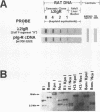
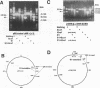
Abstract
An unusual S1-nuclease sensitive microsatellite (STMS) has been found in the single copy, rat polymeric immunoglobulin receptor gene (PIGR) terminal exon. In Fisher rats, elements within or beyond the STMS are expressed variably in the 3' untranslated regions (3'UTRs) of two 'Groups' of PIGR-encoded hepatic mRNAs (pIg-R) during liver regeneration. STMS elements include neighboring constant regions (a 60-bp d[GA]-rich tract with a chi-like octamer, followed by 15 tandem d[GGA] repeats) that merge directly with 36 or 39 tandem d[GAA] repeats (Fisher or Wistar strains, respectively) interrupted by d[AA] between their 5th-6th repeat units. The Wistar STMS is flanked upstream by two regions of nearly contiguous d[CA] or d[CT] repeats in the 3' end of intron 8; and downstream, by a 283 bp 'unit' containing several inversions at its 5' end, and two polyadenylation signals at its 3' end. The 283 nt unit is expressed in Group 1 pIg-R mRNAs; but it is absent in the Group 2 family so that their GAA repeats merge with their poly A tails. In contrast to genomic sequence, GGA triplet repeats are amplified (n > or = 24-26), whereas GAA triplet repeats are truncated variably (n < or = 9-37) and expressed uninterruptedly in both mRNA Groups. These results suggest that 3' end processing of the rat PIGR gene may involve misalignment, slippage and premature termination of RNA polymerase II. The function of this unusual processing and possible roles of chi-like octamers in quiescent or extrahepatic tissues are discussed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Aroeti B., Casanova J., Okamoto C., Cardone M., Pollack A., Tang K., Mostov K. Polymeric immunoglobulin receptor. Int Rev Cytol. 1992;137B:157–168. doi: 10.1016/s0074-7696(08)62603-0. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dubel J. R., Harati Y. Somatic instability of CTG repeat in myotonic dystrophy. Neurology. 1993 Dec;43(12):2674–2678. doi: 10.1212/wnl.43.12.2674. [DOI] [PubMed] [Google Scholar]
- Banting G., Brake B., Braghetta P., Luzio J. P., Stanley K. K. Intracellular targetting signals of polymeric immunoglobulin receptors are highly conserved between species. FEBS Lett. 1989 Aug 28;254(1-2):177–183. doi: 10.1016/0014-5793(89)81034-8. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle V., Atkin N. B. Break points in chromosome #1 abnormalities of 218 human neoplasms. Cancer Genet Cytogenet. 1981 Nov;4(3):215–225. doi: 10.1016/0165-4608(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Kloppel T. M. The role of the liver in translocation of IgA into the gastrointestinal tract. Immunol Invest. 1989 Jan-May;18(1-4):269–285. doi: 10.3109/08820138909112242. [DOI] [PubMed] [Google Scholar]
- Burfeind P., Belgardt B., Szpirer C., Hoyer-Fender S. Structure and chromosomal assignment of a gene encoding the major protein of rat sperm outer dense fibres. Eur J Biochem. 1993 Sep 1;216(2):497–505. doi: 10.1111/j.1432-1033.1993.tb18168.x. [DOI] [PubMed] [Google Scholar]
- Carango P., Noble J. E., Marks H. G., Funanage V. L. Absence of myotonic dystrophy protein kinase (DMPK) mRNA as a result of a triplet repeat expansion in myotonic dystrophy. Genomics. 1993 Nov;18(2):340–348. doi: 10.1006/geno.1993.1474. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Pizzuti A., Fu Y. H., Fenwick R. G., Jr, Nelson D. L. Triplet repeat mutations in human disease. Science. 1992 May 8;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- Collier D. A., Griffin J. A., Wells R. D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J Biol Chem. 1988 May 25;263(15):7397–7405. [PubMed] [Google Scholar]
