Abstract
We report the cloning and characterization of rat α10, a previously unidentified member of the nicotinic acetylcholine receptor (nAChR) subunit gene family. The protein encoded by the α10 nAChR subunit gene is most similar to the rat α9 nAChR, and both α9 and α10 subunit genes are transcribed in adult rat mechanosensory hair cells. Injection of Xenopus laevis oocytes with α10 cRNA alone or in pairwise combinations with either α2-α6 or β2-β4 subunit cRNAs yielded no detectable ACh-gated currents. However, coinjection of α9 and α10 cRNAs resulted in the appearance of an unusual nAChR subtype. Compared with homomeric α9 channels, the α9α10 nAChR subtype displays faster and more extensive agonist-mediated desensitization, a distinct current–voltage relationship, and a biphasic response to changes in extracellular Ca2+ ions. The pharmacological profiles of homomeric α9 and heteromeric α9α10 nAChRs are essentially indistinguishable and closely resemble those reported for endogenous cholinergic eceptors found in vertebrate hair cells. Our data suggest that efferent modulation of hair cell function occurs, at least in part, through heteromeric nAChRs assembled from both α9 and α10 subunits.
Sensory epithelia of the organs responsible for hearing (cochlea) and balance (vestibular labyrinth) share a unique subset of cells that respond to mechanical cues. Designated hair cells, they possess apical mechanoreceptors and specialized basolateral membranes that act in concert to transduce mechanical stimuli into electrical signals (1–3). In the mammalian cochlea there exist two types of hair cells that subserve distinct functions and receive characteristic patterns of innervation (4). The single row of inner hair cells receive nearly all the afferent innervation and are the primary acoustic transducers. The three rows of outer hair cells receive efferent axons from neurons located in the superior olivary complex of the brainstem and form part of a complex “feedback loop” that regulates frequency selectivity and sensitivity (5, 6). Electrical stimulation of the olivocochlear bundle serves to hyperpolarize outer hair cells (7, 8) and reduce auditory afferent output by suppression of basilar membrane motion (9, 10). Acetylcholine (ACh) is the principal neurotransmitter released by medial olivocochlear efferent axons (11), and extant pharmacological and electrophysiological data support a model in which ACh-gated depolarization is followed by activation of small-conductance calcium-activated potassium channels and subsequent hair cell hyperpolarization (12). The outer hair cell nicotinic ACh receptor (nAChR) exhibits an unusual pharmacological profile (see ref. 2 and references therein) that is most similar to that described for receptors assembled in vitro from the nAChR α9 subunit gene (13–16).
Until now, the working hypothesis has been that the α9 nAChR subunit, functioning as a homopentameric ACh-gated channel, is the native hair cell nAChR. However, three findings remain at odds with this hypothesis: the current–voltage relationship, the Ca2+ sensitivity, and the desensitization properties of homomeric α9 nAChRs do not match those seen in isolated hair cells (17, 18). As part of a search for additional nAChR subunit genes (which might modify α9 function), we used segments of the rat α9 nucleotide sequence to query GenBank expressed sequence tag databases. This report describes the cloning, functional characterization, and transcript localization of the rat α10 nAChR subunit. Because both α9 and α10 subunit genes are transcribed in adult rat hair cells, and receptors assembled from α9 and α10 subunits exhibit properties that are indistinguishable from native cochlear hair cell cholinergic receptors, we propose that efferent olivocochlear innervation of outer hair cells is mediated by heteromeric α9α10 nAChRs.
Experimental Procedures
Cloning of the Rat α10 nAChR Subunit.
