Abstract
Systematic studies on Alzheimer's disease (AD)-related pathology that complement clinical and epidemiological data on dementia from low and middle income countries are rare. We report the first large study on AD-related pathology in autopsy service-derived brains from an urban center in India, a low/middle income country, and compare findings with a similar sample from New York. Amyloid-β plaques and neurofibrillary tangles were assessed in 91 brain specimens derived from hospital autopsy cases from Mumbai, India (age 60+ years; mean age 71.1 years, ± 8.3 SD; range 60–107 years) and compared with identically examined age-matched sample obtained in New York. These cases had no known clinical history of dementia. Our study showed that in comparison with the New York sample, the mean brain weight of the Mumbai sample was lower (p = 0.013) and mean diffuse plaque density was higher (p = 0.019), while differences in mean density and counts of neurofibrillary tangles and neuritic plaques were not statistically significant (p > 0.05). Our findings indicate that the burden of AD-related pathology was approximately equivalent in Mumbai and New York samples, which is at variance with expected lower AD-related lesion burden based on the clinical/epidemiological studies suggesting lower prevalence of AD in India.
Keywords: AD-related pathology, Alzheimer's disease, amyloid-β plaques, developing countries, LMIC, neurofibrillary tangles, senile plaques
Introduction
Dementia in old age is a growing healthcare challenge worldwide, as highlighted in the 2009 report of Alzheimer's Disease International [1]. Prevalence of age-related dementia is projected to increase at an even a faster rate in developing countries, or low and/or middle-income countries (LMICs)* due to a more rapid increase in elderly population as compared to the developed countries, or high income countries (HICs)*. This demographic transformation results from dramatically increased life expectancy led by improved socioeconomic conditions and access to better primary medical care [2]. However, medical expertise, resources, and support for healthcare of the elderly, including that for dementia, have not developed in tandem. This situation has prevailed due to limited resource allocation for dementia care and research, and a lack of adequate awareness of dementing diseases among healthcare professionals, health policy makers, and the public [2].
Among the LMICs, India is the second most populous country with over a billion people, where the elderly portion of the population is also large and growing rapidly. India is estimated to have 59.7 million elderly people (65+ years in age) in the year 2010, rising to 86.5 million in 2020 and 124.5 million in 2030 (World Population Prospects: Population database, The 2008 Revision: United Nations Population Division) [3].
Aging is the main risk factor for dementia and thus the number of elderly people suffering from dementia in India will rise in tandem with rising elderly population. Initial epidemiological studies in India suggested low estimates for the prevalence of dementia and Alzheimer's disease (AD), with some showing about one-third of the prevalence [4–7] compared to the United States and other developed countries [8–11]. This was followed by several recent studies based on updated clinical and neuropsychological methodologies [12–21]. Nevertheless, among the present vast elderly population and its projections for exponential growth in the coming few decades, even with these lower prevalence rates, AD and other age-related dementias would affect very large portion of the elderly population.
Epidemiological and neuropsychological investigations in India, similar to other LMICs, face several barriers, including lower literacy levels, sociological factors of stigma of a diagnosis of dementia, and a prevailing notion that memory problems and cognitive loss are natural manifestations of senescence and not due to a disease which might require intervention. Additionally, most elderly people in India live in extended families where they do not need to independently carry out routine, day-to-day functions such as shopping, getting out and about using any modes of transportation, handling financial matters, and maintaining household and its associated responsibilities For these reasons, epidemiological studies investigating cognitive status may not provide precise estimates of prevalence and incidence of dementia. In contrast, the appearance of pathological lesions of amyloid-β (Aβ) plaque deposits and neurofibrillary tangles (NFT) in brain specimens is an outcome of neurobiological mechanisms, therefore pathological studies can offer additional perspectives regarding occurrence of AD. However, there have been very few autopsy confirmations or pathological studies of brain aging and AD in LMICs.
In appraising the extent of AD and other forms of dementia, neuropathological assessment of brain tissue for AD-related changes represents a valuable biologic means to investigate the tendency of this population to develop this disease process. Accordingly, we sought to estimate the burden of AD-pathology prevailing in India and carried out a study on postmortem brain specimens from three large teaching hospitals associated with medical schools in Mumbai, the largest city in India. Here, we present a report on AD-related pathology on 91 autopsy-cases and compared these changes with pathology data of an equal number of age-matched cases from New York, NY.
