Abstract
Background
Interferon-alpha (IFN-α) is a primary pathogenic factor in systemic lupus erythematosus (SLE), and high IFN-α levels may be associated with particular clinical manifestations. The prevalence of individual clinical and serologic features differs significantly by ancestry. We used multivariate and network analyses to detect associations between clinical and serologic disease manifestations and serum IFN-α activity in a large diverse SLE cohort.
Methods
1089 SLE patients were studied (387 African-American, 186 Hispanic-American, and 516 European-American). Presence or absence of ACR clinical criteria for SLE, autoantibodies, and serum IFN-α activity data were analyzed in univariate and multivariate models. Iterative multivariate logistic regression was performed in each background separately to establish the network of associations between variables that were independently significant following Bonferroni correction.
Results
In all ancestral backgrounds, high IFN-α activity was associated with anti-Ro and anti-dsDNA antibodies (p-values 4.6×10−18 and 2.9 × 10−16 respectively). Younger age, non-European ancestry, and anti-RNP were also independently associated with increased serum IFN-α activity (p≤6.7×10−4). We found 14 unique associations between variables in network analysis, and only 7 of these associations were shared by more than one ancestral background. Associations between clinical criteria were different in different ancestral backgrounds, while autoantibody-IFN-α relationships were similar across backgrounds. IFN-α activity and autoantibodies were not associated with ACR clinical features in multivariate models.
Conclusions
Serum IFN-α activity was strongly and consistently associated with autoantibodies, and not independently associated with clinical features in SLE. IFN-α may be more relevant to humoral tolerance and initial pathogenesis than later clinical disease manifestations.
Keywords: systemic lupus erythematosus, interferon alpha, autoantibodies, ancestry
Systemic lupus erythematosus (SLE) is a heterogeneous disease, with diverse autoimmune manifestations commonly affecting the skin, hematologic, musculoskeletal, and renal organ systems. Differences in the prevalence of particular clinical and serologic manifestations of disease by ancestral background have long been appreciated, and it is likely that genetic influences play some role in these differences (1–3). Many lines of evidence support the idea that interferon alpha (IFN-α) is a primary pathogenic factor in SLE, including the development of SLE in patients given recombinant IFN-α to treat viral infections and malignancy (4–6), familial aggregation of high serum IFN-α activity in SLE families (7, 8), and the finding that many SLE-risk genetic variants which function in the IFN-α pathway result in increased IFN-α pathway signaling in patients in vivo (9–15).
Increased expression of IFN-α-induced genes in peripheral blood mononuclear cells (PBMC) is one of the most dominant findings in microarray studies of SLE (16, 17). In cross-sectional studies, SLEDAI (SLE Disease Activity Index) score was correlated with IFN-induced gene expression in PBMC (16, 18), however longitudinal studies have not conclusively demonstrated an association between IFN-α-induced gene expression and flare (19, 20). One study reported that high serum levels of chemokines which can be induced by IFN-α were associated with risk of flare over the ensuing year, and that levels of these chemokines were higher in patients during a disease flare (21). These chemokines can be induced by other SLE-associated cytokines beyond IFN-α, such as IFN-gamma and tumor necrosis factor alpha, and differences between this study and previous studies of IFN-α in SLE may relate to this difference (22). Increased expression of IFN-α-induced genes in PBMC has been associated with specific clinical manifestations including the presence of lupus nephritis and proteinuria, cutaneous manifestations, and presence of anti-Ro, anti-Smith (anti-Sm), anti-RNP, and anti-dsDNA antibodies (16, 18, 23). Anti-dsDNA antibodies have been associated with lupus nephritis (24, 25), and studies have linked anti-Ro antibody to lupus-related skin findings (25, 26). It is not currently clear whether the association between IFN-α and cutaneous and renal disease manifestations in previous studies is primary, or secondary due to an association between autoantibodies and IFN-α. In the case of autoantibodies, previous studies suggest a mechanistic link, in which SLE-associated autoantibody immune complexes may directly trigger IFN-α production in leukocytes when the nucleic acid component of these immune complexes ligate endosomal Toll-like receptors (27, 28). Despite this mechanistic relationship, SLE-associated autoantibodies are not sufficient to result in high circulating levels of IFN-α in humans. We have previously shown that mothers of neonatal lupus patients with anti-Ro or anti-La antibodies who had SLE or Sjögren’s syndrome had high serum IFN-α activity, while healthy mothers of neonatal lupus patients who also had high titer anti-Ro or anti-La antibodies did not have elevated IFN-α activity (29). This suggests that while there is an association between autoantibodies and IFN-α in SLE, disease-associated and individual factors will also be important to this relationship (11, 12).
SLE disease manifestations are highly variable between patients, and the prevalence of individual clinical features differs significantly by ancestry (30–33). Anti-RNP antibodies and anti-Sm antibodies are more common in African-Americans than in European- and Hispanic-Americans (30, 31). Different genetic variants are associated with these autoantibody traits in different ancestral backgrounds (34, 35). African-Americans and Hispanic-Americans tend to have more active SLE, with an earlier age of onset, than European-Americans (31, 36). In general Hispanic-Americans and African-Americans have a higher incidence of SLE-related renal disease than European-Americans, and this has been associated with anti-dsDNA antibodies and anti-RNP antibodies (25, 36, 37).