The human and mouse expressed sequence tag (EST) databases at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) were searched (19) by using nucleotide queries derived from the rat α9 nAChR subunit gene (13). One human EST clone, hAA243627, was obtained from Incyte Genomic Systems (St. Louis) and completely sequenced. Comparison of this clone, encoding amino acids 172–457 (rat α9 subunit sequence numbering), with all known vertebrate nAChR subunits suggested that it encoded a previously uncharacterized nAChR subunit. A fragment of the hAA243627 plasmid was used to screen an adult rat cochlea cDNA library. Three independent full-length clones were isolated. One clone, designated pBRUNO 1.0, encoding the entire rat α10 subunit gene was used in all subsequent experiments. Both DNA strands of pBRUNO 1.0 were sequenced (GenBank accession number AF196344). Genomic clones encoding the mouse α10 gene were isolated from a mouse strain 129/SvJ genomic DNA library by using pBRUNO 1.0 as probe. Assignment of intron–exon boundaries was made by using a combination of cDNA and genomic sequencing and physical mapping of 129/SvJ genomic clones encoding the α10 gene. In situ hybridization experiments using 35S-UTP-labeled antisense and sense configured cRNA probes were carried out essentially as described (13).
Expression of Rat α10 Subunit in Xenopus laevis Oocytes.
Electrophysiological procedures have been described in detail elsewhere (15). Typically, oocytes were injected with 50 nl of RNase-free water containing 0.01–1.0 ng of both α9 and α10 cRNAs (at a 1:1 molar ratio) and maintained in Barth's solution (15) at 17°C. Because comparable amounts of α9 cRNA injected into 1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester-acetoxymethyl ester (BAPTA-AM)-treated oocytes yielded ACh-evoked currents in the low nA range (2–50 nA), homomeric α9 nAChRs were examined after injections of 1–10 ng cRNA per oocyte. During electrophysiological recordings, oocytes were superfused continuously (≈10 ml/min) with normal frog saline containing 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, and 10 mM Hepes buffer (pH 7.2). ACh was applied in the perfusion solution of the oocyte chamber. In experiments where Ca2+ concentrations were varied from nominally 0 to 1.8 mM, the remaining components of the frog saline were held constant, and oocytes were injected with 7.5 ng of a connexin C38 antisense oligonucleotide to reduce activation of nonselective inward current through gap junction hemichannels. Because of desensitization at higher agonist concentrations, experiments with α9α10 nAChRs were performed by using 10 μM ACh. As shown for 10 μM ACh (Fig. 2B), preliminary findings indicate that α9α10 responses to 100 μM ACh were potentiated also by millimolar concentrations of Ca2+ when compared with nominally zero Ca2+. Oocytes were treated with the Ca2+-chelator BAPTA-AM (100 μM) for 3–4 h before electrophysiological recordings to minimize activation of the endogenous Ca2+-sensitive chloride current (15). Concentration-response curves were normalized to the maximal agonist response in each oocyte. For the inhibition curves, antagonists were coapplied with 10 μM ACh, and responses were referred to as a percentage of this value. The mean and SEM of peak current responses are represented. Curve fits and statistical analysis were performed as described (13).
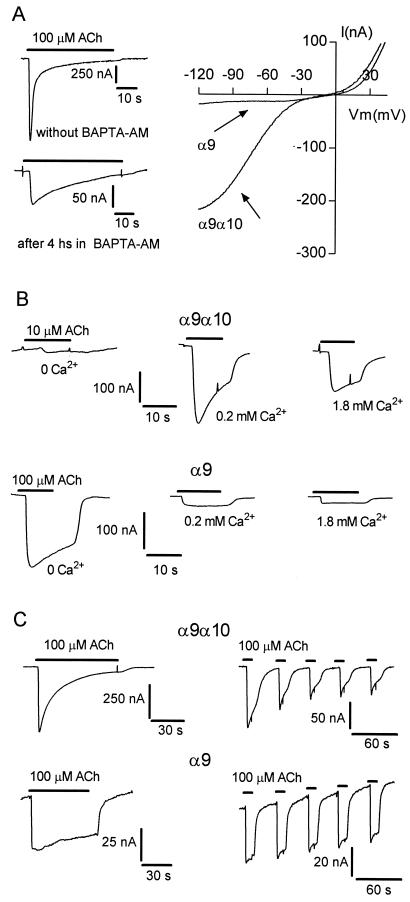
Figure 2.
