Abstract
Tissue factor (TF) is the primary initiator of blood coagulation. In addition to hemostasis, TF can initiate intracellular signaling and promote inflammation and angiogenesis, the key processes underlying the pathogenesis of age-related macular degeneration (AMD). AMD, the leading cause of irreversible blindness among the elderly, involves many genetic and environmental risk factors, including oxidative stress and inflammation. In this study, TF expression was examined in human AMD tissue and in the eyes of a model of AMD, the Ccl2−/−/Cx3cr1−/− (DKO) mouse, as well as in the ARPE-19 cell line after lipopolysaccharide (LPS) and H2O2 stimulation. Total RNA was extracted from tissue samples and further analyzed by real-time RT-PCR. Immunohistochemistry was performed to evaluate TF protein expression. In the human retina, a 32-fold increase of TF mRNA expression was detected in AMD macular lesions compared to normal maculae. TF protein expression was also enhanced in human AMD maculae. Similarly, TF transcript and protein expression were moderately increased in retinal lesions, neuroretinal tissue, and cultured RPE cells of DKO mice compared to age-matched wild-type mice. TF expression level correlated with age in both wild-type and DKO mice. In order to better understand how AMD might lead to enhanced TF expression, 1, 5, and 10 μg/mL LPS as well as 100 and 200 μM H2O2 were used to stimulate ARPE-19 cells for 24 and 2 hours, respectively. LPS treatment consistently increased TF transcript and protein expression. H2O2 alone or in combination with LPS also moderately enhanced TF expression. These results indicate that upregulated TF expression may be associated with AMD, and inflammatory and oxidative stress may contribute to TF expression in AMD eyes.
Keywords: age-related macular degeneration, inflammation, oxidative stress, retina, retinal pigment epithelium, tissue factor
Age-related macular degeneration (AMD) represents a leading cause of irreversible blindness in the elderly.1, 2 Advanced AMD is generally subclassified into geographic atrophy (dry) and neovascular/exudative (wet) AMD. Dry AMD progresses relatively slowly and is characterized by the accumulation of drusen deposits, degeneration and atrophy of both retinal pigment epithelium (RPE) and photoreceptors. Wet AMD leads to sudden and severe vision loss and is characterized by choroidal neovascularization (CNV) - the growth of new blood vessels from the choroid into Bruch's membrane or the subretinal space and retina.
Tissue factor (TF), a transmembrane cell-surface receptor for plasma coagulation factor VII (FVII) and its activated form FVIIa, is the primary initiator of mammalian blood coagulation. Normal endothelium lacks detectable TF expression, whereas vascular subendothelial cells constitutively express TF.3 Upon vascular injury, FVII binds to TF to form the TF/VIIa complex, which initiates the coagulation cascade by activating downstream coagulation factors. In addition to hemostasis, the TF/VIIa complex can also mediate intracellular signaling through protease-activated-receptors, and promote inflammation4, 5 and angiogenesis.6–8
Inflammation and angiogenesis have been implicated in the pathogenesis of AMD. Drusen formation initiates inflammation by stimulating the production of cytokines and reactive oxygen species (ROS), and leads to further RPE and photoreceptor damage. Studies have found an association of AMD with single-nucleotide polymorphisms of the genes coding for complement factor H, factor B, C2, and C3.1, 9 Of interest, coagulation factor X and fibrinogen, which are downstream mediators of blood coagulation closely related to the expression of TF activity, have been detected in AMD lesions as well.10–14 Inflammation elicited by lipopolysaccharide (LPS), TNF-α, IL-1, IL-6, C5a, and many other factors can result in increased cellular expression of TF. Enhanced TF expression may in turn induce expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and macrophage inflammatory protein-2α (MIP-2α/CXCL2α).4, 15
Analogous to the process of tumor angiogenesis, in the later stages of AMD, CNV serves to actually reduce the blood supply to the retina, with diminished transport of macromolecules such as oxygen, thereby creating a hypoxic environment. Hypoxia subsequently induces the expression of vascular endothelial growth factor (VEGF) and promotes further CNV formation.16 In cancer and inflammation, a positive feedback loop exists between VEGF and TF; TF can induce angiogenesis by upregulating VEGF,17–19 and enhanced VEGF can in turn increase TF expression.20
Recent studies have suggested the possibility that TF may be implicated in the pathogenesis of AMD.14 Immunostaining revealed TF expression in RPE cells and macrophages in post-mortem eyes with CNV and in surgically excised CNV specimens,21 TF was expressed strongly in macrophages and variably in RPE cells. In addition, TF staining was observed to be increased in “inflammatory-active” versus “inflammatory-inactive” CNV. Belting and colleagues reported that TF phosphorylation was associated with pathological neovascular vessels but not with normal vessels in human diabetic retina,6 indicating a potential role of TF in CNV formation. Furthermore, targeting TF has been proposed as a potential immunotherapy for treating CNV. Administration of a FVII-Fc chimeric antibody, which targets TF, selectively obliterated CNV without any side effects in laser-induced mouse and pig models, which simulate neovascular AMD.22, 23 Taken together, these findings suggest that aberrant expression or activity of intraocular TF may play a role in AMD pathogenesis and that TF may serve as an effective therapeutic target for pathological neovascular lesions in AMD retina.