In the present study, we analyze the largest series of serum IFN-α activity data available in SLE patients to date, to determine associations with serologic and clinical factors. Given the inter-related nature of many of the clinical and serologic characteristics in SLE, we use multivariate logistic regression to account for between-variable relationships. Also, given the strong precedent for differences in the prevalence of serologic and clinical features by ancestral background, each self-reported ancestral background is analyzed separately. As expected, we detect a number of significant relationships between the various clinical variables, and present the associations discovered in network diagrams to illustrate the patterns of association in the different ancestral backgrounds studied. Interestingly, relationships between serologic parameters and serum IFN-α activity were relatively similar across ancestral backgrounds, while relationships between other clinical variables were heterogeneous by ancestral background. These data support the concept that IFN-α is a common pathologic feature of SLE that is shared across ancestral backgrounds, while the factors underlying SLE clinical manifestations might differ by ancestry.
Patients and Methods
Patients, samples, and data
Serum samples were obtained from 1089 SLE patients (387 African-American, 186 Hispanic-American, and 516 European-American) from the Lupus Family Registry and Repository (LFRR) at the Oklahoma Medical Research Foundation (n=875), the Translational Research Initiative in the Department of Medicine at the University of Chicago Medical Center (n=156), and Rush University Medical Center (n=58). Data regarding the presence or absence of ACR criteria for the diagnosis of SLE (38, 39), and the presence or absence of SLE-associated autoantibodies including ANA, anti-Ro, anti-La, anti-Sm, anti-RNP, and anti-dsDNA were available for all of these subjects. Samples were collected and studied at any point after the formal diagnosis of SLE, from newly diagnosed patients to those who are many years post-diagnosis, and this is consistent across all of the above registries.
Measurement of serum IFN-α activity
We have developed a sensitive and reproducible bioassay to detect serum IFN-α activity (7, 40), as ELISA methods for detection of IFN-α in human serum have been complicated by both low sensitivity and low specificity (41). In this bioassay, reporter cells (WISH cells, ATCC #CCL125) are used to measure the ability of sera to cause IFN-induced gene transcription. The reporter cells are cultured with patient sera for 6 hours and then lysed, and three canonical IFN-α-induced transcripts (IFIT-1, MX-1, and PKR) are measured using rtPCR. Relative expression data from the three transcripts are then normalized using the mean and standard deviation of healthy donor sera from a multi-ancestral cohort (n=141) run in the same assay (7), and data are presented as an IFN-α activity score. To bin the IFN results into high vs. low, we used a cutoff point for the IFN-α activity score of two standard deviations above the mean of healthy donors. This categorical variable is used in the IFN-α high vs. low stratified analyses and the multivariate logistic regression analyses presented below. The IFN-induced transcriptional activity in the reporter cells can be blocked with anti-IFN-α monoclonal antibodies, and no common significant functional inhibitors have been detected to date (7). The demographics of the healthy donors were similar to that of the SLE patients as follows: 70% female, 9% Asian-American, 14% African-American, 60% European-American, and 17% Hispanic-American ancestry, and the mean age +/− SD was 40.8 +/− 13 years. In the controls, we did not see significant differences in serum IFN-α activity by ancestry (p=0.18 by Kruskall-Wallis one-way ANOVA). Additionally, there were no significant differences in serum IFN-α activity related to age or sex in the controls (8).
Measurement of autoantibodies
Antibodies to anti-Ro, anti-La, anti-Sm, and anti-RNP were measured by precipitin method in the OMRF samples at the OMRF Clinical Immunology Laboratory, and by ELISA methods (INOVA Diagnostics, San Diego, CA) in the University of Chicago and Rush University samples at the University of Chicago clinical laboratory. Standard clinical lab cutoff points were used to categorize samples as positive or negative. Anti-dsDNA antibodies were measured using Crithidia luciliae immunofluorescence in all samples at all sites, and detectable fluorescence was considered positive. There were no significant differences in antibody prevalence between study sites within each ancestral background; however the majority of the samples were measured at OMRF and the number of subjects from the other collections is relatively small when split by ancestral background for analysis of heterogeneity. Subset analysis of the autoantibody associations in this smaller group of subjects (n=214) in whom the anti-Ro, anti-La, anti-Sm, and anti-RNP autoantibodies were measured by ELISA showed a similar pattern of association with serum IFN-α activity as that observed in the overall cohort (data not shown). These data and previously published studies examining autoantibody data measured by ELISA and serum IFN-α activity (7, 29) suggest that positive results for these four autoantibodies by ELISA would be similarly predictive of high serum IFN-α activity in SLE as would positive results using the precipitin technique.
Statistical Analysis
The cohort was stratified by ancestral background into 387 African-American patients, 186 Hispanic-Americans and 516 European-Americans. Serum IFN-α activity data for each of these subjects was binned as high vs. low, using a cut-off point of two standard deviations above the mean of healthy donors to separate high vs. low. In the univariate analyses, Chi-square test statistic was used to compare differences in proportions of the clinical characteristics between the three ancestral backgrounds (42). The multivariate logistic regression presented in Table 3 was performed including all variables except IFN-α activity as predictor variables and IFN-α activity as the outcome variable, and p-values which remained significant following a Bonferroni correction for multiple comparisons (p<0.00278) were considered significant. In this model, we also tested for any first-order interactions between self-reported ancestry and other variables significantly associated with serum IFN-α activity. Interaction variables were generated as the product of the ancestry variable and each significant predictor variable. Interaction variables were then tested in regression models with the two original predictor variables using serum IFN-α activity as the outcome variable, and p-values for the interaction variables in these models were assessed.
Table 3.