α9α10 nAChRs exhibit distinct functional properties. (A, Left) Representative electrophysiological responses to 100 μM ACh obtained in an oocyte expressing the α9α10 nAChR before (Upper) and after (Lower) a 4-h incubation in 100 μM BAPTA-AM. (Right) Voltage ramps (2 sec from −120 to +50 mV, at Vhold = −70 mV) were applied 20 sec after the onset of 100 μM ACh application. (B) Representative responses elicited by ACh (Vhold = −90 mV) in the presence of different concentrations of extracellular Ca2+ for oocytes expressing α9α10 nAChRs (Upper) and α9 nAChRs (Lower). (C) Responses to ACh either in the continued presence of agonist or after repeated challenges with agonist (10-sec pulses with 40-sec intervals). (Upper) α9α10 nAChRs. (Lower) α9 nAChRs.
Results
Cloning of the Rat α10 nAChR Subunit Gene.
cDNA clones encoding the rat α10 nAChR subunit gene were isolated from an adult rat cochlea library as described in Experimental Procedures. Fig. 1 shows the nucleotide and deduced amino acid sequence of one such clone, designated pBRUNO 1.0. This α10 cDNA is 2,169 bp in length and contains the entire coding region of the α10 gene as well as portions of the 5′ (63 bp) and 3′ (765 bp) untranslated regions. The protein encoded by the rat α10 gene has characteristics that are reminiscent of other members of the nAChR gene family. First, the α10 protein has substantial overall sequence identity (34–57%) with previously cloned nAChR α-subunits. Second, the hydrophobicity profile suggests the presence of a signal peptide as well as four strongly hydrophobic domains that, as with other nAChR subunits, are likely to be membrane-spanning regions. Third, although every member of the nAChR subunit gene family has cysteines near positions 153 and 167 (α10 numbering, see Fig. 1), the contiguous Cys-217 and -218 present in α10 represent a canonical motif present in all agonist binding (or α-type) nAChR subunits. Finally, the protein encoded by the rat α10 subunit gene is most similar to α9 (57% overall identity). Moreover, a combination of physical mapping and sequencing of mouse α10 genomic clones reveals that both α9 and α10 genes have identical intron–exon boundaries (shown in Fig. 1).
Figure 1.
Nucleotide and deduced amino acid sequences of a cDNA clone encoding the rat nAChR α10 subunit. The deduced amino acid sequence is shown above the nucleotide sequence. Amino acids are presented as the standard single-letter code. Functional domains are underlined and include a possible signal peptide and four putative transmembrane regions. Filled circles (●) are placed above extracellular cysteine residues that are conserved in nAChR agonist-binding (or α-type) subunits. The calculated molecular mass of the mature nonglycosylated form of the rat α10 protein is 47,127 Da. Intron positions are indicated by an arrowhead, and the asterisk marks the translation termination codon.
Functional Characterization of the Rat nAChR α10 Subunit.
To determine whether the protein encoded by the α10 subunit gene could assemble into a functional nAChR, α10 cRNA was injected into X. laevis oocytes. Two-electrode voltage-clamped oocytes did not respond to 100 μM ACh (n = 48). In addition, oocytes injected with α10 cRNA were unresponsive to 100 μM nicotine, muscarine, glutamate, serotonin, γ-aminobutyric acid, adenosine, epinephrine, histamine, and dopamine. Moreover, pairwise injections of α10 cRNA with cRNAs transcribed from plasmids encoding the nAChR α2-α6 or β2-β4 subunits did not result in detectable ACh-gated currents (n = 5–12 for each combination). Preliminary findings (n = 4) show that ACh-gated currents obtained with α7- and α7α10-injected oocytes exhibit identical rapid desensitization kinetics and possess inward-rectifying current–voltage relationships characteristic of homomeric α7 nAChRs.