Materials and Methods
Sample selection
The brain specimens were derived from autopsy services of BYL Nair Ch. Hospital, KEM Hospital, and LTM General Hospital, and were collected in the brain bank of the Mount Sinai/Nair Hospital Brain Aging and Dementia Research Project (funded by a NIH/NIA/FIC grant). The subjects of this study lived primarily in densely populated neighboring localities consisting of populations with lower income and literacy levels. We followed a protocol to obtain consent to examine brain specimens for research and training purposes that was approved by the BYL Nair Ch. Hospital Ethics Committee, KEM Hospital Ethics Committee, and LTM General Hospital Ethics Committee. The research protocol stated that consent to offer brain specimens from the routine hospital autopsy services should be voluntary and after the next of kin(s) had already provided consent for hospital autopsy. Therefore, after the consent for general autopsy was received, our pathology residents approached the next of kin to request brain donation for research purposes. For this study, we examined 91 autopsy brain specimens from elderly decedents 60 years or older, which were accessioned in the period from February 2006 to May 2009 [M/F: 63/28; age range 60–107 years; mean age 71.1 years ±8.3 SD; median age 70 years; mean PMI (postmortem interval) 9.9 hours, ±5.3 SD]. Based on medical chart reviews, these cases were free of clinical history of dementia. In addition, the cases met exclusion criteria for optimum sample collection for our AD-related pathology, which included history of brain trauma and large brain lesions including acute brain infarcts, brain hemorrhage or tumors, and infective diseases (to ensure safety of the laboratory staff). Final autopsy diagnosis/cause of death of these cases included fulminant infections leading to septicemia/septic shock (32), hypovolemic shock from massive internal hemorrhage (16), pneumonia/bronchopneumonia (21), ischemic heart disease/congestive heart failure (13), cancer (7), malaria (1), and unknown (1).
For comparison of the neuropathological findings, autopsy brains of 91 age-matched autopsy cases [age-match ± 3 years; M/F: 50/41; age range 59–108 years; mean age 71.0 ± 9.3 SD; median age 71 years; mean PMI 24.5 hours, ±18.3 SD] were derived from the autopsy service of the Mount Sinai Hospital and affiliated medical facilities and were collectively banked in the Mount Sinai Brain Bank for Neurodegenerative Disorders and Brain-Aging Studies. These cases had no history of dementia according to chart review. The patient population was predominantly of a middle or higher income socio-economical group from metropolitan New York City, an urban center of a HIC. Final autopsy diagnosis/cause of death of these cases included ischemic heart disease/congestive heart failure (41), cancer (23), pneumonia/bronchopneumonia (14), fatal multiple injuries without brain trauma (5), pulmonary embolism (4), septicemia/septic shock (1), hepatic failure (1), and chronic renal failure (2).
Neuropathology methods
The brain specimens of the Mumbai sample from all three Mumbai hospitals were sampled and processed in a centralized brain bank/pathology laboratory at the BYL Nair Hospital in an identical fashion to that of Mount Sinai Brain Bank/Neuropathology Laboratory for Neurodegenerative Diseases. Neuropathological procedures were carried out using the brain examination protocol of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) [22] for selection of brain tissue samples, processing/neurohistological staining of paraffin sections and for assessment and quantification of AD-related lesions. For this study, tissue blocks were sampled for paraffin sectioning from the following areas: middle frontal gyrus (Brodmann area 9), orbital frontal cortex (Brodmann area 45/47), superior/middle temporal gyrus (Brodmann area 21/22), inferior parietal lobule (Brodmann area 7), calcarine cortex (Brodmann area 17/18), the rostral and caudal hippocampus, and midbrain. Paraffin sections were cut at 5-micron thickness for hematoxylin and eosin stain and at 8-micron thickness for modified Bielschowsky silver stain. Immunohistochemistry preparations were also carried out on 8 micron thick sections using antibodies directed against Aβ (Dako Corp., Carpinteria, CA) and abnormal tau (TG3, a gift of Dr. P. Davies, Albert Einstein School of Medicine, Bronx, New York) for NFT formation. The presence and extent of AD-related changes were assessed in the sections stained with modified Bielschowsky's silver stain, blind in respect to the patient information about source of the specimen, age, and clinical history, by pathologists (Drs. Purohit and Batheja) experienced in dementia-related neuropathologic studies. The assessment included estimates (using a 4-point scale: absent, sparse, moderate, and severe, as shown in the CERAD protocol [22]) of the grades of density of senile plaques (SP) that showed dystrophic neurites and an amyloid core, and of diffuse plaques (DP) and NFT. This lesion density assessment was carried out using a medium high power (X20) objective lens producing a visual field measuring 0.785 mm2. The densities of SP in the neocortex and of NFT in hippocampus and entorhinal cortex were also assessed quantitatively at the above mentioned magnification. The SP and NFT were counted in five medium high (X20) power fields, and then the average converted to number per mm2 area as follows: for SP, areas with high neocortical SP density were identified in modified Bielschowsky's silver stained sections, and visual counts of SP were carried out in the five microscopic fields in each of the five neocortical regions. From these results, a mean neocortical SP-count was calculated per mm2 area. Quantitative NFT assessment was carried out in hippocampus and entorhinal cortex as follows: in sections from rostral and caudal hippocampus, the areas with most numerous NFT (or dense clusters) were identified in hippocampus and the adjacent entorhinal cortex, and the lesion counts of these clusters were converted as counts per mm2 area for hippocampus and entorhinal cortex.
In addition to estimating the degree of SP and NFT formation employing the CERAD neuropathological criteria [22], we also determined NFT distribution using the Braak staging method [23]. For neuropathological diagnosis of AD, we employed CERAD diagnostic criteria [22] as well as the consensus recommendation for postmortem diagnosis of AD by the National Institute for Aging/Reagan Institute Working Group [24].