TF expression in normal and diseased eyes as well as its association with increasing age has not been previously demonstrated. In addition, it still remains unclear whether TF can serve as a target or marker in early and dry AMD. In the current study, we examined TF expression in human AMD retina, as well as in the eyes of the murine model of AMD, Ccl2−/−/Cx3cr1−/− double-knockout (DKO) mice, which spontaneously develop focal, progressive retinal lesions and elevated A2E levels as early as 4–6 weeks of age.24 TF expression under the control of inflammatory and oxidative stress was also evaluated in vitro in ARPE-19 cells after stimulation with LPS and H2O2.
MATERIALS AND METHODS
Human eye sections
The study was approved by the National Eye Institute Institutional Review Board for human subjects. Archived paraffin-embedded sections cut through the macula of age-matched two non-AMD and 4 AMD eyes were selected. The AMD retinas demonstrated macular disciform scars or neovascular AMD, indicating end-stage of the disease.
Animals
The development of DKO mice was described previously.24, 25 The DKO mice and age-matched wild type (WT) control (C57Bl/6) mice were bred in-house. All animal experiments were performed under protocols approved by the NEI Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Both WT and DKO had young (one-month-old mice) and old (one-year-old) subgroups. Three mice of each subgroup were used in experiments.
Cell culture
Adult human RPE cells (ARPE-19) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in DMEM/F12 medium (1:1) (Sigma, St Louis, MO) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 1% L-glutamine-penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO), 1% 100× MEM non-essential amino acids (Invitrogen, Carlsbad, CA), and 1% N1 growth medium supplement (Sigma-Aldrich, St. Louis, MO). The cells were cultured at 37 °C in humidified 5% CO2 condition and split when 90% confluent.
Microdissection
Human retinal cells in the macular area, including RPE, were manually microdissected from paraffin-embedded AMD eye sections. Retinal neuronal and RPE cells from normal maculae were also microdissected from non-AMD human eye sections to compare TF mRNA expression levels. Retinal lesion sites of DKO mice and comparable retinal areas from WT mice were microdissected from frozen sections. Total RNA was isolated from the microdissected cells following the instructions of the Picopure™ RNA Isolation Kit (Arcturus Bioscience, Mountain View, CA).
Dissection of mouse neuroretinal tissue and RPE
The neuroretinal tissue and RPE of DKO and WT mice was dissected following a protocol published previously.26 In brief, a limbal puncture was made in an enucleated eye. Cutting around the limbus, the cornea, lens, and vitreous were removed. Growth medium was injected to dislodge the neuroretina from the RPE. The neuroretina was plucked off and snap-frozen for RNA isolation. One hundred μl of 0.25% EDTA-trypsin (Invitrogen, Carlsbad, NY) was added to each posterior eyecup and incubated at 37°C for 45 minutes. The RPE cells were dislodged by injecting growth media. The collected RPE was spun at 2000 rpm for 5 minutes. The cell pellet was resuspended in 5ml growth medium and plated onto a 96-well cell culture plate (Costar, Corning. Inc., Corning, NY) at 5% CO2/37 °C. The first change of growth medium was performed after 72 hours. Thereafter, growth medium was changed every other day and cells split in a 1:3 ratio into new 25 cm2 flasks upon reaching 90% confluence. The cells were used for experiments at passages between 4 and 7.
Detection of TF transcripts by quantitative real time RT-PCR
Total RNA was isolated from microdissected retinal tissue, cultured mouse RPE cells and ARPE-19 cells using the PureLink Micro-to-Midi RNA purification system according to the manufacturer's protocol (Invitrogen, Carlsbad, NY). Equal amounts of RNA were reverse transcribed with Superscript II RNase H Reverse Transcriptase (Invitrogen, Carlsbad, NY). RT-PCR was performed on the resulting cDNA using Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA). The comparative cycle threshold value (Ct) method, representing log transformation, was used to establish relative quantification of the fold changes in gene expression using Stratagene Mx3000P QPCR System (SABioscience Corporation, Frederick, MD). Fold changes were normalized first by the level of β-actin. The average fold change in different subgroups was again normalized to the level of young WT or control cell. TF primers were purchased from SABiosciences Corporation. For an internal control, β-actin was amplified with a sense primer (5'-TCCCCCAACTTGAGATGTATGAAG-3') and an antisense primer (5'-AACTGGTCTCAAGTCAGTGTACAGG-3') (Sigma-Genosys, Woodlands, TX).