However, oocytes injected with both α9 and α10 cRNAs responded to superfused ACh with robust depolarizing responses that ranged from 0.2 to 5 μA (Fig. 2A). Because injection of oocytes with comparable amounts of α9 transcripts alone typically yielded maximal responses of ≈50 nA, the coinjection data revealed a marked synergism and suggested an interaction between the α9 and α10 subunits. Such an interaction may include the formation of heteromeric α-subunit-containing AChRs assembled from the α9 and α10 genes. To explore this possibility, we compared the responses of oocytes injected with α9 cRNA alone (homomeric α9 nAChRs) and those injected with an admixture of α9 and α10 cRNAs (heteromeric α9α10 nAChRs). Treatment of α9α10-injected oocytes with the Ca2+-chelator BAPTA-AM resulted in an 88.3 ± 2.7% decrease (n = 10) in ACh-evoked currents. As previously described for homomeric α9 receptors (15), this result is compatible with the presence of Ca2+ permeable α9α10 nAChRs and subsequent stimulation of endogenous Ca2+-activated chloride channels. To minimize the contribution of the Cl− current, all subsequent experiments were performed with BAPTA-AM-treated oocytes.
Fig. 2A shows representative current–voltage responses obtained by applying 2-sec voltage ramps (−120 to +50 mV) 20 sec after superfusion of ACh. The apparent reversal potentials, −11 ± 1.3 mV (n = 22) for α9α10 receptors and −10 ± 1 mV (n = 46) for α9 receptors (15), were nearly identical. Near the reversal potential, both α9 and α9α10 channels showed considerable rectification, although α9α10 channels pass substantially more current at hyperpolarized potentials. Indeed, the ratio of ACh-elicited currents at +40 mV with respect to that at −90 mV is 0.81 ± 0.07 for α9α10-expressing oocytes and 3.1 ± 0.53 (P < 0.01) for α9-expressing oocytes.
Extracellular Ca2+ ions modulate the activity of several nAChRs (see ref. 15 and references therein). Indeed, homomeric α9 nAChRs are blocked by Ca2+ in a voltage-dependent fashion with an IC50 of 100 μM (15). Fig. 2B shows responses of α9- and α9α10-injected oocytes to ACh in the presence of increasing concentrations of Ca2+ ions. As previously noted, such treatment results in a pronounced inhibition of current through homomeric α9 channels (15). However, currents through heteromeric α9α10 channels showed a more complex response to extracellular calcium; responses were negligible under nominally Ca2+-free conditions, potentiated by 0.2 mM Ca2+, and decreased by millimolar concentrations of Ca2+ ions.
As illustrated in Fig. 2C, homomeric α9 and heteromeric α9α10 channels differed with respect to their desensitization kinetics. In α9α10-expressing oocytes, responses were reduced markedly after continuous application of 100 μM ACh. Moreover, a progressive desensitization was observed after 10-sec pulses of 100 μM ACh delivered at 40-sec intervals. In contrast, ACh-evoked currents in α9-expressing oocytes did not desensitize significantly during continuous or intermittent application of agonist. At a holding potential of −70 mV, the current remaining after 20 sec in the presence of ACh was 57.1 ± 4.3% of peak values for α9α10 nAChRs (n = 6) and 94.9 ± 1.6% for α9 nAChRs (n = 12).