- Cunningham P. R., Weitzmann C. J., Ofengand J. SP6 RNA polymerase stutters when initiating from an AAA... sequence. Nucleic Acids Res. 1991 Sep 11;19(17):4669–4673. doi: 10.1093/nar/19.17.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dayn A., Samadashwily G. M., Mirkin S. M. Intramolecular DNA triplexes: unusual sequence requirements and influence on DNA polymerization. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11406–11410. doi: 10.1073/pnas.89.23.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher D. L., Mostov K. E. Alternate splicing of rabbit polymeric immunoglobulin receptor. Mol Cell Biol. 1986 Jul;6(7):2712–2715. doi: 10.1128/mcb.6.7.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher U., Chowdhury K., Gruss P. Isolation and characterization of murine transcriptional control elements using a "shotgun" method. DNA. 1987 Aug;6(4):307–316. doi: 10.1089/dna.1987.6.307. [DOI] [PubMed] [Google Scholar]
- Drewe J. A., Verma S., Frech G., Joho R. H. Distinct spatial and temporal expression patterns of K+ channel mRNAs from different subfamilies. J Neurosci. 1992 Feb;12(2):538–548. doi: 10.1523/JNEUROSCI.12-02-00538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. C., Skowron P., Van Etten J. L., Smith L. M., Mead D. A. Rapid shotgun cloning utilizing the two base recognition endonuclease CviJI. Nucleic Acids Res. 1992 Jul 25;20(14):3753–3762. doi: 10.1093/nar/20.14.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huling S., Fournier G. R., Feren A., Chuntharapai A., Jones A. L. Ontogeny of the secretory immune system: maturation of a functional polymeric immunoglobulin receptor regulated by gene expression. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4260–4264. doi: 10.1073/pnas.89.10.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. Analysis of the signals for transcription termination by purified RNA polymerase II. Biochemistry. 1990 Jan 9;29(1):269–278. doi: 10.1021/bi00453a037. [DOI] [PubMed] [Google Scholar]
- Koch K. S., Fletcher R. G., Grond M. P., Leffert H. L. A 43-base-pair complementary DNA sequence homology and triplet repeat motif among putative polymeric immunoglobulin receptor messenger RNAs in regenerating rat liver. Hepatology. 1993 Jul;18(1):226–228. [PubMed] [Google Scholar]
- Krajci P., Gedde-Dahl T., Jr, Høyheim B., Rogde S., Olaisen B., Brandtzaeg P. The gene encoding human transmembrane secretory component (locus PIGR) is linked to D1S58 on chromosome 1. Hum Genet. 1992 Nov;90(3):215–219. doi: 10.1007/BF00220065. [DOI] [PubMed] [Google Scholar]
- Krajci P., Kvale D., Taskén K., Brandtzaeg P. Molecular cloning and exon-intron mapping of the gene encoding human transmembrane secretory component (the poly-Ig receptor) Eur J Immunol. 1992 Sep;22(9):2309–2315. doi: 10.1002/eji.1830220920. [DOI] [PubMed] [Google Scholar]
- Krajci P., Solberg R., Sandberg M., Oyen O., Jahnsen T., Brandtzaeg P. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem Biophys Res Commun. 1989 Feb 15;158(3):783–789. doi: 10.1016/0006-291x(89)92790-3. [DOI] [PubMed] [Google Scholar]
- Krowczynska A. M., Rudders R. A., Krontiris T. G. The human minisatellite consensus at breakpoints of oncogene translocations. Nucleic Acids Res. 1990 Mar 11;18(5):1121–1127. doi: 10.1093/nar/18.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Misalignment-mediated DNA synthesis errors. Biochemistry. 1990 Sep 4;29(35):8003–8011. doi: 10.1021/bi00487a001. [DOI] [PubMed] [Google Scholar]
- Lin B., Nasir J., MacDonald H., Hutchinson G., Graham R. K., Rommens J. M., Hayden M. R. Sequence of the murine Huntington disease gene: evidence for conservation, alternate splicing and polymorphism in a triplet (CCG) repeat [corrected]. Hum Mol Genet. 1994 Jan;3(1):85–92. doi: 10.1093/hmg/3.1.85. [DOI] [PubMed] [Google Scholar]
