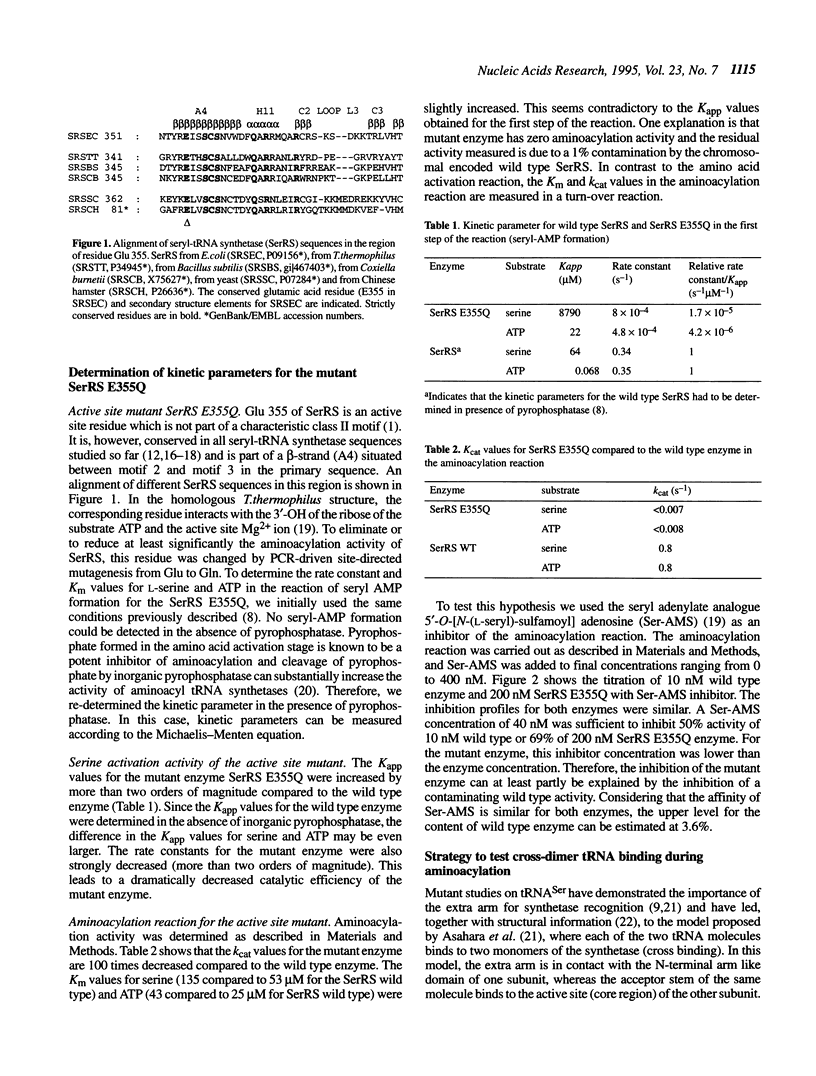
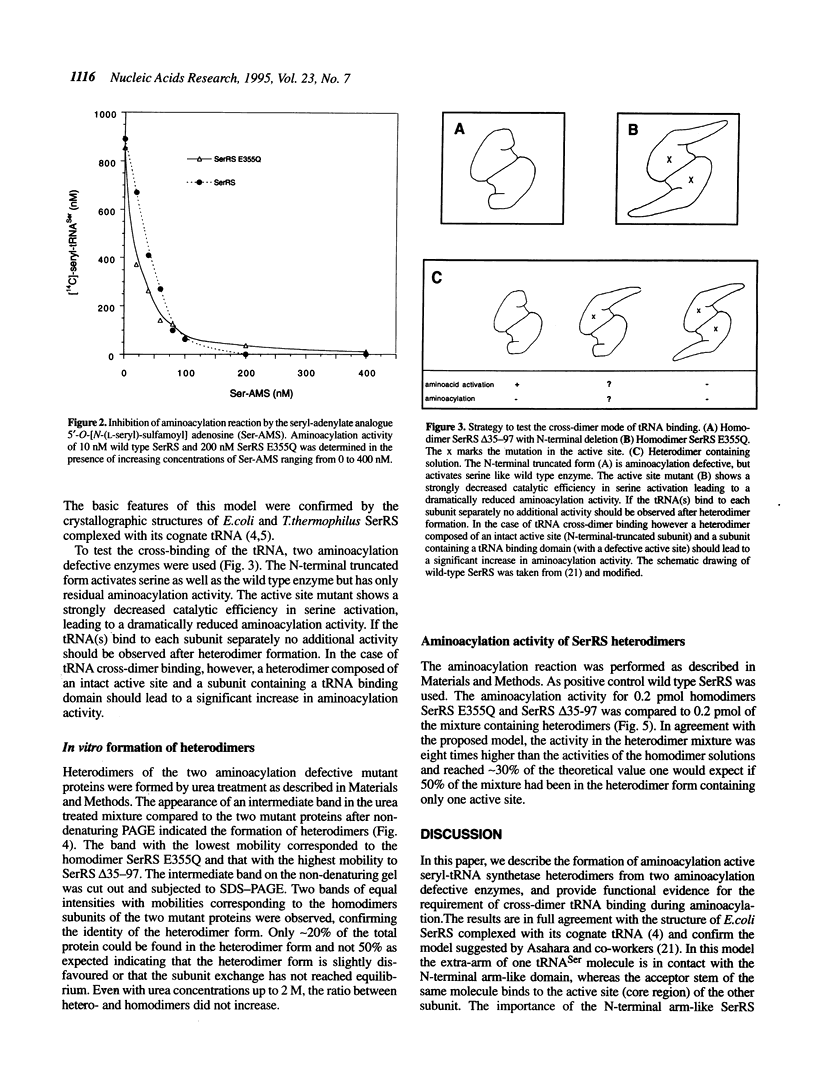
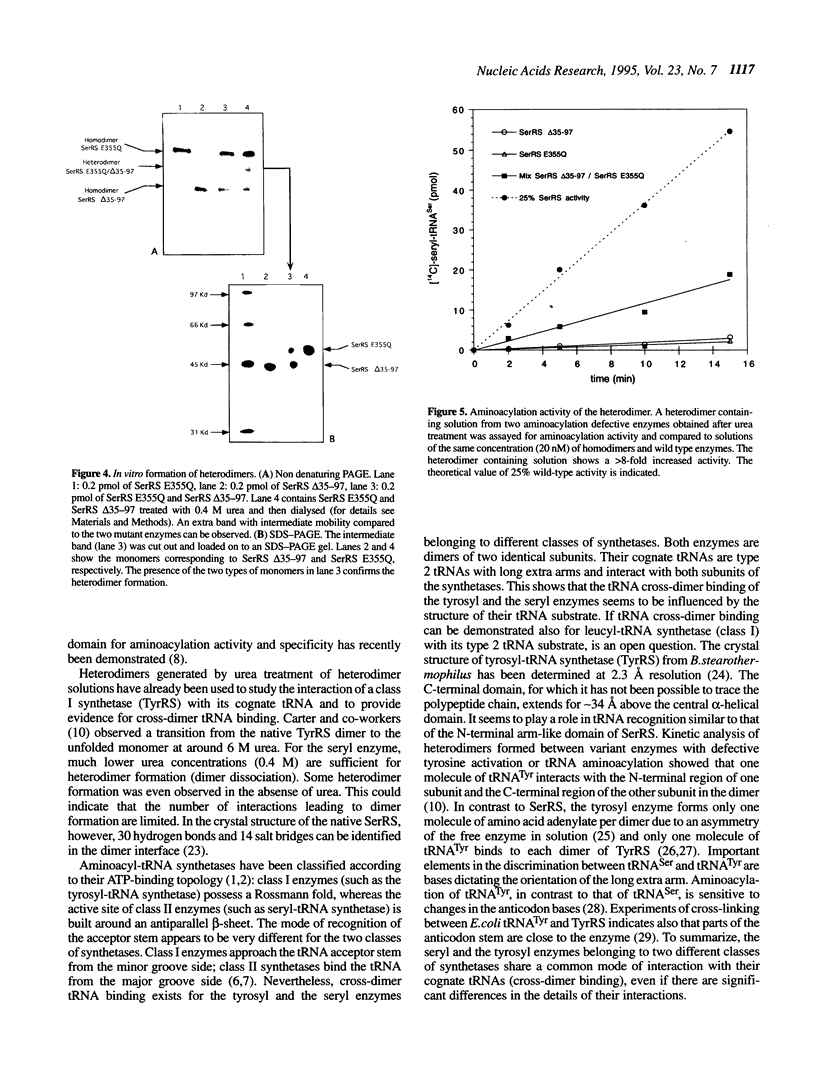
Abstract
Escherichia coli seryl-tRNA synthetase (SerRS) is a homo-dimeric class II aminoacyl-tRNA synthetase. Each subunit is composed of two distinct domains: the N-terminal domain is a 60 A long, arm-like coiled coil structure built up of two antiparallel alpha-helices, whereas the C-terminal domain, the catalytic core, is an alpha-beta structure overlying a seven-stranded antiparallel beta-sheet. Deletion of the arm-like domain (SerRS delta 35-97) does not affect the amino acid activation step of the reaction, but reduces aminoacylation activity by more than three orders of magnitude. In the present study, it was shown that the formation of heterodimers from two aminoacylation defective homodimers, the N-terminal deletion and an active site mutant (SerRS E355Q), restored charging activity. The aminoacylation activity in a mixture containing the heterodimers was compared to that of solutions containing the same concentrations of homodimer. The activity of the mixture was eight times higher than the activities of the homodimer solutions, and reached 50% of the theoretical value that would be expected if 50% of the mixture was in the heterodimer form and assuming that a heterodimer contains only one active site. These results are in full agreement with the structural analysis of E. coli SerRS complexed with its cognate tRNA and provide functional evidence for the cross-dimer binding of tRNA in solution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman E. J., Joachimiak A., Klinghofer V., Sigler P. B. Directly photocrosslinked nucleotides joining transfer RNA to aminoacyl-tRNA synthetase in methionine and tyrosine systems. J Mol Biol. 1985 Jan 5;181(1):93–102. doi: 10.1016/0022-2836(85)90327-4. [DOI] [PubMed] [Google Scholar]