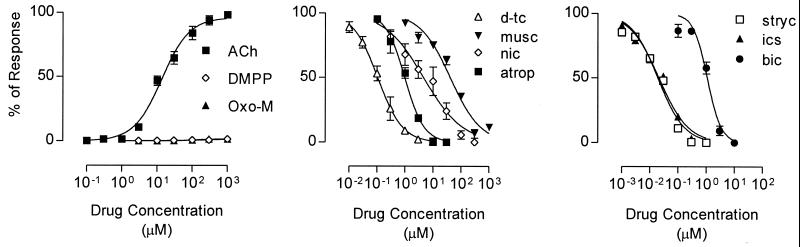
The pharmacological properties of α9α10 nAChRs are presented in Fig. 3, and the results compared with homomeric α9 and native hair cell nAChRs are shown in Table 1. ACh had an apparent affinity of 13.8 ± 1.7 μM (n = 5) and a Hill coefficient of 1.1 (Fig. 3, Left). As with α9 nAChRs (13, 16), α9α10 nAChRs did not respond to nicotine or muscarine (data not shown); however, both the nicotinic agonist 1,1-dimethyl-4-phenylpiperazinium (DMPP) and the muscarinic agonist oxotremorine M (Oxo-M) induced small inward currents (≈1% of the maximum ACh response). Although neither nicotine nor muscarine activated α9α10 nAChRs, both of these cholinergic agonists reduced ACh-evoked currents in α9α10-expressing oocytes (Fig. 3, Middle). Furthermore, the rank order of potency (d-tubocuraine > atropine > nicotine > muscarine) was the same for both α9- and α9α10-receptor subtypes. Interestingly, several compounds that act as antagonists at noncholinergic receptors such as strychnine (glycine receptors), bicuculline (γ-aminobutyric acid type A receptors), and ICS-205,930 (ligand-gated serotonin receptors) are potent inhibitors of both α9 (14) and α9α10 nAChRs (Fig. 3, Right and Table 1). Finally, as described for α9 nAChRs (13), the α9α10 subtype is blocked reversibly by nanomolar concentrations of α-bungarotoxin (α-BgTx; data not shown). Pretreatment of α9α10-expressing oocytes with 100 nM α-BgTx results in a 96.6 ± 1.2% decrease (n = 3) in the amplitude of the response elicited by 10 μM ACh. After a 10-min wash in the absence of α-BgTx, 68.2 ± 6.3% of the initial response is recovered.
Figure 3.
Pharmacological responses of oocytes expressing the α9α10 nAChR. (Left) Concentration-response curves for ACh 1, 1-dimethyl-4-phenylpiperazinium (DMPP) and oxotremorine methiodide (Oxo-M). (Middle and Right) Inhibition of ACh-evoked responses in the presence of a variety of cholinergic and noncholinergic receptor compounds. The estimated IC50 values for each drug are summarized in Table 1.
Table 1.
Comparison of the pharmacological properties of native cochlear hair-cell AChRs and nAChRs assembled from rat α9 and α9α10 subunits
| Compound | Hair cells | α9 | α9α10 |
|---|---|---|---|
| Agonists (EC50) | |||
| Acetylcholine | 7–22 μM | 11.4 μM | 13.8 μM |
| Carbachol | 87 μM, partial agonist | 64 μM, partial agonist | na |
| DMPP | Partial agonist | Partial agonist | Partial agonist |
| Oxotremorine | Partial agonist | Partial agonist | Partial agonist |
| Antagonists (IC50) | |||
| Nicotine | μM range | 31.5 μM | 3.9 μM |
| d-tubocurarine | nM range | 300 nM | 110 nM |
| α-bungarotoxin | nM range | nM range | nM range |
| Atropine | μM range | 1 μM | 1 μM |
| Muscarine | na | 75–83 μM | 41 μM |
| Strychnine | nM range | 18 nM | 20 nM |
| Bicuculline | μM range | 0.8 μM | 1 μM |
| ICS-205,930 | na | 20 nM | 20 nM |
Localization of α10 nAChR Subunit Gene Expression.