- Lu X. P., Koch K. S., Lew D. J., Dulic V., Pines J., Reed S. I., Hunter T., Leffert H. L. Induction of cyclin mRNA and cyclin-associated histone H1 kinase during liver regeneration. J Biol Chem. 1992 Feb 15;267(5):2841–2844. [PubMed] [Google Scholar]
- Mahadevan M. S., Amemiya C., Jansen G., Sabourin L., Baird S., Neville C. E., Wormskamp N., Segers B., Batzer M., Lamerdin J. Structure and genomic sequence of the myotonic dystrophy (DM kinase) gene. Hum Mol Genet. 1993 Mar;2(3):299–304. doi: 10.1093/hmg/2.3.299. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Mazanec M. B., Kaetzel C. S., Lamm M. E., Fletcher D., Nedrud J. G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- NISHIMURA S., JACOB T. M., KHORANA H. G. SYNTHETIC DEOXYRIBOPOLYNUCLEOTIDES AS TEMPLATES FOR RIBONUCLEIC ACID POLYMERASE: THE FORMATION AND CHARACTERIZATION OF A RIBOPOLYNUCLEOTIDE WITH A REPEATING TRINUCLEOTIDE SEQUENCE. Proc Natl Acad Sci U S A. 1964 Dec;52:1494–1501. doi: 10.1073/pnas.52.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Zepf N. E. The murine Slp gene. Additional evidence that sex-limited protein has no biologic function. J Immunol. 1991 Oct 15;147(8):2756–2763. [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Richards J. E., Gilliam A. C., Shen A., Tucker P. W., Blattner F. R. Unusual sequences in the murine immunoglobulin mu-delta heavy-chain region. Nature. 1983 Dec 1;306(5942):483–487. doi: 10.1038/306483a0. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Rhoads R. E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989 Nov 11;17(21):8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Serikawa T., Kuramoto T., Hilbert P., Mori M., Yamada J., Dubay C. J., Lindpainter K., Ganten D., Guénet J. L., Lathrop G. M. Rat gene mapping using PCR-analyzed microsatellites. Genetics. 1992 Jul;131(3):701–721. doi: 10.1093/genetics/131.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlaczck I., Epplen C., Riess O., Epplen J. T. Simple repetitive (GAA)n loci in the human genome. Electrophoresis. 1993 Oct;14(10):973–977. doi: 10.1002/elps.11501401155. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Wells R. D. DNA structure, mutations, and human genetic disease. Curr Opin Biotechnol. 1992 Dec;3(6):612–622. doi: 10.1016/0958-1669(92)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Hotspots of homologous recombination. Experientia. 1994 Mar 15;50(3):234–241. doi: 10.1007/BF01924006. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R., Richards R. I. Anticipation legitimized: unstable DNA to the rescue. Am J Hum Genet. 1992 Jul;51(1):7–9. [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D., Piatigorsky J. Primary structure and lens-specific expression of genes for an intermediate filament protein and a beta-tubulin in cephalopods. Biochim Biophys Acta. 1993 Nov 16;1216(2):245–254. doi: 10.1016/0167-4781(93)90151-3. [DOI] [PubMed] [Google Scholar]
- Usui H., Kuwano R., Maeda T., Araki K., Sakimura K., Kushiya E., Takahashi Y. ID sequences in the genes of three brain-specific proteins. Biochem Int. 1987 Oct;15(4):809–816. [PubMed] [Google Scholar]
- Vanin E. F. Processed pseudogenes: characteristics and evolution. Annu Rev Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Amirhaeri S., Kang S., Wells R. D., Griffith J. D. Preferential nucleosome assembly at DNA triplet repeats from the myotonic dystrophy gene. Science. 1994 Jul 29;265(5172):669–671. doi: 10.1126/science.8036515. [DOI] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Wels J. A., Word C. J., Rimm D., Der-Balan G. P., Martinez H. M., Tucker P. W., Blattner F. R. Structural analysis of the murine IgG3 constant region gene. EMBO J. 1984 Sep;3(9):2041–2046. doi: 10.1002/j.1460-2075.1984.tb02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]