Statistical methods
We used the SPSS 17.0.0 (2008) package for data analysis. We performed analysis of covariance, comparing samples from Mumbai and NY, controlling for age and gender. We also performed repeated measures analysis of covariance by comparing gender- and age-matched samples from Mumbai and NY, controlling for the average age of the pair and for the gender of both subjects. We carried out these analyses for brain weights, NFT grades and count in hippocampus, NFT grades and counts in entorhinal cortex, counts of SP in neocortex, density grades of diffuse plaques, age-adjusted CERAD scores of SP and the Braak scores for NFT.
Results
AD-related pathology
This study evaluated AD-related neuropathological lesions, including DP, SP, and NFT in brain specimens of the Mumbai sample (Figs 1,2, and 3) and compared these with the NY-derived samples. DP, consisting of fine fibrils of silver-stain positive deposits, were the most frequent lesions in both Mumbai and NY samples. Neuritic/amyloid core plaques (SP), characterized by coarse silver-stain positive fibers as well as thick and short darkly stained dystrophic neurites and dense cores of Aβ were encountered less frequently. NFT were identified as dark brown or almost black silver-stained structures in the neurons, often conforming to the shapes of the neurons. Both SP and NFT were better visualized by immunohistochemistry. The frequency and extent of SP and NFT in the brain specimens are shown and analyzed in Table 1. We also carried out statistical analysis of brain weight and AD-related pathology of SP, DP, and NFT in the age-matched Mumbai and NY samples (see Table 2 for detail), which revealed statistically significant differences only in brain weight and in density grades of DP. In the Mumbai samples, brain weight was lower (mean weight 1034.34 gram, SEM 14.92 versus 1209.03 g, SEM 14.42; F = 6.41, p = 0.013) and DP density grades were higher (mean of DP grade 1.7912, SEM 0.22 verus 0.78, SEM 0.11; F = 5.67, p = 0.019). Although mean SP count in the Mumbai sample was higher than that of the NY sample, the difference was not statistically significant (mean SP counts 1.15/mm2, SEM 0.31 versus 0.59/mm2, SEM 0.18; F = 0.79, p = 0.37). Differences in the NFT counts in the hippocampus and entorhinal cortex were not statistically significant, nor were the Braak scales (Table 2).
Fig. 1.
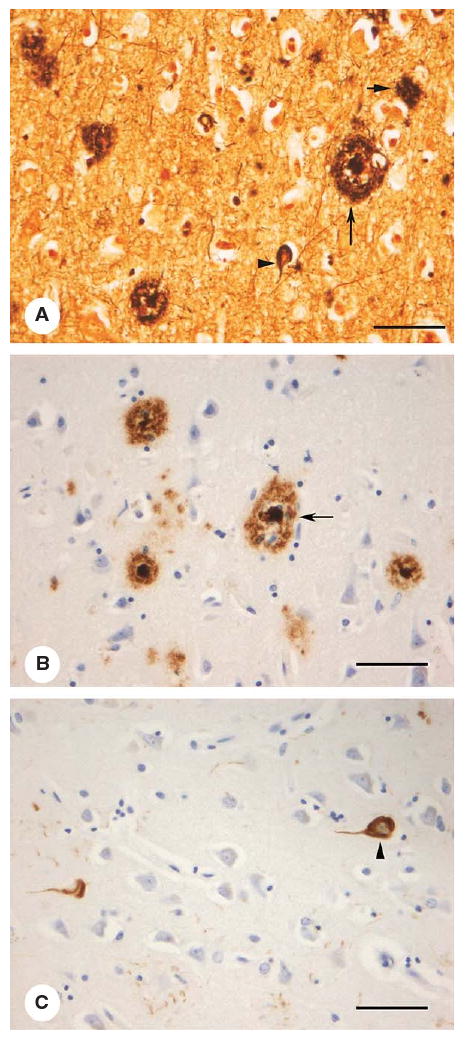
Histological images of AD-related lesions in neocortex: (A) modified Bielschowsky's silver stain, (B) Aβ immunohistochemistry demonstrating amyloid plaques and (C) hyperphosphorylated tau immunohistochemistry for neurofibrillary tangles. Senile plaques (SP, large arrow); diffuse plaque (DP, small arrow), and neurofibrillary tangles (NFT, arrowheads) (bar = 150 μm).
Fig. 2.
