs with genes that encode cytokines such as IL-12.26
Overall, these findings indicate that treatments using TCRs directed against NY-ESO-1 are effective at mediating tumor regression in patients with metastatic melanoma and synovial cell sarcoma and suggest that TCRs directed against NY-ESO-1 and additional CT antigens should be explored for the treatment of patients with common epithelial cancers.
Acknowledgment
We thank Arnold Mixon, Shawn Farid, and Laura Devillier for carrying out fluorescence activated cell sorting analysis of patient samples as well as members of the surgery branch tumor infiltrating lymphocytes laboratory, the immunotherapy nurses, and the fellows for their assistance with our research.
Appendix
Fig A1.
Titration of response to NY-ESO-1 peptide. Soluble interferon gamma (IFN-γ) levels were measured in overnight cocultures of 105 cells from samples of infused transduced T cells from patients with (A) melanoma and (B) synovial cell sarcoma with 105 T2 cells that had been pulsed with the NY-ESO-1:157-165 peptide. Responses to the negative control gp100:154-162 peptide were all less than 100 pg/mL of IFN-γ.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00670748.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Paul F. Robbins, Richard A. Morgan, Steven A. Feldman, James C. Yang, Richard M. Sherry, Mark E. Dudley, John R. Wunderlich, Lee J. Helman, Crystal L. Mackall, Udai S. Kammula, Marybeth S. Hughes, Nicholas P. Restifo, Mona El-Gamil,Steven A. Rosenberg
Administrative support: Azam V. Nahvi, Catherine L. Levy,Carolyn Laurencot
Provision of study materials or patients: Steven A. Feldman, James C. Yang, Mark E. Dudley, John R. Wunderlich, Azam V. Nahvi, Lee J. Helman, Crystal L. Mackall, Udai S. Kammula, Marybeth S. Hughes, Mark Raffeld, Chyi-Chia Richard Lee, Catherine L. Levy, Yong F. Li, Mona El-Gamil, Susan L. Schwarz, Steven A. Rosenberg
Collection and assembly of data: Paul F. Robbins, Richard M. Sherry, Mark Raffeld, Chyi-Chia Richard Lee, Catherine L. Levy, Yong F. Li, Mona El-Gamil, Susan L. Schwarz
Data analysis and interpretation: Paul F. Robbins, Richard A. Morgan, Steven A. Feldman, Richard M. Sherry, Crystal L. Mackall, Udai S. Kammula, Nicholas P. Restifo, Mark Raffeld, Chyi-Chia Richard Lee, Mona El-Gamil, Susan L. Schwarz, Carolyn Laurencot,Steven A. Rosenberg
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrow C, Browning J, MacGregor D, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 8.Goydos JS, Patel M, Shih W. NY-ESO-1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res. 2001;98:76–80. doi: 10.1006/jsre.2001.6148. [DOI] [PubMed] [Google Scholar]
- 9.Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 10.Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan HA, Svobodova S, Macgregor D, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10:8396–8404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 12.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff SL, Robbins PF, El-Gamil M, et al. No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma. J Immunother. 2009;32:884–885. doi: 10.1097/CJI.0b013e3181affbf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns WR, Zheng Z, Rosenberg SA, et al. Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114:2888–2899. doi: 10.1182/blood-2009-01-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamers CH, Langeveld SC, Groot-van Ruijven CM, et al. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56:1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bender A, Karbach J, Neumann A, et al. LUD 00-009: Phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun. 2007;7:16. [PMC free article] [PubMed] [Google Scholar]
- 21.Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jäger E, Karbach J, Gnjatic S, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholaou T, Ebert LM, Davis ID, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 24.Caballero OL, Chen YT. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fred Hutchinson Cancer Research Center. Patient's own infection-fighting T cells put late-stage melanoma into long-term remission—without chemotherapy or radiation. http://www.fhcrc.org/about/ne/news/2008/06/18/T_cells.html.
- 26.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]