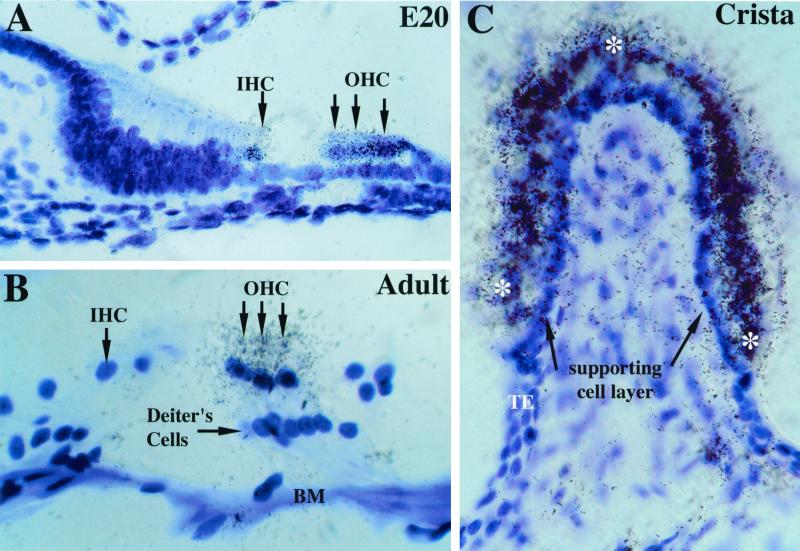
In situ hybridization experiments showed expression of α10 transcripts in sensory organs of the rat inner ear. By using sections obtained during development (embryonic day 20), α10 signal was observed over both inner and outer hair cells (Fig. 4A). At postnatal day 13, both inner and outer hair cells continued to transcribe the α10 gene, but by postnatal day 21 (and through 4 months of age), α10 transcripts were observed only over outer hair cells (Fig. 4B). In all sections examined, little or no signal above background was detected over Deiters cells (Fig. 4B) or neurons of the spiral ganglia (data not shown). In adult rats, hybridization signal over outer hair cells was observed in cross sections of the organ of Corti from base to apex (data not shown). In the vestibular labyrinth, α10 transcripts were detected in sensory epithelia of the otolithic organs (saccular and utricular maculae; data not shown) and the semicircular canals (ampullary crista; Fig. 4C). Silver grains were deposited over hair cells in both organs but were not detected over supporting cell layers or in Scarpa's ganglion (data not shown).
Figure 4.
α10 nAChR subunit gene expression in cochlear and vestibular hair cells. (A) At embryonic day 20, both inner and outer hair cells (arrows) were decorated with silver grains after emulsion dipping of slides. No other structures in the cochlea were labeled (magnification ×630). (B) In adults (2–4 months old), α10 mRNA was detected only in outer hair cells and not in inner hair cells. BM, basilar membrane; IHC, inner hair cells; OHC, outer hair cells (magnification ×1,000). (C) Adult cristae express α10 transcripts as evidenced by the dense accumulation of silver grains over the entire surface (see asterisks) of the sensory end organ. Note the lack of positive reaction at both the transitional epithelium (TE) and the supporting cell layer (black arrows) underlying the hair cell layer (magnification ×630).
Previous reports have shown that the α9 gene is transcribed in the pars tuberalis of the hypophyseal gland, sternohyoid and tongue muscle, nasal epithelium, bone marrow cells, and embryonic blood cells (13, 20). However, by using in situ hybridization, we could not detect α10 transcripts in any of the above-mentioned adult rat tissues. Finally, in situ hybridization has failed to detect α9 and α10 transcripts in adult rodent brain.
Discussion
The Rat α10 Subunit Modifies α9 nAChR Function.
This report describes the cloning and functional characterization of α10, the 17th member of the nAChR subunit gene family. Although the protein encoded by the rat α10 gene does not form homomeric ACh-activated channels in Xenopus oocytes, the extant electrophysiological data argue that it does form heteromeric α9α10 receptors when coexpressed with the rat α9 subunit. Forming heteromeric channels with select nAChR α or β subunits is not a general property of the α10 subunit, however, because oocytes injected with pairwise combinations of α10 cRNA and cRNA transcribed from plasmids encoding either the α2-α6 or β2-β4 subunit genes did not result in the appearance of detectable ACh-evoked currents.
Coinjection of Xenopus oocytes with α9 and α10 cRNAs results in the appearance of ACh-gated channels possessing properties that are distinct from α9-injected oocytes. In particular, α9α10-injected oocytes show ≈100-fold larger currents, have a unique current–voltage relationship, exhibit more rapid and complete desensitization kinetics, and show a biphasic response to changes in extracellular Ca2+ ions. It seems reasonable to postulate that the synergy observed in coinjected oocytes is a consequence of a direct interaction between α9 and α10 subunits. The large increase in current amplitude seen in α9α10-injected oocytes may result from an increased efficiency of α9α10-receptor assembly and/or insertion into the oocyte membrane, a slower rate of receptor turnover, altered biophysical properties of the α9α10 heteromeric channels, or a combination of these traits.