Output from multivariate regression to detect independent associations with high IFN-α activity across all ancestral backgrounds
| β-coefficient | Standard error | p-value | |
|---|---|---|---|
| Non-European Ancestry | 0.534 | 0.157 | 6.7 × 10−4 |
| Age at Recruitment | −0.025 | 0.0056 | 9.7 × 10−6 |
| Photosensitivity | −0.224 | 0.151 | 0.14 |
| Discoid Rash | 0.236 | 0.192 | 0.22 |
| Malar Rash | 0.304 | 0.151 | 0.044 |
| Oral Ulcers | −0.089 | 0.155 | 0.56 |
| Serositis | −0.007 | 0.149 | 0.96 |
| Hematologic | 0.230 | 0.152 | 0.13 |
| Neurologic | −0.187 | 0.215 | 0.38 |
| Renal Disease | −0.052 | 0.154 | 0.74 |
| Arthritis | −0.220 | 0.176 | 0.21 |
| Anti-dsDNA | 0.859 | 0.158 | 6.5 × 10−8 |
| Anti-Sm | 0.247 | 0.290 | 0.39 |
| Anti-RNP | 1.132 | 0.195 | 7.8 × 10−9 |
| Anti-Ro | 0.985 | 0.182 | 7.3 × 10−8 |
| Anti-La | 0.398 | 0.300 | 0.19 |
coefficient = beta coefficient from the logistic regression model, p-value calculated from the t-statistic. Bolded entries have a p-value that would remain <0.05 following a Bonferroni correction for the number of variables tested in this analysis.
In the network analysis, iterative backward logistic regression modeling was performed in each ancestral background separately. Positive ANA test is one of the ACR clinical criteria for SLE, but was not included in the regression models, as this variable was almost uniformly positive in the SLE cohorts. Also, the “immunologic disorder” ACR criterion was not included in the multivariate regression models because we included both anti-dsDNA and anti-Sm in these models, and the immunologic disorder criterion is largely derived from these two variables. The iterative logistic regression models used each variable as an outcome variable serially, with all of the other variables used as predictor variables (variables tested were 9 of the 11 ACR criteria excluding ANA and immunologic, 5 SLE-associated autoantibodies, and IFN-α high vs. low). Variables from this initial analysis with a p-value below 0.20 were then carried forward in next-step logistic regression models, and variables with p-values greater than this threshold were discarded. On repeat regression analysis, results with p<0.00357 were considered significant to allow for a Bonferroni correction for multiple comparisons accounting for the number of variables tested (43). The magnitude of positive and inverse correlations was estimated by calculating the odds-ratio for each association. Some variables did not fit a logistic model (for example, anti-La is almost never found without anti-Ro, and thus these variables are “perfect predictors” and do not fit a logistic curve). In this case, Chi-square or Fisher’s exact analysis was used to assess the relationships between these “perfect predictor” variables.
Results
Prevalence of SLE criteria, autoantibodies, and high serum IFN-α in different ancestral backgrounds
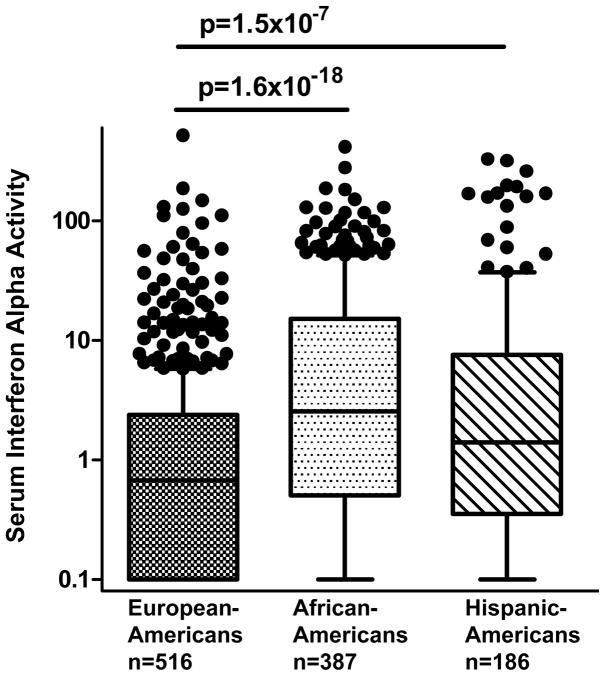
When comparing the prevalence of different ACR criteria and autoantibodies across ancestral backgrounds, we observed a number of significant differences, similar to previous studies (Table 1) (32, 33, 36, 37, 44). Hematological manifestations and discoid rash were more common in African-Americans than in the other two cohorts. Photosensitivity, oral ulcers and malar rash were more common in European-Americans and Hispanic-Americans. When IFN-α activity was assessed categorically as high vs. low, African-Americans and Hispanic-Americans had a greater proportion of high IFN-α subjects than European-Americans (Table 1). In accordance with this result, quantitative serum IFN-α activity was significantly lower in European-Americans than all non-European ancestral backgrounds (Figure 1).
Table 1.