Immunohistochemistry
Immunohistochemical (IHC) analysis was carried out using avidin-biotin-complex immunoperoxidase technique. Paraffin-embedded human eye sections were deparaffinized in xylene and rehydrated in a graded series of ethanol before IHC. Enucleated WT and DKO mouse eyes were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Inc., Torrance, CA), snap-frozen, and cut via the papillary-optic nerve plane. Cultured mouse RPE cells and ARPE-19 cells were plated overnight on 8-well glass LabTek chamber slides (Nunc, Rochester, NY) at 10,000 cells/well.
The specimens were fixed in acetone for 7 minutes and rinsed in Tris-buffer saline (0.05 M, pH 7.4). Endogenous peroxide activity was blocked in 0.3% hydrogen peroxide (H2O2) for 15 minutes. The slides were then immersed in 10% goat serum to block unspecific background staining and incubated with primary antibodies for 1 hour at room temperature. The primary antibody against mouse TF (polyclonal IgG rabbit antibody, American Diagnostica, Greenwhich, CT) was diluted to 1:100, and the primary antibody against human TF (4509 monoclonal mouse antibody, American Diagnostica, Greenwhich, CT) to 1:2000. The secondary antibody was biotin-conjugated, goat anti-rabbit IgG (Vector laboratories, Burlingame, CA), and the chromagen was 3,3'-diaminobenzidine (Biocare Medical, Concord, CA). The slides were counterstained with 1% methyl green. IHC was performed three times for each specimen (the eyes and the cultured cells). Representative images were taken for illustrations.
LPS and H2O2 stimulation of ARPE-19 cells
ARPE-19 cells grown to 90% confluence in 25 cm2 flasks were incubated in fresh growth medium without FBS for 24 hours, and subsequently with various concentrations of LPS and H2O2. Five mg Salmonella typhimurium LPS stock (DIFCO, Livonia, MI) was used at final concentrations of 1, 5, and 10 μg/mL in growth media for 24 hours. In order to induce oxidative stress, 100 and 200 μM H2O2-conditioned media was added for 2 hours by dissolving 30% H2O2 stock solution (Fisher Scientific, Fair lawn, NJ) in growth media just before use. Afterwards, the stimulated cells were examined for TF mRNA and protein expression via RT-PCR and IHC, as described above.
Determination of ARPE-19 cell viability by crystal violet staining method
ARPE-19 cells were seeded in 96-well culture plate in growth medium with FBS. After the cells became adherent, growth medium without FBS was added for 24 hours. After LPS and H2O2 stimulation for the indicated time periods, the cells were washed with phosphate buffered saline (PBS), stained with crystal violet staining solution (0.4% crystal violet, 20% ethanol) for 20 minutes, washed three times with PBS, and air-dried for one hour. The cells were then incubated with 200 μl of extraction buffer (10% methanol, 10% acetic acid) for 15 minutes. 100 μl of the dye mixture was transferred to a 96-well plate and, and viability was determined by measuring the optical density at 590 nm using an ELISA plate reader (BioTek, Burlington, VT). The cell viability was greater than 90% in all experiments.
Statistical analysis
Differences in TF mRNA levels among groups were evaluated by one-way analysis of variance (ANOVA) using SPSS version 16.0 (SPSS Inc., Chicago, IL) with the level of significance set at P<0.05. The values are presented as means ±SD.
RESULTS
Detection of TF expression in human AMD retina
IHC result showed diffuse immunoreactivity for TF, which was expressed in the entire neuroretina of both non-AMD (Figure 1a) and AMD (Figure 1b) eyes, in particular in the inner and outer plexiform layers as well as in the nerve fiber layers (see the arrows) as reported previously.14 However, immunoreactivity for TF was much stronger in AMD retina, when compared to non-AMD retina. Within the same eye, the macula showed higher TF expression than the periphery (data not shown). Detectable RT-PCR result was only available for microdissected cells from one non-AMD and one advanced wet AMD retina. The expression of the TF transcript in AMD lesions was 32-fold higher than the expression level in the non-AMD normal retinal area (Figure 1c).
Figure 1. TF expression in human non-AMD and AMD retina.