- Airas R. K., Cramer F. Pyrophosphate-caused inhibition of the aminoacylation of tRNA by the leucyl-tRNA synthetase from Neurospora crassa. Eur J Biochem. 1986 Oct 15;160(2):291–296. doi: 10.1111/j.1432-1033.1986.tb09970.x. [DOI] [PubMed] [Google Scholar]
- Belrhali H., Yaremchuk A., Tukalo M., Larsen K., Berthet-Colominas C., Leberman R., Beijer B., Sproat B., Als-Nielsen J., Grübel G. Crystal structures at 2.5 angstrom resolution of seryl-tRNA synthetase complexed with two analogs of seryl adenylate. Science. 1994 Mar 11;263(5152):1432–1436. doi: 10.1126/science.8128224. [DOI] [PubMed] [Google Scholar]
- Biou V., Yaremchuk A., Tukalo M., Cusack S. The 2.9 A crystal structure of T. thermophilus seryl-tRNA synthetase complexed with tRNA(Ser). Science. 1994 Mar 11;263(5152):1404–1410. doi: 10.1126/science.8128220. [DOI] [PubMed] [Google Scholar]
- Borel F., Vincent C., Leberman R., Härtlein M. Seryl-tRNA synthetase from Escherichia coli: implication of its N-terminal domain in aminoacylation activity and specificity. Nucleic Acids Res. 1994 Aug 11;22(15):2963–2969. doi: 10.1093/nar/22.15.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick P., Bhat T. N., Blow D. M. Structure of tyrosyl-tRNA synthetase refined at 2.3 A resolution. Interaction of the enzyme with the tyrosyl adenylate intermediate. J Mol Biol. 1989 Jul 5;208(1):83–98. doi: 10.1016/0022-2836(89)90090-9. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Construction of heterodimer tyrosyl-tRNA synthetase shows tRNATyr interacts with both subunits. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1189–1192. doi: 10.1073/pnas.83.