Prevalence of clinical characteristics, autoantibodies, and serum IFN-α activity in SLE patients stratified by ancestral background.
| European Americans (%) | Hispanic Americans (%) | African Americans (%) | Χ2 | p-value | |
|---|---|---|---|---|---|
| Female | 88.6 | 87.1 | 92.0 | 4.19 | 0.12 |
| Age (yrs mean+/−SD) | 45.5+/−14.1 | 38.4+/−14.2 | 41.9+/−12.4 | - | 3.5 × 10−13 |
| Photosensitivity | 57.2 | 48.4 | 33.9 | 47.36 | 5.2 × 10−11 |
| Discoid Rash | 10.2 | 13.4 | 25.8 | 39.25 | 3.0 × 10−9 |
| Malar Rash | 59.5 | 50.5 | 42.9 | 23.92 | 6.4 × 10−6 |
| Oral Ulcer | 38.4 | 34.4 | 26.6 | 13.24 | 1.3 × 10−3 |
| Serositis | 42.4 | 40.3 | 35.9 | 3.67 | 0.16 |
| Hematologic | 57.9 | 57.0 | 72.1 | 21.36 | 2.3 × 10−5 |
| Immunologic | 78.5 | 80.1 | 82.9 | 2.79 | 0.25 |
| Neurologic | 12.8 | 9.14 | 15.2 | 3.65 | 0.16 |
| Renal Disease | 29.1 | 43.0 | 42.4 | 20.63 | 3.3 × 10−5 |
| Arthritis | 83.3 | 60.8 | 78.6 | 39.20 | 3.1 × 10−9 |
| ANA | 99.0 | 97.3 | 99.2 | 2.71 | 0.27 |
| Anti-dsDNA | 27.3 | 28.5 | 30.2 | 0.78 | 0.68 |
| Anti-Sm | 3.10 | 9.14 | 18.1 | 56.27 | 6.0 × 10−13 |
| Anti-RNP | 9.50 | 21.0 | 45.7 | 156.98 | 8.2 × 10−35 |
| Anti-Ro | 21.7 | 25.3 | 32.8 | 13.60 | 1.1 × 10−3 |
| Anti-La | 6.40 | 9.68 | 8.79 | 2.29 | 0.32 |
| High IFN | 27.7 | 44.1 | 53.5 | 61.97 | 3.5 × 10−14 |
% indicates the percentage of subjects in which the specified disease feature is present, except Age which is reported as a mean years of age at recruitment +/− the standard deviation (SD). ANA = antinuclear antibody test, Χ2 = Chi-square test statistic. P-values are derived from the Chi-square test statistic with two degrees of freedom for all rows, except age which is calculated using one-way ANOVA with a p-value calculated from Fisher’s F value.
Figure 1.
Serum IFN-α alpha activity in SLE patients stratified by self-reported ancestry. Serum IFN-α activity is represented in relative units on the Y-axis, as described in the Methods. Line is drawn at the median, boxes show the interquartile range, and error bars represent the 90th and 10th percentiles respectively. Two-column t-tests and p-value calculations were done using the non-parametric Mann-Whitney U test.
Univariate analysis of the prevalence of clinical features and autoantibodies in SLE patients stratified by ancestry and high vs. low IFN-α
As shown in Table 2, IFN-α activity was strongly associated with autoantibodies in univariate analyses, regardless of patient ancestry. High IFN-α activity subjects of all ancestral backgrounds had significantly higher proportion of positive tests for anti-dsDNA and anti-Ro autoantibodies when compared with low IFN-α activity subjects [Cochran-Mantel-Haenzel meta-analysis across ancestral backgrounds for anti-dsDNA OR=3.04 (2.32–3.98), p-value=2.9×10−16 and for anti-Ro OR=3.35 (2.53–4.43), p-value=4.6×10−18]. In contrast, when testing for potential differences in the prevalence of ACR clinical criteria in the high vs. low IFN-α activity groups, only two significant differences were observed that would exceed a Bonferroni correction for the number of variables tested. In European-Americans, hematologic manifestations were more common in the high IFN-α activity group, and in African-Americans immunologic disorder was more common in high IFN-α activity subjects. In the case of the immunologic disorder association, both anti-dsDNA and anti-Sm are components of this criterion, and it is likely that the association of this criterion with high IFN-α activity in African-Americans is secondary to autoantibody associations. The “hematologic” ACR criterion is a composite measure incorporating anemia, leukopenia, and lymphopenia. We had data available regarding these individual cytopenias in 894 subjects (294/387 AA, 446/516 EA, 154/186 HA), and we analyzed these data for association with serum IFN-α activity. We did not find any significant correlations between serum IFN-α activity and individual cytopenias in our patients which would withstand multiple comparisons correction (minimum p-value was 0.023 for association of anemia with high IFN-α activity in European-Americans).
Table 2.
Prevalence of clinical characteristics and autoantibodies in SLE patients stratified by high vs. low IFN-α activity in each ancestral background.