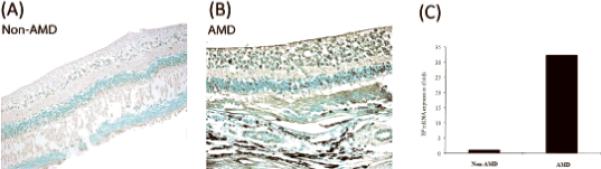
A representative section stained with the avidin– biotin-complex method is illustrated (A) in human non-AMD retina and (b) in human AMD retina (advanced wet AMD). In all samples evaluated to date, IHC analysis shows more intense immunoreactivity against TF (brown-blackish staining indicated by arrows) in AMD compared to non-AMD retina. (c) RT-PCR was performed on one AMD retina and one non-AMD retina to quantify relative TF mRNA expression. Microdissected retinal lesions in AMD retina demonstrated a 32-fold increase in TF transcript level as compared to normal maculae from non-AMD retina.
Evaluation of TF expression in WT and DKO mouse eyes
Knowing that elevated TF expression might be associated with the pathogenesis of AMD, we investigated whether TF expression is also increased in the eyes from the DKO murine model of AMD. Frozen sections of age-matched WT and DKO mouse retina were immunostained for TF expression (Figure 2a and 2b, respectively). Moderately higher TF expression was observed in all cells of the DKO retina as compared with those in the WT retina; in particular the plexiform layers and the nerve fiber layer were most heavily endowed with TF expression (see the asterisks).
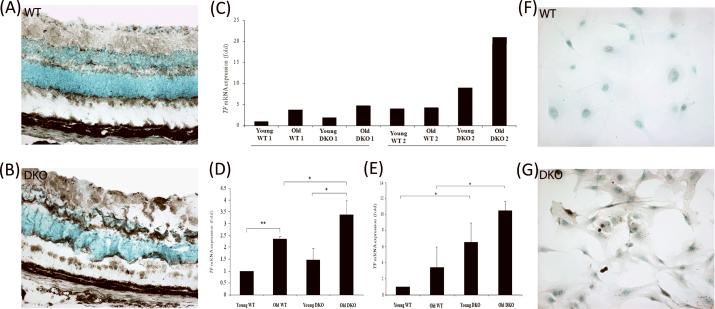
Figure 2. TF expression in WT and DKO mouse eyes.
(A and B) IHC demonstrated more intense TF staining (asterisks) in DKO retina compared to WT retina. (C) TF mRNA levels were analyzed and compared to that in young WT. Retinal lesions of DKO mice express higher TF mRNA levels than is observed in the normal retinal area of age-matched WT mice. Two sets of mice were analyzed and the retinal tissue from the second set of DKO mice (with more lesions) exhibited a higher TF expression level than was observed with the first set of animals. (d) TF mRNA expression in neuroretinal tissue increased in DKO mice as compared with age-matched WT mice. TF expression increased with age in WT and DKO mouse retina (*p<0.05 and **p<0.01; n = 3). (e) TF mRNA expression in RPE cells isolated from the mice also increased with age. DKO RPE cells demonstrated significantly higher TF transcript level compared to age-matched WT cells (*p value<0.05; n=3). (f and g) TF antigen expression was elevated in cultured RPE cells isolated from WT mice compared to RPE cells isolated from DKO mice.
In order to investigate whether TF is particularly upregulated in the retinal lesion sites and, therefore, potentially contributes to AMD pathogenesis, retinal cells in the lesions were microdissected from frozen sections of age-matched WT and DKO retina for quantitative RT-PCR (Figure 2c). The frozen sections and funduscopic pictures of the second samples of DKO retina demonstrated more lesions than the first DKO samples (data not shown). The RT-PCR results demonstrated a trend toward higher TF mRNA expression in DKO lesions compared to WT normal retina, which was more prominent in the second samples of mice. In the first samples of mice, the expression of TF in young and old DKO mice were 1.93 and 4.69-fold greater, respectively, as compared to the expression level observed in the retina of young WT mice, whereas the second samples of young and old DKO mice demonstrated 9.00 and 20.95-fold increase, respectively, in TF transcript level, compared to that in young WT mice from the first samples of experiments. In addition, TF transcript levels increased in an age-related fashion in both WT and DKO mice, indicating a possible link between age and TF expression in the retina.
The whole neuroretinal tissue (a much larger surface area than the microdissected retinal cells in the focal lesions) was removed from age-matched DKO and WT mice for RNA isolation to evaluate generalized TF expression level (Figure 2d). TF mRNA level increased significantly as a function of age in both WT and DKO mice from 1.00 to 2.35±0.1 in WT (p<0.01) and from 1.46±0.50 to 3.39±0.59 in DKO (p<0.05). The older subgroup of DKO mice had a significantly higher mean TF mRNA level than was observed in the older WT mice (p<0.05). TF mRNA was increased slightly in young DKO mice compared to young WT but this difference was not statistically significant.