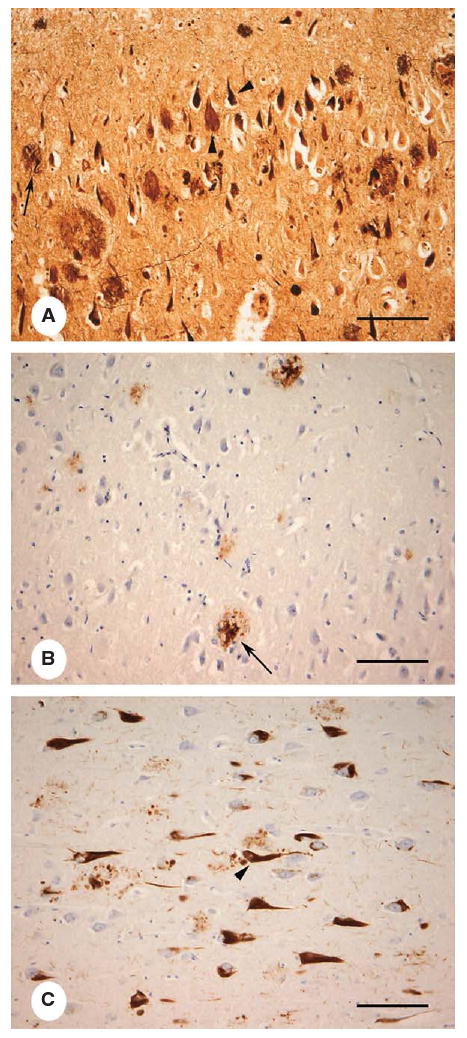
Histological images of AD-related lesions in the layer CA1 of the hippocampus by modified Bielschowsky's silver stain (A), Aβ immunohistochemistry demonstrating amyloid plaques (B) and hyperphosphorylated tau immunohistochemistry for neurofibrillary tangles (C) (bar = 120 μm).
Fig. 3.
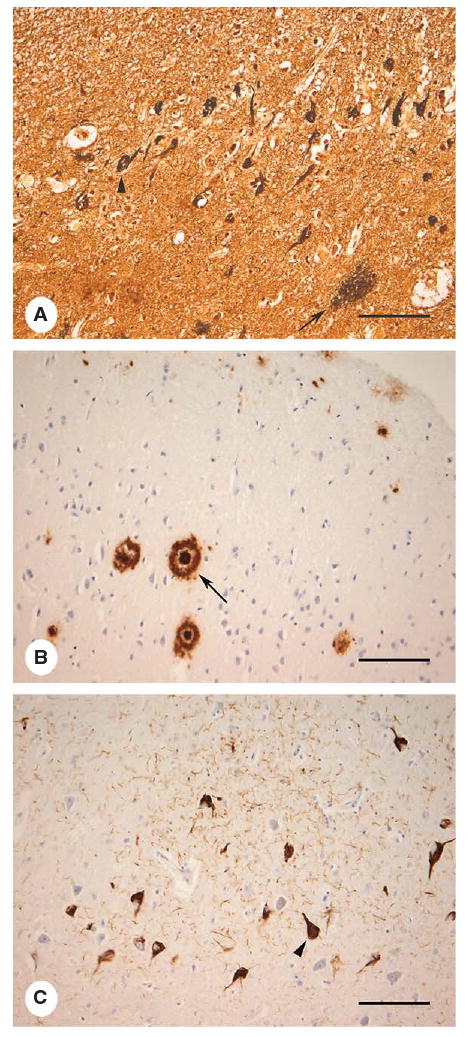
Histological images of AD-related pathology in the entorhinal cortex by modified Bielschowsky's silver stain (A), Aβ immunohistochemistry demonstrating amyloid plaques (B) and hyperphosphorylated tau immunohistochemistry for neurofibrillary tangles (C). Senile plaques (SP, large arrow); diffuse plaque (DP, small arrow), and neurofibrillary tangles (NFT, arrowheads) (bar = 120 μm).
Table 1.
Alzheimer's disease-related pathology in the Mumbai and New York samples, grouped by age and gender (n = 91, number of specimens in each sample)
| Age range | Number of cases (by gender) | Brain weight (g) Mean (SD) |
Senile plaques counts: /mm2 Mean (SD) |
Neurofibrillary tangles Braak score: Mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mumbai | NY | Mumbai | NY | Mumbai | NY | Mumbai | NY | ||
| 60–69y | M | 27 | 22 | 1086.9 (115.7) | 1285.8 (117.3) | 0.39 (1.35) | 0.20 (0.67) | 0.3 (0.61) | 0.52 (0.65) |
| F | 8 | 13 | 1046.8 (156.1) | 1158.5 (108.6) | 0 | 0.55 (1.78) | 0.25 (0.71) | 0.38 (0.65) | |
| 70–79y | M | 26 | 19 | 991.7 (146.2) | 1245.4 (158.2) | 1.36 (3.3) | 0.09 (0.38) | 1.31 (1.23) | 1.26 (0.93) |
| F | 12 | 19 | 979.1 (160.0) | 1181.1 (149.3) | 1.13 (2.92) | 0.91 (2.0) | 1.33 (1.67) | 1.21 (1.32) | |
| 80+ y | M | 10 | 9 | 1082.3 (132.3) | 1200.7 (158.6) | 2.8 (5.39) | 1.78 (2.7) | 2.60 (1.8) | 2.30 (1.1) |
| F | 8 | 9 | 1006.2 (133.3) | 1085.0 (143.9) | 2.04 (2.84) | 0.83 (1.6) | 3.00 (1.8) | 2.0 (1.22) | |
Abbreviation: NY = New York; M = Male, F = Female.
Table 2.