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Cusack S., Härtlein M., Leberman R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991 Jul 11;19(13):3489–3498. doi: 10.1093/nar/19.13.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessen P., Zaccaï G., Blanquet S. Neutron scattering studies of escherichia coli tyrosyl-trna synthetase and of its interaction with trna tyr. J Mol Biol. 1982 Aug 25;159(4):651–664. doi: 10.1016/0022-2836(82)90106-1. [DOI] [PubMed] [Google Scholar]
- Dock-Bregeon A. C., Westhof E., Giegé R., Moras D. Solution structure of a tRNA with a large variable region: yeast tRNASer. J Mol Biol. 1989 Apr 20;206(4):707–722. doi: 10.1016/0022-2836(89)90578-0. [DOI] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fujinaga M., Berthet-Colominas C., Yaremchuk A. D., Tukalo M. A., Cusack S. Refined crystal structure of the seryl-tRNA synthetase from Thermus thermophilus at 2.5 A resolution. J Mol Biol. 1993 Nov 5;234(1):222–233. doi: 10.1006/jmbi.1993.1576. [DOI] [PubMed] [Google Scholar]
- Herlitze S., Koenen M. A general and rapid mutagenesis method using polymerase chain reaction. Gene. 1990 Jul 2;91(1):143–147. doi: 10.1016/0378-1119(90)90177-s. [DOI] [PubMed] [Google Scholar]
- Himeno H., Hasegawa T., Ueda T., Watanabe K., Shimizu M. Conversion of aminoacylation specificity from tRNA(Tyr) to tRNA(Ser) in vitro. Nucleic Acids Res. 1990 Dec 11;18(23):6815–6819. doi: 10.1093/nar/18.23.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlein M., Madern D., Leberman R. Cloning and characterization of the gene for Escherichia coli seryl-tRNA synthetase. Nucleic Acids Res. 1987 Feb 11;15(3):1005–1017. doi: 10.1093/nar/15.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes R., Fersht A. R. Tyrosyl-tRNA synthetase from Escherichia coli. Stoichiometry of ligand binding and half-of-the-sites reactivity in aminoacylation. Biochemistry. 1975 Jul 29;14(15):3344–3350. doi: 10.1021/bi00686a009. [DOI] [PubMed] [Google Scholar]