Lastly, mice RPE cells were dissected and cultured for IHC and RT-PCR. Compared to age-matched WT RPE cells, RPE cells from both young and old subgroups of DKO mice expressing significantly higher TF transcript levels, were observed in both young (p<0.05) and old (p<0.05) subgroups of DKO (Figure 2e; n=3). Stronger immunoreactivity for TF was observed in DKO RPE cells (Figure 2g), as compared to WT (Figure 2f). Similar to the previous results, TF mRNA level in RPE cells showed a positive association with age in both WT and DKO; increasing from 1.00 to 6.53±2.42 in cells from the WT mice and from 3.39±2.57 to 10.47±1.19 in cells from the DKO mice; however, this difference was not statistically significant.
LPS and H2O2-mediated induction of TF expression in ARPE-19 cells
ARPE-19 cells were stimulated with 1, 5, and 10 μg/mL of LPS for 24 hours. Moderately increased TF antigen expression was observed in ARPE-19 cells stimulated with 10 μg/mL LPS (Figure 3b) compared to non-stimulated control cells (Figure 3a). TF mRNA expression in the cultured cells demonstrated a dose-dependent increase with LPS treatment (Figure 3c). LPS doses of 1, 5 and 10 μg/ml induced, respectively, 2.01±1.27, 2.17±0.15, and 3.45±0.16 fold increase in TF transcript level in the cells. The results of the 5 μg/mL and 10 μg/mL doses of LPS stimulation were found to be statistically significant (p<0.05 and p<0.01, respectively).
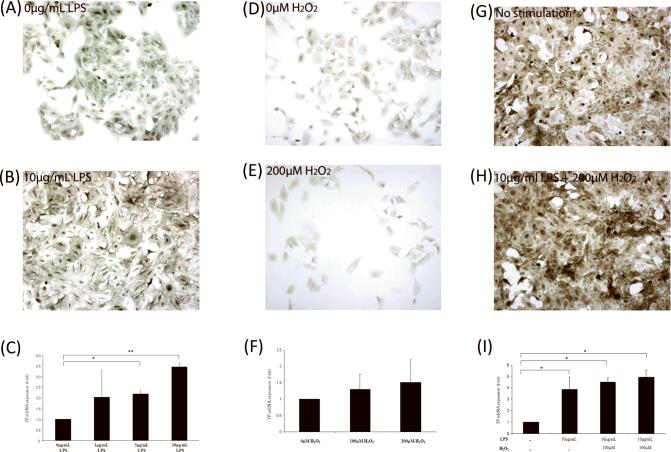
Figure 3. TF expression in ARPE-19 cells.
(a and b) ARPE-19 cells stimulated with 10μg/mL LPS demonstrated increased TF protein expression compared to non-stimulated control cells. (c) Dose-response curve for LPS; increased TF mRNA expression with increasing concentration of LPS (*p<0.05 and **p<0.01; n = 3). There was no statistically significant difference among 1, 5 and 10μg treatments. (d and e) TF protein expression in ARPE-19 cells increased when stimulated with H2O2 compared to control cells. (f) TF mRNA expression slightly increased upon H2O2 stimulation (n=3). (g and h) Stimulation with 10μg LPS and 200μM H2O2 induced a moderate increase in TF protein expression compared to the control. The result of the combination of stimulants was similar to that of LPS treatment alone. (i) LPS alone increased TF mRNA expression by 3.88-fold over the baseline level, similar to the previous results. Addition of H2O2 further enhanced TF transcript level but the difference was not statistically significant (*p value<0.01; n=3).
Stimulation with 200 μM H2O2 for 2 hours (Figure 3e) did not induce noticeable TF protein expression compared to the control cells (Figure 3d). TF mRNA expression was increased 1.30±0.44 and 1.52±0.69-fold over the control cells (Figure 3f) when stimulated by 100 and 200 μM H2O2, respectively. No statistical significance was observed.
Finally, ARPE-19 cells pre-stimulated with 10 μg/mL LPS for 24 hours and then induced with previously selected doses of H2O2 for 2 hours demonstrated increased TF protein expression (Figure 3h) compared to control (Figure 3g). RT-PCR data showed that 10 μg/mL LPS treatment alone induced 3.88±1.07 fold significant increase in TF transcript expression (Figure 3i: p<0.01). Addition of 100 and 200 μM H2O2 induced a further increase in TF mRNA expression levels to 4.51±0.35 (p<0.01) and 4.95±0.60 (p<0.01), respectively (Figure 3i). These data suggest that enhanced TF expression in human AMD and DKO eyes may be, in part, induced by inflammatory signals (e.g. simulated by LPS) and oxidative stress (e.g. simulated by H2O2).