Statistical analysis of Alzheimer's disease-related pathology in all cases* of Mumbai and New York samples
| Mumbai sample Mean (SEM) |
New York sample Mean (SEM) |
F | p | |
|---|---|---|---|---|
| Brain weight | 1034.34 g (14.92) | 1209.03 g (14.42) | 6.41 | 0.013 |
| DP in Neocortex (grade) | 1.79 (0.22) | 0.78 (0.11) | 5.67 | 0.019 |
| NFT in Hippocampus (grade) | 1.24 (0.18) | 1.16 (0.18) | 0.09 | 0.76, ns |
| NFT in Hippocampus (count/mm2) | 4.41 (0.87) | 3.78 (0.78) | 0.01 | 0.95, ns |
| NFT in EC (grade) | 1.39 (0.20) | 1.32 (0.16) | 1.33 | 0.25, ns |
| NFT in EC (count/mm2) | 4.06 (0.71) | 3.81 (0.63) | 0.80 | 0.37, ns |
| SP in Neocortex (count/mm2) | 1.15 (0.31) | 0.59 (0.18) | 0.79 | 0.37, ns |
| Braak stage for NFT | 1.01 (0.14) | 1.11 (0.12) | 1.93 | 0.16, ns |
| SP, Age adjusted CERAD score | 0.80 (0.17) | 0.39 (0.11) | 0.42 | 0.51, ns |
n = 91; controlled for ages and genders.
Abbreviations: DP = diffuse plaques; NFT = neurofibrillary tangles; EC = entorhinal cortex; SP = senile plaques; CERAD = consortium to establish a registry for Alzheimer's disease.
We also compared the frequency of cases with SP, DP, and NFT in the Mumbai and NY samples (see Fig. 4), which revealed that the Mumbai sample showed AD-related lesions in about the same number of cases as in the NY sample, but overall there were greater number of cases with moderate or severe degrees of SP and NFT formation. Neocortical NFT were present in 8 cases of Mumbai sample (in three cases they were in a moderate to severe degree). In the NY sample, only three cases showed neocortical NFT, which were sparse in degree.
Fig. 4.
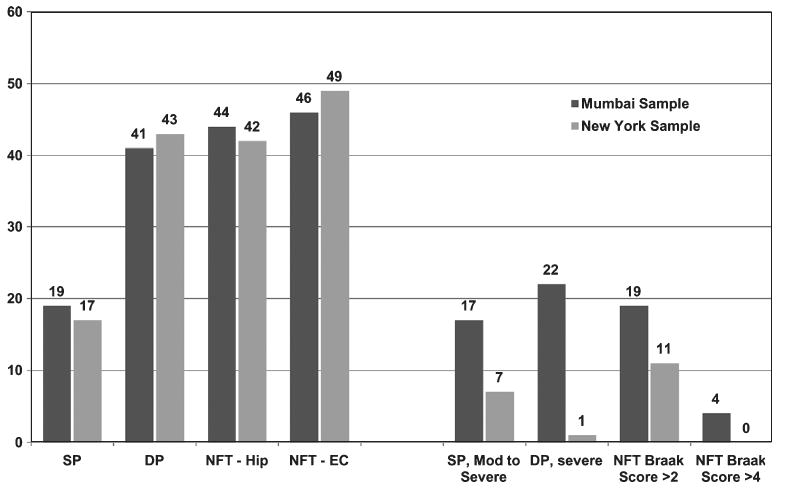
Comparison of AD related pathology in 91 age-matched cases of the Mumbai and New York samples. SP, neuritic/amyloid core plaques; DP, diffuse amyloid plaques; NFT, neurofibrillary tangles; NC, neocortex; Hip, hippocampus; EC, entorhinal cortex.
We further investigated the basis of relatively more cases in Mumbai sample showing higher degree of AD-pathology by carrying out another comparative analysis in a set of subgroups of the 82 cases from Mumbai sample and 89 cases from NY sample after excluding the cases that showed CERAD's “C” grade SP score (which indicated diagnosis of AD; CERAD criteria [22] for neuropathological diagnosis; see below in “Diagnosis of AD” section for further details). We analyzed SP, DP, and NFT lesion loads on 76 pairs (M = 49, F = 27) from this subgroup, derived after age and gender-matched pairing. This analysis (Table 3) also showed statistically significant differences only in the brain weights (p = 0.000) and DP (p = 0.013). Although there was no statistically significant difference in the density or counts of SP or NFT (p = ns) in the subgroup from Mumbai sample, there was marked reduction in the mean SP counts [from 1.15/mm2 (SEM 0.31) in all 91 cases to 0.35/mm2 (SEM 0.14) in the cases of this smaller subgroup of 76 cases] and in the age-adjusted SP scores [from 0.80 (SEM 0.17) to 0.38 (SEM 0.10)].
Table 3.