- Lunel C., Buttin G., de Saint Vincent B. R. A seryl-tRNA synthetase gene is coamplified with the adenylate deaminase 2 gene in coformycin resistant Chinese hamster fibroblasts. Nucleic Acids Res. 1992 May 25;20(10):2597–2597. doi: 10.1093/nar/20.10.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona J. J., Swanson R. N., Rould M. A., Steitz T. A., Söll D. Structural basis for misaminoacylation by mutant E. coli glutaminyl-tRNA synthetase enzymes. Science. 1989 Dec 1;246(4934):1152–1154. doi: 10.1126/science.2686030. [DOI] [PubMed] [Google Scholar]
- Price S., Cusack S., Borel F., Berthet-Colominas C., Leberman R. Crystallization of the seryl-tRNA synthetase:tRNAS(ser) complex of Escherichia coli. FEBS Lett. 1993 Jun 14;324(2):167–170. doi: 10.1016/0014-5793(93)81386-e. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Saks M. E. Contributions of discrete tRNA(Ser) domains to aminoacylation by E.coli seryl-tRNA synthetase: a kinetic analysis using model RNA substrates. Nucleic Acids Res. 1993 Sep 25;21(19):4467–4475. doi: 10.1093/nar/21.19.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. H., Fersht A. R. Asymmetry of tyrosyl-tRNA synthetase in solution. Biochemistry. 1988 Feb 9;27(3):1041–1049. doi: 10.1021/bi00403a029. [DOI] [PubMed] [Google Scholar]
- Weygand-Durasevic I., Johnson-Burke D., Söll D. Cloning and characterization of the gene coding for cytoplasmic seryl-tRNA synthetase from Saccharomyces cerevisiae. Nucleic Acids Res. 1987 Mar 11;15(5):1887–1904. doi: 10.1093/nar/15.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]