| African-Americans High IFN (%) | African-Americans Low IFN (%) | p-value | European-Americans High IFN (%) | European-Americans Low IFN (%) | p-value | Hispanic-Americans High IFN (%) | Hispanic-Americans Low IFN (%) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Female | 92.3 | 91.7 | 0.85 | 84.6 | 90.1 | 0.08 | 85.4 | 88.5 | 0.66 |
| Age (mean years) | 39.9 | 44.2 | 5.3 × 10−4 | 40.9 | 47.2 | 4.5 × 10−6 | 34.9 | 41.2 | 2.6 × 10−3 |
| Photosensitivity | 30.9 | 37.2 | 0.19 | 60.1 | 56.0 | 0.40 | 37.8 | 56.7 | 0.010 |
| Discoid Rash | 24.6 | 27.2 | 0.56 | 11.9 | 9.65 | 0.45 | 14.6 | 12.5 | 0.67 |
| Malar Rash | 45.4 | 40.0 | 0.28 | 64.3 | 57.6 | 0.17 | 51.2 | 50.0 | 0.87 |
| Oral Ulcers | 28.0 | 25.0 | 0.50 | 35.0 | 39.7 | 0.32 | 32.9 | 35.6 | 0.71 |
| Serositis | 36.2 | 35.6 | 0.89 | 37.8 | 44.2 | 0.18 | 47.6 | 34.6 | 0.074 |
| Hematologic | 73.9 | 70.0 | 0.39 | 68.5 | 53.9 | 2.6 × 10−3 | 57.3 | 56.7 | 0.94 |
| Immunologic | 88.4 | 76.7 | 2.2×10−3 | 85.3 | 75.9 | 0.019 | 82.9 | 77.9 | 0.46 |
| Neurologic | 14.5 | 16.1 | 0.66 | 9.09 | 14.2 | 0.12 | 11.0 | 7.69 | 0.44 |
| Renal Disease | 42.0 | 42.8 | 0.88 | 29.4 | 29.0 | 0.92 | 45.1 | 41.3 | 0.61 |
| Arthritis | 78.7 | 78.3 | 0.92 | 79.7 | 84.7 | 0.17 | 52.4 | 67.3 | 0.039 |
| ANA | 99.0 | 99.4 | 0.65 | 99.3 | 98.9 | 0.70 | 100 | 95.2 | 0.044 |
| Anti-dsDNA | 40.1 | 18.9 | 5.9 × 10−6 | 45.5 | 20.4 | 1.0 × 10−8 | 42.7 | 17.3 | 1.4 × 10−4 |
| Anti-Sm | 26.1 | 8.89 | 1.2 × 10−5 | 8.9 | 1.9 | 8.8 × 10−4 | 13.4 | 5.77 | 0.072 |
| Anti-RNP | 58.9 | 30.6 | 2.0 × 10−8 | 7.69 | 10.2 | 0.39 | 29.2 | 14.4 | 0.014 |
| Anti-Ro | 44.9 | 18.9 | 5.0 × 10−8 | 33.6 | 17.2 | 5.2 × 10−5 | 41.5 | 12.5 | 6.4 × 10−6 |
| Anti-La | 13.0 | 3.89 | 1.5 × 10−3 | 11.9 | 4.29 | 3.9 × 10−3 | 15.9 | 4.81 | 0.011 |
% indicates the percentage of subjects in which the specified disease feature is present, Age (mean years) = average of the subjects ages at recruitment, ANA = antinuclear antibody test, p-value is derived from the Chi-square test statistic with one degree of freedom. Bolded entries have a p-value that would remain <0.05 following a Bonferroni correction for the number of variables tested in this analysis.
Multivariate Regression to Detect Independent Associations with High IFN-α
While we found that non-European-Americans have higher serum IFN-α activity than European-Americans, we also show that anti-Ro, anti-RNP and anti-Sm antibodies were all significantly more common in the African- and Hispanic-American cohorts, and the non-European ancestry subjects were younger than the European ancestry subjects. Given the previously reported correlations between autoantibodies and younger age with increased serum IFN-α activity, these two variables could partially explain the higher serum IFN-α activity seen in these populations. To test this possibility we performed a multivariate logistic regression on all subjects using IFN-α high vs. low as the outcome variable and including all other variables as predictors, including age at recruitment as well as European vs. non-European ancestry. In this analysis, the following variables were independently associated with increased serum IFN-α activity: non-European ancestry, younger age at time of sample, anti-Ro, anti-RNP, and anti-dsDNA (Table 3). Given that ancestral background was associated with both serum IFN-α (Figure 1) and the prevalence of autoantibodies (Table 1), we attempted to further categorize ancestral background as either an effect modifier or a confounder in the analysis. We examined first-order interactions between ancestral background and autoantibodies upon serum IFN-α activity high vs. low, and there were no significant first-order interactions (all p>0.09, data not shown). This would suggest that ancestral background is a confounder and not an effect modifier, which was controlled in the logistic model presented in Table 3 by inclusion as a variable. Subsequent network models were performed in each ancestral background separately.
Network analysis of clinical variables, autoantibodies, and IFN-α in SLE
In order to determine relationships between the ACR criteria for SLE, autoantibodies, and serum IFN-α activity, we used an iterative multivariate logistic regression strategy as described in the Methods section. The magnitude of the statistically significant associations was determined by odds-ratio calculations, and these relationships were represented in network diagrams. Of over one trillion possible associations, we found 14 unique associations, forming network maps of relatively sparse density in each background (Figure 2). Of these 14 associations, only 7 were shared by more than one different ancestral background, significantly differing from a model in which each of the associations would be shared by at least two of the three ancestral backgrounds (p = 5.8×10−3). Interestingly, associations between IFN-α activity and serologic parameters were strikingly similar across the different ancestral backgrounds. IFN-α activity was positively correlated with anti-dsDNA and anti-Ro antibodies in all three backgrounds. As expected, anti-Ro antibodies were strongly correlated with anti-La antibodies and anti-RNP antibodies were correlated with anti-Smith antibodies across all three ancestries.
Figure 2.