DISCUSSION
Tissue factor is a ubiquitous cell surface protein that regulates the activation of mammalian coagulation. TF is expressed constitutively only on the surface of cells involved in so-called “barrier functions” -e.g. fibroblasts, smooth muscle cells, pericytes, trophoblasts, etc.-in anatomic locations where the prevention of bleeding is critical (e.g. subendothelium, central nervous system, lung, placenta and perhaps, in the eye). In the membrane of other cells, such as endothelial cells and circulating monocytes, TF exists in an encrypted form, devoid of procoagulant properties. In response to specific receptor binding to TF of its natural, high-affinity ligand (FVII and/or FVIIa), or to cell stimulation by inflammatory mediators such as IL-1β or TNF-α and/or interaction with proteases released from inflammatory or malignant cells, cellular TF becomes “de-encrypted” and is able to function as an important regulator of signal transduction. Indeed, TF expression has now been linked to oncogene-mediated malignant transformation of cells, atherogenesis, chronic inflammation and a variety of other pathogenic processes.27, 28 It was with this knowledge of the rather ubiquitous relationship of TF expression to inflammatory, degenerative and malignant processes that we sought information regarding TF in AMD.
To our knowledge, this is the first study that demonstrates the association of intraocular TF expression with increasing age and compares TF expression levels of normal with AMD eyes, as well as WT and DKO eyes. ARPE-19 cells were also used as an in vitro model in order to understand better how the microenvironment of AMD might induce TF expression. Although TF has been implicated in AMD,14 in most of the published studies, investigators have examined TF in human CNV specimens and laser-induced CNV models, which reflect principally the neovascular component of AMD.21–23 In addition, it remains unclear whether TF expression induced by laser injury has direct relevance to evaluating the role of TF expression in AMD or simply represents a response to acute injury or wound healing regardless of underlying retinal disease.
Enhanced expression of TF mRNA and protein was observed in human advanced wet AMD retina compared to non-AMD retina (Figures 1a, 1b, 1c). Due to the availability of a limited number of AMD cases, we cannot be certain if our findings are representative of all AMD cases; a question, which remains unanswered and requires validation. As an alternative, we examined TF expression in the human RPE cell line, ARPE-19, after creating an environment that mimics that of AMD, which will be discussed later.
In the current study, use of the Ccl2−/−/Cx3cr1−/− murine model of retinal lesions allowed more definitive investigation of the potential association of TF expression with early and also likely dry AMD. DKO mice spontaneously develop retinal lesions without exogenous stimulation, and thus may provide a model, which may closely represent the likely expression and distribution of TF in AMD, in the absence of additional variables such as physical injury and the wound healing response. Therefore, the DKO model enables us to define the effects of age on retinal TF expression independent of laser photocoagulation-induced injury. WT and DKO mice were divided into young and old groups to explore the potential association of TF expression with increasing age. A similar age-dependent increase in TF expression was observed in WT and DKO neuroretinal tissue (Figures 2c, 2d, 2e), which was statistically significant (Figure 2d and 2e). The apparently higher TF transcript levels in young DKO mice vs. young WT were not significantly different, whereas the pair wise comparison in old DKO mice vs. old WT was significantly different. Since young DKO mice start to develop lesions at 4–6 weeks of age and old DKO mice show confluent lesions and photoreceptor atrophy,24, 25 it may be reasonable to postulate that TF expression in the neuroretinal tissue does not increase in the early stage of lesion development but becomes significant in later stages.
Moderately higher TF antigen expression was observed in DKO mice (Figure 2b), indicating a potential link between TF and AMD. Furthermore, TF mRNA level closely corresponded to the number and severity of retinal lesions in DKO mice; the first samples of young and old DKO mice presented with a small focal lesion and retinal degeneration, respectively, whereas the second samples of young and old DKO mice had 3 focal lesions with RPE vacuolization, photoreceptor degeneration and atrophy, respectively. AMD is a focal disease, in which alterations in gene expression in lesion tissue appears to be highly relevant for determining the pathogenic mechanisms of the disease. Therefore, upregulated TF expression in the retinal lesions suggests that TF may play a role in AMD pathogenesis.
Cultured RPE cells dissected from age-matched WT and DKO eyes also demonstrated an age-related increase in TF transcript level. Both young and old DKO RPE showed statistically significant increases in TF transcript levels, as compared to age-matched WT RPE. RPE cells have been reported to express variable levels of TF in human CNV specimens.21 Our data confirms that RPE cells are capable of expressing TF, and extends previous observations by demonstrating that TF may be involved in both dry and wet AMD pathology.
Given that inflammatory and oxidative stress, the key contributors to aging and AMD, are capable of inducing TF expression, ARPE-19 cells were stimulated with LPS (inflammation) and H2O2 (oxidative stress) to simulate a microenvironment consistent with that of AMD and investigate these effect on TF expression. LPS has been used to induce TF expression in monocytes, platelets, and human umbilical vein endothelial cells.29–32 In ARPE-19 cells, LPS consistently enhanced TF protein and transcript expression, confirming that a pro-inflammatory environment can induce TF expression in these cells from the eye as well.