Paired-sample statistical analysis of AD-related pathology of subsets generated by excluding cases indicating neuropathological diagnosis of AD (CERAD criteria) and further excluding gender/age mismatches
| Mumbai sample (n = 76) Mean (SEM) |
New York sample (n = 76) Mean (SEM) |
F | p | |
|---|---|---|---|---|
| Brain weight | 1045.95 g (14.67) | 1221.38 g (17.27) | 42.73 | 0.000 |
| DP (grades) | 1.42 (0.23) | 0.77 (0.12) | 6.50 | 0.013 |
| NFT in Hippocampus (grade) | 1.27 (0.20) | 1.15 (0.19) | 0.32 | 0.57, ns |
| NFT in Hippocampus (count/mm2) | 4.18 (0.85) | 3.86 (0.89) | 0.20 | 0.65, ns |
| NFT in EC (grades) | 1.25 (0.21) | 1.42 (0.18) | 0.07 | 0.79, ns |
| NFT in EC (count/mm2) | 3.68 (0.78) | 4.13 (0.73) | 0.01 | 0.92, ns |
| SP in Neocortex (count/mm2) | 0.35 (0.14) | 0.38 (0.12) | 0.01 | 0.90, ns |
| Braak stage for NFT | 1.02 (0.15) | 1.14 (0.12) | 0.30 | 0.58, ns |
| SP, Age adjusted CERAD score | 0.30 (0.10) | 0.23 (0.07) | 0.10 | 0.78, ns |
Abbreviations: AD = Alzheimer's disease; DP = diffuse plaques; EC = entorhinal cortex; NFT=neurofibrillary tangles; SP = senile plaques; CERAD = consortium to establish a registry for Alzheimer's disease [22].
Diagnosis of AD
In a considerable number of brains in the Mumbai sample, the above described AD-related pathology (or changes of pathological aging) was present to such an extent that could raise a possibility of AD, considering that a diagnosis of dementia in those cases might have remained clinically unrecognized ante-mortem. We applied CERAD criteria [22] in these cases and found that in the Mumbai sample, there were 9 cases with an age-adjusted CERAD SP score of “C” indicating a diagnosis of AD, while the NY sample had only two such cases. Applying the NIA/Reagan Institute Criteria [24] based on the degree of SP and NFT pathology, we found two cases in the Mumbai sample with high probability of AD (CERAD SP score “C” and Braak scale “V/VI”) and another 6 cases with intermediate probability of AD (CERAD SP score of “B” and Braak scale “III/IV”). In contrast, none of the NY cases had SP and NFT lesion loads that would suggest a diagnosis of high probability of AD and just one case with intermediate probability of AD.
Discussion
The data reported here demonstrates that the degree and extent of AD-related pathology in the brains derived from an urban Indian population are remarkably similar to that in the brains obtained in NY, another urban center but in a high income country (HIC). AD-related pathology was encountered in a similar number of cases in Mumbai and NY samples. We also found that the number of cases in the Mumbai sample showing pathology at the level equivalent to a neuropathological diagnosis of AD was considerably higher than that seen in the NY sample. It is possible that clinical diagnosis of dementia or AD in these cases had escaped ante-mortem detection, since recognition or reporting of dementia as a clinical entity can be uncommon, if not rare, in the population from which the autopsy specimens were derived.
To date, there have been several case reports of neuropathologically-confirmed cases of AD and four studies on pathology of AD from India [25–29] and other LMICs [30–33]. One of these studies reported a total absence of AD-pathology [32] and another reported only 6% of the cases with SP and 88% showing NFT [28]. A smaller study (32 cases) from East Africa [33] showed diffuse or dense amyloid deposits and NFT comparable to age-matched cases from Cleveland, USA. Yasha and colleagues [29] in a study on AD-pathology in 53 autopsy-derived cases collected over 10 years in Bangalore, India, suggested that the AD related lesions were similar to that reported in the HIC, although they did not conduct a direct comparison. In our study, we employed rigorous study design, neuropathological protocol, and statistical methods, and recruited a large sample of consecutive autopsy cases and compared it to the findings of age-matched cases from HIC, employing an identical study protocols.
Our results are in contrast with impressions provided by some epidemiological and clinical studies [5, 6], particularly in rural populations and among early reports [4]. These studies suggest that environmental factors and dietary patterns in rural or other subgroups of India's populace are responsible for protection against dementia. Alternately, the cognitive demands may be limited in rural areas of LMICs, and may mitigate detection of dementia. Furthermore, it is possible that rural prevalence of dementia may be low due to different factors for survival/mortality issues [2]. More recent studies suggest higher rates of dementia but report that up to 50% is non-Alzheimer type with significant attribution to vascular disease [4, 12, 18]. Neuropathological studies such as this could differentiate these possibilities by determining if reported lower incidence is associated with lower pathology and in future work, determining if non-Alzheimer pathology is present and to what extent.
In a recent review, Kalaria et al. [2] observe that the existing retrospective and prospective clinical studies reporting that prevalence estimates of dementia and AD vary widely between and within individual countries, possibly due to subjective, cultural and contextual factors operating within clinical and epidemiological methodology. This may lead to an underestimate of the dementia burden [2]. However, neuropathological studies on autopsy brain specimens can provide culture-independent insights into the extent of dementing diseases, since the pathological lesions are the outcome of biological phenomena. Further, neuropathology can help identify or confirm specific diagnostic types of dementia.