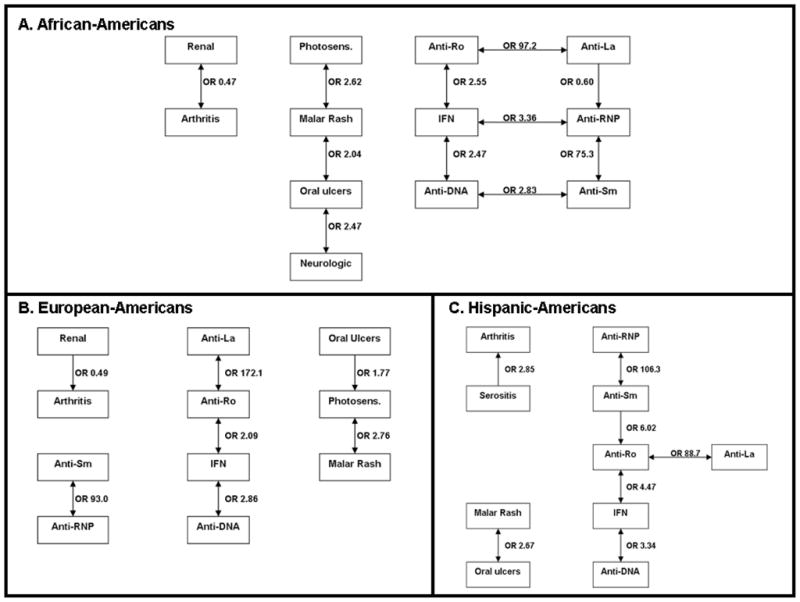
Network diagram showing significant associations between ACR clinical criteria, autoantibodies, and serum IFN-α activity in SLE patients of each ancestral background. A. shows African-American patients, B. shows European-American patients, and C. shows Hispanic-American patients. Each of the boxes indicates the presence of the given feature, and IFN indicates high IFN-α activity, and the odds ratios indicate the direction of the association. Thus, an odds ratio greater than one for an association between “Anti-Ro” and “IFN” means that the presence of anti-Ro antibodies is associated with high IFN-α activity. Connecting lines indicate associations, and arrowheads indicate directionality of the associations (arrowhead touching the box of the outcome variable that is associated with the given predictor variable, most are bidirectional associations). OR = Odds ratio, Photosens. = photosensitivity, anti-DNA = anti-dsDNA
As shown in Figure 2A, in African-Americans high IFN-α activity was associated with the presence of anti-dsDNA and anti-Ro antibodies, in addition to anti-RNP. Anti-dsDNA was positively correlated with anti-Sm antibodies, and anti-RNP was inversely correlated with anti-La, forming a robust network of serologic associations in this background. Malar rash was linked to photosensitivity and oral ulcers, and oral ulcers were associated with neurologic manifestations. Arthritis and renal manifestations were inversely correlated. In European-Americans, high IFN-α activity was also linked to anti-dsDNA and anti-Ro antibodies (Figure 2B). Anti-Ro antibodies were highly correlated with anti-La antibodies, and anti-Sm antibodies were associated with anti-RNP antibodies as expected. The skin manifestations (photosensitivity, oral ulcers, and malar rash) were associated with each other (Figure 2B), similar to African-Americans. In this background, arthritis was also inversely correlated with renal manifestations. In Hispanic-Americans, autoantibody-IFN-α associations were very similar to the other ancestral backgrounds, although in this background anti-Sm antibodies were correlated with anti-Ro antibodies (Figure 2C). As in African-Americans, malar rash is linked to oral ulcers, and a novel association between arthritis and serositis was observed in this ancestral background.
We did not observe similar associations between serology and clinical features in this study as have been previously described, such as anti-Ro with cutaneous disease or anti-dsDNA with renal disease (24–26). It is possible that these manifestations could be mediated in part by IFN-α, and that inclusion of serum IFN-α activity in our multivariate models abolished the observed associations. To test this hypothesis, we analyzed these particular associations between serology and clinical manifestations leaving IFN-α activity out of the model. Only one association which exceeded our threshold p-value was observed between anti-Ro and photosensitivity in Hispanic-Americans with an odds-ratio of 6.47 (data not shown). This would suggest that inclusion of IFN-α activity in our model was not obscuring previously reported associations between serology and clinical manifestations. Given that we have previously reported an association between age and serum IFN-α activity (8) and that age differed significantly between the ancestral backgrounds studied, we tested whether age could be confounding the network of association by including age as a covariate in the regressions. This re-analysis of the network of associations with IFN-α including age as a covariate resulted in the same associations (data not shown), suggesting that age differences between different ancestral backgrounds were not causing the differences observed in the patterns of association between ancestral backgrounds.
Discussion
By studying a large cohort of SLE patients, we were able to show that IFN-α levels vary significantly by ancestral background, and demonstrate some shared and some distinct patterns of association between serum IFN-α activity, autoantibodies, and clinical manifestations in SLE patients of different ancestral backgrounds. Both the similarities and the differences between ancestral backgrounds are interesting, as SLE is a highly heterogeneous condition and a better understanding of this heterogeneity could inform our approach to diagnosis and treatment. The association between SLE-associated autoantibodies and high IFN-α activity was remarkably similar across all of the ancestral backgrounds studied. Consistent with previous data, we found that SLE-associated autoantibodies were more common in African-American and Hispanic-American SLE patients than in European-American SLE patients, and serum IFN-α activity were also higher in African- and Hispanic-American SLE patients. Studies support the idea that SLE-autoantibody immune complexes can directly trigger IFN-α production, likely via ligation of endsomal Toll-like receptors (27, 28), and these data provide a potential mechanistic explanation for the strong shared association we observed. It was interesting that despite controlling for all of the variables analyzed in this study, some residual association between non-European ancestry and higher serum IFN-α activity remained, suggesting that some genetic or other unaccounted for factors result in this difference in serum IFN-α activity by ancestry.