Although the presence of the α10 subunit modifies key biophysical characteristics of α9 nAChRs, homomeric α9 and heteromeric α9α10 nAChRs exhibit remarkably similar pharmacological profiles (with the notable exception of a 10-fold difference in IC50 values for nicotine; see Table 1). It is possible, given the similarity of the α9 and α10 extracellular domains (≈80% identical or conserved residues), that cholinergic ligand-binding sites located near the α9/α9 and α9/α10 subunit interfaces are functionally equivalent in both receptor subtypes. Similar pharmacological profiles might be observed also if the α9 subunit harbored sites for interaction with cholinergic ligands while the α10 subunit served as a “structural subunit” to modulate calcium sensitivity, rectification, or desensitization kinetics.
α9α10 nAChRs in Native Hair Cells: Functional Implications.
The signature pharmacology of the nAChR at the efferent olivocochlear hair cell synapse (7, 21, 22) was sufficiently distinct that before the cloning and characterization of the α9 nAChR subunit, none of the extant recombinant nAChRs were likely candidates to fill the role. Since then, however, characterization of recombinant α9 nAChRs (14–16) and generation of a transgenic α9−/− null mutant mouse (23) has provided a combination of data compelling enough to propose a central role for α9-containing nAChRs in the cholinergic biology of mechanosensory hair cells.
Is the native outer hair cell nAChR an α9 homomer or an α9α10 heteromer? Although the pharmacological data aren't sufficient to distinguish among the two subtypes, the biophysical data strongly support the α9α10 heteromer. First, the Ca2+ modulation of chick (24) and guinea pig (18) hair cell responses most closely resembles α9α10 nAChRs. Although ACh-evoked responses in native and α9α10 nAChRs are potentiated by external Ca2+, α9 receptors show a voltage-dependent block at all concentrations tested (15). Second, the current–voltage relationship for the α9α10 nAChR is most similar to that described for the native rat hair cell nAChR (17). Although aspects of the current–voltage responses vary among species, both hair cell and α9α10 channels pass current in inward and outward directions. This property differs from other native and recombinant neuronal nAChRs, which show a marked inward rectification at depolarized potentials. Finally, although homomeric α9 channels show no significant desensitization, native rat (17) and guinea pig (18) hair cell nAChRs desensitize in a manner reminiscent of that reported here for the α9α10 nAChR.
Are there other nAChR subunit genes transcribed in hair cells that might partner with either α9 or α10? A reverse transcription–PCR study using microdissected adult rat cochlea suggests that the α7 gene also may be transcribed in the organ of Corti, albeit at levels that were undetectable in parallel in situ hybridization experiments (25). No other nAChR α or β subunits have been detected in hair cells. In the absence of subunit-specific antibodies, we have been unable to determine whether heteromeric α9α10 complexes actually exist in vivo; however, given the remarkably concordant physiology for heteromeric α9α10 and native hair cell nAChRs, we favor the presence of an α9α10 nAChR subtype.
Recent whole-cell recordings from immature rat (postnatal day 7–13) apical inner hair cells show that both synaptic currents and responses to ACh are mediated by a channel with a pharmacological profile very much like α9 and α9α10 nAChRs (26). As the inner ear matures and hearing begins (near the second postnatal week), α10 expression by neonatal inner hair cells begins to disappear, and by postnatal day 21 all responses to ACh are lost. Because α9 transcripts are present in rat inner hair cells from embryonic day 16 through adulthood, it is possible that the loss of ACh sensitivity is caused by postnatal cessation of α10 transcription (Fig. 4B) and subsequent loss of heteromeric α9α10 nAChRs.
In summary, the data presented in this report are consistent with the formation of an nAChR assembled from two α subunits. This heteromeric α9α10 subtype retains the unique pharmacology of homomeric α9 nAChRs and, in concert with appropriate biophysical properties and cellular localization, is a strong candidate for the nAChR subtype present at synapses formed between olivocochlear axons and mechanosensory hair cells of the inner ear. Such synapses continue to act as singularly instructive prototypes for documenting the role of postsynaptic nAChRs in central sensory systems.