Compared to LPS treatment, H2O2 stimulation induced a slight increase in TF mRNA and protein level (Figures 3d 3e, 3f). Both human and murine TF genes contain one NFκB and two AP-1 binding sites, which are required for optimal gene transcription,33–36 and H2O2 is known to induce the translocation of NFκB and initiate AP-1 binding. However, Penn et al., using smooth muscle cell culture, also observed that H2O2 did not significantly increase TF mRNA or cell surface TF antigen levels, but rather enhanced the activity of preexisting, latent TF.37 Of note, apoptosis is known as an important contributor to blood or plaque thrombogenicity, and colocalization of cellular and extracellular TF expression with apoptotic death was found in the lipid core of atherosclerotic plaques.38 In AMD, apoptotic processes in RPE and photoreceptors induced by oxidative stress may contribute to enhanced TF expression. Thus, different cell types and sources of oxidative stress, as well as duration and severity of the stress, seem to play a role in regulating TF expression and activity.
Although we have limited studies directly linking TF to AMD, several pathological roles of TF in AMD can be proposed based on previous studies of TF expression in other analogous human diseases. Increased TF expression is a hallmark of many inflammatory conditions, such as sepsis, atherosclerosis, and the antiphospholipid syndrome. Redecha et al. showed that anaphylatoxin C5a, which is also observed in drusen, induced TF expression on the surface of neutrophils in antiphospholipid-treated mice.39 Enhancement of ROS production by TF has been described in human macrophages.40 Furthermore, inflammation-active human CNV showed much more intense TF reactivity than inflammation-inactive CNV.21 Thus, increased TF expression in AMD may induce RPE and photoreceptor damage by upregulating both inflammation and ROS production.
Previous studies on the pathobiological role of TF in inflammation and malignancy suggest that enhanced TF gene expression, perhaps with activation of localized blood coagulation, may contribute to AMD development. However, whether activation of TF signaling is also relevant to AMD has not yet been determined. TF was shown to be selectively phosphorylated in association with pathological neovascular but not normal vessels in diabetic retinopathy.6 In order to identify whether TF is an essential participating factor in the pathogenesis of AMD, it will be important to evaluate the level of TF phosphorylation in AMD and DKO retina, in addition to TF expression. In the present studies, we have demonstrated that TF expression increases in human AMD maculae and DKO mouse eyes compared to non-AMD maculae and WT eyes, and that upregulated TF expression may be associated with AMD. Our data also suggest that inflammatory and oxidative stress may lead to enhanced TF expression in aging eyes as well as AMD eyes. A more complete understanding of the role of TF expression and function in AMD may provide a further rationale for potentially targeting TF in the therapy of AMD.
Acknowledgments
Financial Support: The study was supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
List of Abbreviations
- AMD
Age-related macular degeneration
- CNV
Choroidal neovascularization
- DKO
Ccl2−/−/Cx3cr1−/− double-deficient mouse
- IHC
Immunohistochemistry
- LPS
Lipopolysaccharide
- ROS
Reactive oxygen species
- RPE
Retinal pigment epitheliu
- TF
Tissue factor
- VEGF
Vascular endothelium growth factor
- WT
Wild type
Footnotes
Conflict of interest Dr. Rickles declares a proprietary interest in the development of TF-targeting therapies by Pharmacyclics, Inc. and Genmab. The other authors have no proprietary or commercial interest in any materials discussed in this manuscript.