We acknowledge there are limitations to our study. Autopsy studies can be subjected to bias, in particular in sample selection from a restricted pool of autopsies, which in this case was from a subgroup of the population who had reached the hospital with serious and acute illnesses. This group may not fully represent the urban population of Mumbai, in general, or the whole population of the country. In reference to the comparative data of Mumbai and NY samples, we achieve maximum comparability by ensuring that sample collection was carried out with very little sample bias or selection preference that would influence AD-related pathology. Another limitation arose due to an absence of information on cognitive status that was not obtainable retrospectively from the decedent's families, particularly on the cases showing moderate to severe AD-related pathology. If this had been done, it would have facilitated an interesting clinicopathological correlation. Even then, such information would not have provided any significant data to comment on prevalence of dementia in the study population. Therefore, we do not attempt to offer precise estimates of AD-related pathology in the urban population of Mumbai, or of the country at large, but our study indicates that AD-related pathology is apparent in a pattern similar to that in the urban population of NY.
The significant extent of AD-related pathology found in our study cases implies that the prevalence of dementia and AD in India could be similar to the HICs. In absence of correlative studies of neuropathological findings on clinically evaluated cases of dementia, this study may be regarded as a surrogate attempt, but not a substitute of clinicopathological studies on cases of dementia or of more inclusive neurobiological investigations and collaborative research on AD and other dementing neurodegenerative disorders. Such investigations in India (and other LMICs) could provide better indication of the true extent of dementia burden, help plan for future health care needs and also offer neurobiological perspectives that underlie lifestyle and environmental risk/protective factors for AD affecting these cultures.
Acknowledgments
Dr. A. Shah (Neurology Dept, BYL Nair Ch. Hospital, Mumbai site Co-PI and Director of the project) and Dr. Pinto (Department of Psychiatry, BYL Nair Ch. Hospital, Mumbai) carried out administrative functions for the project in Mumbai. Dr. Puranik, Dr, Shenoy and Dr. Shinde (BYL Nair Ch. Hospital), Dr. Kandalkar and Dr. Goel (KEM Hospital) and Dr. Kalgutkar and Dr. Vartak (LTM General Hospital) played important role in establishing the brain bank and acquisition of brain specimens from hospital autopsy services. Laboratory teams led by A. Kadam/V. Kate (Mumbai) and M. Thybulle (NY) provided vital technical support and helped develop the project laboratory in Nair Hospital. Major grant funding was from the NIH's National Institute for Aging and Fogarty International Center (5R01 AG 028188: Cognitive Loss in the Elderly in Mumbai, India; PI D. Purohit) and additional support was from NIH grant funding to Mount Sinai Alzheimer's Disease Research Center (2P50 AG05138-21; PI M. Sano). RNK's research projects are supported by the Medical Research Council, UK and the Alzheimer's Research Trust, UK.
Footnotes
LMIC and HIC are alternative terms devised by the World Bank (http://data.worldbank.org/about/country-classification) for developing and developed countries respectively, as defined by economic status.
Authors' disclosures available online (http://www.j-alz.com/disclosures/view.php?id=686).
References
- 1.Prince M, Jackson J. [December 14, 2010];World Alzheimer Report, Alzheimer Disease International. 2009 http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf.
- 2.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, World Health Organization WHO Statistical Information System (WHOSIS) [December 14, 2010]; http://www.who.int/whosis/database/life_tables/life_tables.cfm.
- 4.Shaji S, Promodu K, Abraham T, Roy KJ, Verghese A. An epidemiological study of dementia in a rural community in Kerala, India. Br J Psychiatry. 1996;168:745–749. doi: 10.1192/bjp.168.6.745. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar S, Kumar S, Thara R. Prevalence of dementia in a rural setting: a report from India. Int J Geriatr Psychiatry. 1997;12:702–707. doi: 10.1002/(sici)1099-1166(199707)12:7<702::aid-gps489>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51:1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 7.Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13:439–450. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- 8.Shibayama H, Kasahara Y, Kobayashi H. Prevalence of dementia in a Japanese elderly population. Acta Psychiatr Scand. 1986;74:144–151. doi: 10.1111/j.1600-0447.1986.tb10598.x. [DOI] [PubMed] [Google Scholar]
- 9.Livingston G, Sax K, Willison J, Blizard B, Mann A. The Gospel Oak Study stage II: the diagnosis of dementia in the community. Psychol Med. 1990;20:881–891. doi: 10.1017/s0033291700036588. [DOI] [PubMed] [Google Scholar]