Acknowledgments
This work was supported by grants from the University of California Los Angeles Faculty Frontiers of Science, the Stein–Oppenheimer Foundation, and the National Institute of Drug Abuse (DA11836 to J.B.), an International Research Scholar grant from the Howard Hughes Medical Institute and Agencia Nacional de Promoción Científica y Tecnológica, Argentina (to A.B.E.), and a grant from the National Institute on Deafness and Other Communication Disorders (DC02871 to S.H.).
Abbreviations
- ACh
acetylcholine
- nAChR
nicotinic ACh receptor
- BAPTA-AM
1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester-acetoxymethyl ester
Note Added in Proof.
During preparation of this manuscript, two other laboratories submitted the human α10 nAChR subunit gene to GenBank under accession numbers AF199235 and AJ278118.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF196344).
References
- 1.Hudspeth A J. Nature (London) 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs P A. Curr Opin Neurobiol. 1996;6:514–519. doi: 10.1016/s0959-4388(96)80058-4. [DOI] [PubMed] [Google Scholar]
- 3.Guth P S, Norris C H. Hear Res. 1996;98:1–8. doi: 10.1016/0378-5955(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 4.Warr W B. Organization of Olivocochlaer Efferent Systems in Mammals/The Mammalian Auditory Pathway: Neuroanatomy. New York: Springer; 1992. [Google Scholar]
- 5.Guinan J J, Jr, Stankovic K M. J Acoust Soc Am. 1996;100:1680–1690. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- 6.Ulfendahl M, Flock A. Curr Opin Neurobiol. 1998;8:475–479. doi: 10.1016/s0959-4388(98)80034-2. [DOI] [PubMed] [Google Scholar]
- 7.Housley G D, Ashmore J F. Proc R Soc London. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs P A, Murrow B W. J Neurosci. 1992;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sridhar T S, Liberman M C, Brown M C, Sewell W F. J Neurosci. 1995;15:3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugasu E, Russell I J. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eybalin M. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 12.Yuhas W A, Fuchs P A. J Comp Physiol. 1999;185:455–462. doi: 10.1007/s003590050406. [DOI] [PubMed] [Google Scholar]
- 13.Elgoyhen A B, Johnson D S, Boulter J, Vetter D E, Heinemann S. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 14.Rothlin C V, Katz E, Verbitsky M, Elgoyhen A B. Mol Pharmacol. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- 15.Katz E, Verbitsky M, Rothlin C V, Vetter D E, Heinemann S F, Elgoyhen A B. Hear Res. 2000;141:117–128. doi: 10.1016/s0378-5955(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 16.Verbitsky M, Rothlin C V, Katz E, Elgoyhen A B. Neuropharmacology. 2000;39:2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 17.Dulon D, Lenoir M. Eur J Neurosci. 1996;8:1945–1952. doi: 10.1111/j.1460-9568.1996.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 18.Blanchet C, Eróstegui C, Sugasawa M, Dulon D. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 20.Zuo J, Treadaway J, Buckner T W, Fritzsch B. Proc Natl Acad Sci USA. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs P A, Murrow B W. Proc R Soc London. 1992;248:35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- 22.Erostegui C, Norris C H, Bobbin R P. Hear Res. 1994;74:135–147. doi: 10.1016/0378-5955(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 23.Vetter D E, Liberman M C, Mann J, Barhanin J, Boulter J, Brown M C, Saffiote-Kolman J, Heinemann S F, Elgoyhen A B. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- 24.McNiven A I, Yuhas W A, Fuchs P A. Audit Neurosci. 1996;2:63–77. [Google Scholar]
- 25.Morley B J, Li H S, Hiel H, Drescher D G, Elgoyhen A B. Mol Brain Res. 1998;53:78–87. doi: 10.1016/s0169-328x(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 26.Glowatzki E, Fuchs P A. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]