REFERENCES
- 1.Coleman HR, Chan CC, Ferris FL, 3rd, et al. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 4.Chu AJ. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005;440:123–132. doi: 10.1016/j.abb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Chu AJ. Role of tissue factor in thrombosis. Coagulation-inflammation-thrombosis circuit. Front Biosci. 2006;11:256–271. doi: 10.2741/1796. [DOI] [PubMed] [Google Scholar]
- 6.Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Bierhaus A, Schiekofer S, et al. Tissue factor--a receptor involved in the control of cellular properties, including angiogenesis. Thromb Haemost. 2001;86:334–345. [PubMed] [Google Scholar]
- 8.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–1550. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 9.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DH, Talaga KC, Rivest AJ, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 13.Mullins RF, Russell SR, Anderson DH, et al. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb J. 2000;14:835–846. [PubMed] [Google Scholar]
- 14.Cho Y, Rickles FR, Parver LM, et al. The potential pathophysiological role of tissue factor in age-related macular degeneration. Expert Rev Ophthalmol. 2010;5:27–34. [Google Scholar]
- 15.Chu AJ. Tissue factor upregulation drives a thrombosis-inflammation circuit in relation to cardiovascular complications. Cell Biochem Funct. 2006;24:173–192. doi: 10.1002/cbf.1200. [DOI] [PubMed] [Google Scholar]
- 16.Yang XM, Wang YS, Zhang J, et al. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:1873–1879. doi: 10.1167/iovs.08-2591. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Shoji M, Chen J, et al. Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci U S A. 1999;96:8663–8668. doi: 10.1073/pnas.96.15.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Deng Y, Luther T, et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiarugi V, Ruggiero M, Magnelli L. Molecular polarity in endothelial cells and tumor-induced angiogenesis. Oncol Res. 2000;12:1–4. doi: 10.3727/000000001108747372. [DOI] [PubMed] [Google Scholar]
- 20.Takano S, Tsuboi K, Tomono Y, et al. Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br J Cancer. 2000;82:1967–1973. doi: 10.1054/bjoc.2000.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- 22.Bora PS, Hu Z, Tezel TH, et al. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc Natl Acad Sci U S A. 2003;100:2679–2684. doi: 10.1073/pnas.0438014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tezel TH, Bodek E, Sonmez K, et al. Targeting tissue factor for immunotherapy of choroidal neovascularization by intravitreal delivery of factor VII-Fc chimeric antibody. Ocul Immunol Inflamm. 2007;15:3–10. doi: 10.1080/09273940601147760. [DOI] [PubMed] [Google Scholar]
- 24.Tuo J, Bojanowski CM, Zhou M, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CC, Ross RJ, Shen D, et al. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–128. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma V, Sauer T, Chan CC, et al. Constancy of ERp29 expression in cultured retinal pigment epithelial cells in the Ccl2/Cx3cr1 deficient mouse model of age-related macular degeneration. Curr Eye Res. 2008;33:701–707. doi: 10.1080/02713680802236185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorak HF, Rickles FR. Malignancy and hemostasis. In: Colman RW, Hirsh J, Marder VJ, et al., editors. Hemostasis and thrombosis: Basic principles and clinical practice. 5th ed. Lippincott-Raven; Philadelphia, PA: 2006. pp. 851–873. [Google Scholar]
- 28.Rickles FR. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb. 2006;35:103–110. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 29.Norris LA, Weldon S, Nugent A, et al. LPS induced tissue factor expression in the THP-1 monocyte cell line is attenuated by conjugated linoleic acid. Thromb Res. 2006;117:475–480. doi: 10.1016/j.thromres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Meszaros K, Aberle S, Dedrick R, et al. Monocyte tissue factor induction by lipopolysaccharide (LPS): dependence on LPS-binding protein and CD14, and inhibition by a recombinant fragment of bactericidal/permeability-increasing protein. Blood. 1994;83:2516–2525. [PubMed] [Google Scholar]
- 31.Stephens AC, Ranlall NF, Rivers RP. Suppression of HUVEC tissue factor synthesis by antisense oligodeoxynucleotide. Thromb Res. 2008;122:99–107. doi: 10.1016/j.thromres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Panes O, Matus V, Saez CG, et al. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 33.Brisseau GF, Dackiw AP, Cheung PY, et al. Posttranscriptional regulation of macrophage tissue factor expression by antioxidants. Blood. 1995;85:1025–1035. [PubMed] [Google Scholar]
- 34.Mackman N. Regulation of tissue factor gene expression in human monocytic and endothelial cells. Haemostasis. 1996;26(Suppl 1):17–19. doi: 10.1159/000217234. [DOI] [PubMed] [Google Scholar]
- 35.Oeth P, Parry GC, Mackman N. Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol. 1997;17:365–374. doi: 10.1161/01.atv.17.2.365. [DOI] [PubMed] [Google Scholar]
- 36.Moll T, Czyz M, Holzmuller H, et al. Regulation of the tissue factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Sp1-like transcription factors. J Biol Chem. 1995;270:3849–3857. doi: 10.1074/jbc.270.8.3849. [DOI] [PubMed] [Google Scholar]
- 37.Penn MS, Patel CV, Cui MZ, et al. LDL increases inactive tissue factor on vascular smooth muscle cell surfaces: hydrogen peroxide activates latent cell surface tissue factor. Circulation. 1999;99:1753–1759. doi: 10.1161/01.cir.99.13.1753. [DOI] [PubMed] [Google Scholar]
- 38.Mallat Z, Tedgui A. Current perspective on the role of apoptosis in atherothrombotic disease. Circ Res. 2001;88:998–1003. doi: 10.1161/hh1001.090571. [DOI] [PubMed] [Google Scholar]
- 39.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham MA, Romas P, Hutchinson P, et al. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413–3420. [PubMed] [Google Scholar]