- 10.Aronson MK, Ooi WL, Geva DL, Masur D, Blau A, Frishman W. Dementia. Age-dependent incidence, prevalence, and mortality in the old old. Arch Intern Med. 1991;151:989–992. doi: 10.1001/archinte.151.5.989. [DOI] [PubMed] [Google Scholar]
- 11.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, Gureje O, Rodenberg CA, Baiyewu O, Musick BS. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 12.Shaji S, Bose S, Verghese A. Prevalence of dementia in an urban population in Kerala, India. Br J Psychiatry. 2005;186:136–140. doi: 10.1192/bjp.186.2.136. [DOI] [PubMed] [Google Scholar]
- 13.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Biswas A, Roy T, Banerjee TK, Mukherjee CS, Raut DK, Chaudhuri A. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163–172. [PubMed] [Google Scholar]
- 15.Raina S, Razdan S, Pandita KK. Prevalence of dementia among Kashmiri migrants. Ann Indian Acad Neurol. 2008;11:106–108. doi: 10.4103/0972-2327.41878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llibre Rodriguez JJ, Ferri CP, Acosta D, Guerra M, Huang Y, Jacob KS, Krishnamoorthy ES, Salas A, Sosa AL, Acosta I, Dewey ME, Gaona C, Jotheeswaran AT, Li S, Rodriguez D, Rodriguez G, Kumar PS, Valhuerdi A, Prince M. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das SK, Biswas A, Roy J, Bose P, Roy T, Banerjee TK, Mukherjee C, Raut DK, Chowdhury A, Hazra A. Prevalence of major neurological disorders among geriatric population in the metropolitan city of Kolkata. J Assoc Physicians India. 2008;56:175–181. [PubMed] [Google Scholar]
- 18.Banerjee TK, Mukherjee CS, Dutt A, Shekhar A, Hazra A. Cognitive dysfunction in an urban Indian population – some observations. Neuroepidemiology. 2008;31:109–114. doi: 10.1159/000146252. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari SC, Tripathi RK, Kumar A. Applicability of the Mini-mental State Examination (MMSE) and the Hindi Mental State Examination (HMSE) to the urban elderly in India: a pilot study. Int Psychogeriatr. 2009;21:123–128. doi: 10.1017/S1041610208007916. [DOI] [PubMed] [Google Scholar]
- 20.Mathuranath PS, Cherian PJ, Mathew R, Kumar S, George A, Alexander A, Ranjith N, Sarma PS. Dementia in Kerala, South India: prevalence and influence of age, education and gender. Int J Geriatr Psychiatry. 2010;25:290–297. doi: 10.1002/gps.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CH, Mizuno T, Elston R, Kariuki MM, Hall K, Unverzagt F, Hendrie H, Gatere S, Kioy P, Patel NB, Friedland RP, Kalaria RN. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol Aging. 2008;31:732–740. doi: 10.1016/j.neurobiolaging.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The consortium to establish a registry for Alzheimer's disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-284. [DOI] [PubMed] [Google Scholar]
- 24.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 25.Shankar SK, Chandra PS, Rao TS. Alzheimer's disease-histological, ultrastructural, and immunochemical study of an autopsy-proven case. Indian J Psychiatry. 1988;30:291–298. [PMC free article] [PubMed] [Google Scholar]
- 26.Satishchandra P, Yasha TC, Shankar L, Santosh V, Das S, Swamy HS, Shankar SK. Familial Alzheimer disease: first report from India. Alzheimer Dis Assoc Disord. 1997;11:107–109. doi: 10.1097/00002093-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Somasundaram O. Alzheimer's disease. J Indian Med Assoc. 1974;63:66–68. [PubMed] [Google Scholar]
- 28.Barodawala SA, Ghadi PS. A progress report on the prevalence of Alzheimer's lesions in Bombay hospital population. Curr Sci. 1992;63:449–455. [Google Scholar]
- 29.Yasha TC, Shankar L, Santosh V, Das S, Shankar SK. Histopathological & immunohistochemical evaluation of ageing changes in normal human brain. Indian J Med Res. 1997;105:141–150. [PubMed] [Google Scholar]
- 30.Osuntokun BO, Ogunniyi A, Akang EE, Aghadiuno PU, Ilori A, Bamgboye EA, Beyreuther K, Masters C. Beta A4-amyloid in the brains of non-demented Nigerian Africans. Lancet. 1994;343:56. doi: 10.1016/s0140-6736(94)90910-5. [DOI] [PubMed] [Google Scholar]
- 31.Dani SU, Pittella JE, Boehme A, Hori A, Schneider B. Progressive formation of neuritic plaques and neurofibrillary tangles is exponentially related to age and neuronal size. A morphometric study of three geographically distinct series of aging people. Dement Geriatr Cogn Disord. 1997;8:217–227. doi: 10.1159/000106634. [DOI] [PubMed] [Google Scholar]
- 32.Osuntokun BO, Ogunniyi A, Junaid TA, Lekwauwa UG. Autopsy survey for Alzheimer's disease in Nigerian Africans: a preliminary report. Afr J Med Med Sci. 1995;24:75–79. [PubMed] [Google Scholar]
- 33.Ogeng'o JA, Cohen DL, Sayi JG, Matuja WB, Chande HM, Kitinya JN, Kimani JK, Friedland RP, Mori H, Kalaria RN. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly east Africans. Brain Pathol. 1996;6:101–107. doi: 10.1111/j.1750-3639.1996.tb00790.x. [DOI] [PubMed] [Google Scholar]