Previous studies have correlated increased expression of IFN-induced genes in SLE with presence of renal disease, cutaneous manifestations, anti-Ro, anti-Sm, anti-RNP, and anti-dsDNA antibodies (16, 18, 23). While we strongly confirm the previously reported serologic associations, strikingly we did not find any associations between clinical characteristics and IFN-α activity or autoantibodies. Certain clinical characteristics were positively correlated with each other, and autoantibodies were correlated with high IFN-α activity and with each other, but these two groups of associations were not interconnected in the network diagrams. We may not have detected these associations between clinical and serum factors due to the stringent statistical significance we required in our analysis to allow for multiple comparisons. However if this is the case, then the associations between serology and high IFN-α activity are at least much stronger than any potential association between clinical features and high IFN-α activity. The ACR criterion for renal involvement in SLE does not differentiate well between current and prior disease. Thus, our study cannot exclude an association between IFN-α activity and acute renal disease. Similarly, the cutaneous ACR criteria could refer to present or past skin involvement. In each ancestral background, cutaneous manifestations were associated with each other in some way. This seems logical that ACR criteria which assess the same organ system are correlated with each other, and this may indicate a shared pathogenesis.
A strength of the present analysis is that the large cohort of SLE subjects from diverse ancestral backgrounds allowed us to stratify the analyses by ancestry, and account for between-variable correlations with multivariate analyses in each ancestral background. Heterogeneity in the ancestral composition of an SLE cohort could result in confounding of an IFN-α-clinical correlation study, as both the prevalence of high IFN-α activity as well as the prevalence of particular clinical manifestations differ significantly by ancestry. For example, both renal disease and high IFN-α activity are more common in African-and Hispanic-Americans, and without control for ancestry these two variables could be seen as correlated in the overall mixed ancestry cohort when they may actually be co-linear in a subset of the cohort.
Limitations of the study include that disease activity was not assessed in these subjects, and could not be included as a covariate. These data would have been interesting as we could have tested whether the previously reported associations between IFN-α activity and disease activity were dependent or independent of autoantibody traits. In particular, anti-dsDNA antibodies would be an important potential confounder clearly related to both IFN-α activity and SLEDAI score. Other autoantibodies associated with high IFN-α activity, such as anti-Ro, tend to be stable, and in this case large temporal variation in IFN-α due to changes in disease activity would tend to obscure this correlation. Additionally, work from a number of investigators supports relative stability of IFN-α in SLE serum over time (19, 20). Age was included in this study as “age at time of sample”, and data regarding age at SLE onset were not fully available. In younger patients these two variables will likely be correlated, but this would not be the case in older patients, and thus we cannot assess any effects related to disease duration in this study. Immunosuppression and its possible effect on IFN expression were not controlled in the study. However, prior studies have shown that while pulse glucocorticoids suppress IFN-α and IFN-α-induced gene expression, patients treated with azathioprine or mycophenolate mofetil did not have lower IFN-α-induced gene expression in PBMCs when compared to patients not receiving these medications (18). Data regarding hydroxychloroquine would be interesting to analyze in our cohort if these data were available, as studies have suggested that anti-malarial agents decrease intracellular Toll-like receptor signaling (45), and these agents are commonly used in SLE. Previous work suggests a non-significant trend (p=0.06) toward an association between hydroxychloroquine treatment and lower IFN-α-induced gene expression in SLE patient PBMC in a multivariate model (18).
While our analysis did not show a direct link between clinical manifestations of SLE and IFN-α activity or autoantibodies, this may be because there are intermediate factors relating these disease characteristics which have not yet been identified or included in our analysis. IFN-α is known to be a primary pathogenic factor in SLE (7, 9), but may not directly influence the late disease manifestations. Instead, IFN-α may be involved in the early pathogenesis of SLE through a break in humoral self-tolerance, allowing the production of pathogenic SLE-associated autoantibodies. Previous studies have shown that autoantibodies can develop years prior to the disease onset of SLE, and typically develop in a predictable fashion (46). Our analysis showed that IFN-α activity was strongly associated with autoantibodies in SLE patients, and this association was strikingly consistent despite the known differences in the prevalence of both autoantibodies and clinical features in different ancestral backgrounds. Our data support a model in which IFN-α is involved in early humoral loss of self-tolerance which is more highly conserved in SLE pathogenesis, and IFN-α may be less involved in the later diversification of the autoimmune response against various organs which is more variable between persons and ancestral backgrounds.
Acknowledgments
Funding Sources: CE Weckerle – American College of Rheumatology Rheumatology Scientist Development Award; JA Kelly - Lupus Family Registry and Repository – NIH AR62277, research grants from NIH (AR42460, AI24717); TO Utset – Lupus Clinical Trials Consortium, consulting fees from Genentech; JA James - research grants from NIH (RR15577, AR48940, AR045084, AR053483, AR058554, AI082714), Mary Kirkland Scholar, and Lou Kerr Chair in Biomedical Research; JB Harley - Lupus Family Registry and Repository – NIH AR62277, research grants from NIH (AR42460, AI53747, AI31584, DE15223, RR20143, AI24717, AI62629, AR48940, AI83194, and AR49084), and research grants from the US Department of Veterans Affairs, Alliance for Lupus Research, and Rheuminations, Inc.; TB Niewold – NIH K08 AI083790, NIH P30 DK42086, NIAID Clinical Research Loan Repayment AI071651, NIH CTSA Core Subsidy Grant and CTSA Pilot Grants from UL1 RR024999, Lupus Research Institute Novel Research Grant, Alliance for Lupus Research Target Identification in Lupus Grant, Arthritis National Research Foundation Eng Tan Scholar Award.
The authors would like to acknowledge the contribution of Gail R. Bruner, RN at the Lupus Family Registry and Repository, and all of the patients who donated to the registries that were studied, without whom the work would not have been possible.
Footnotes
Financial Disclosures and Conflict of Interest: The authors report no financial conflict of interest.
References
- 1.Hopkinson ND, Doherty M, Powell RJ. Clinical features and race-specific incidence/prevalence rates of systemic lupus erythematosus in a geographically complete cohort of patients. Ann Rheum Dis. 1994;53(10):675–80. doi: 10.1136/ard.53.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kariuki SN, Franek BS, Mikolaitis RA, Utset TO, Jolly M, Skol AD, et al. Promoter Variant of PIK3C3 Is Associated with Autoimmunity against Ro and Sm Epitopes in African-American Lupus Patients. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/826434. Article ID 826434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodolce JP, Kolodziej LE, Rhee L, Kariuki SN, Franek BS, McGreal NM, et al. African-derived genetic polymorphisms in TNFAIP3 mediate risk for autoimmunity. J Immunol. 2010;184(12):7001–9. doi: 10.4049/jimmunol.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227(3):207–10. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Niewold TB, Swedler WI. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol. 2005;24(2):178–81. doi: 10.1007/s10067-004-1024-2. [DOI] [PubMed] [Google Scholar]
- 6.Niewold TB. Interferon alpha-induced lupus: proof of principle. J Clin Rheumatol. 2008;14(3):131–2. doi: 10.1097/RHU.0b013e318177627d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum. 2008;58(7):2113–9. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kariuki SN, Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res. 2010;155(3):109–117. doi: 10.1016/j.trsl.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10(5):487–94. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M, et al. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthritis Rheum. 2010;62(2):553–561. doi: 10.1002/art.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: Autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182(1):34–8. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harley ITW, Niewold TB, Stormont RM, Kaufman KM, Glenn SB, Franek BS, et al. The role of genetic variation near interferon-kappa in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/706825. Article ID 706825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58(9):2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36(8):481–90. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 18.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 19.Landolt-Marticorena C, Bonventi G, Lubovich A, Ferguson C, Unnithan T, Su J, et al. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2009;68(9):1440–6. doi: 10.1136/ard.2008.093146. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Singh S, Tesfasyone H, Dedrick R, Fry K, Lal P, et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus. 2009;18(11):980–9. doi: 10.1177/0961203309105529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60(10):3098–107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crow MK, Kirou KA. Interferon-induced versus chemokine transcripts as lupus biomarkers. Arthritis Res Ther. 2008;10(6):126. doi: 10.1186/ar2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 24.Bastian HM, Alarcon GS, Roseman JM, McGwin G, Jr, Vila LM, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007;46(4):683–9. doi: 10.1093/rheumatology/kel347. [DOI] [PubMed] [Google Scholar]
- 25.Bastian HM, Roseman JM, McGwin G, Jr, Alarcon GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11(3):152–60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 26.Sontheimer RD, Maddison PJ, Reichlin M, Jordon RE, Stastny P, Gilliam JN. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97(5):664–71. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- 27.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50(6):1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 28.Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren's syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54(6):1917–27. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- 29.Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum. 2008;58(2):541–6. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molina JF, Molina J, Garcia C, Gharavi AE, Wilson WA, Espinoza LR. Ethnic differences in the clinical expression of systemic lupus erythematosus: a comparative study between African-Americans and Latin Americans. Lupus. 1997;6(1):63–7. doi: 10.1177/096120339700600109. [DOI] [PubMed] [Google Scholar]
- 31.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11(3):161–7. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 32.Quintero-Del-Rio AI, Bacino D, Kelly J, Aberle T, Harley JB. Familial systemic lupus erythematosus: a comparison of clinical manifestations and antibody presentation in three ethnic groups. Cell Mol Biol (Noisy-le-grand) 2001;47(7):1223–7. [PubMed] [Google Scholar]
- 33.Duran S, Apte M, Alarcon GS, Marion MC, Edberg JC, Kimberly RP, et al. Features associated with, and the impact of, hemolytic anemia in patients with systemic lupus erythematosus: LX, results from a multiethnic cohort. Arthritis Rheum. 2008;59(9):1332–40. doi: 10.1002/art.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kariuki SN, Franek BS, Kumar AA, Arrington J, Mikolaitis RA, Utset TO, et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(4):R151. doi: 10.1186/ar3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos PS, Kelly JA, Gray-McGuire C, Bruner GR, Leiran AN, Meyer CM, et al. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7(5):417–32. doi: 10.1038/sj.gene.6364316. [DOI] [PubMed] [Google Scholar]
- 36.Alarcon GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8(3):197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 37.Uribe AG, McGwin G, Jr, Reveille JD, Alarcon GS. What have we learned from a 10-year experience with the LUMINA (Lupus in Minorities; Nature vs. nurture) cohort? Where are we heading? Autoimmun Rev. 2004;3(4):321–9. doi: 10.1016/j.autrev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 39.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 40.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 41.Jabs WJ, Hennig C, Zawatzky R, Kirchner H. Failure to detect antiviral activity in serum and plasma of healthy individuals displaying high activity in ELISA for IFN-alpha and IFN-beta. J Interferon Cytokine Res. 1999;19(5):463–9. doi: 10.1089/107999099313901. [DOI] [PubMed] [Google Scholar]
- 42.Preacher KJ. Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fit and independence [Computer software] 2001. [Google Scholar]
- 43.Abdi H. Bonferroni and Šidák corrections for multiple comparisons. In: Salkind NJ, editor. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- 44.Li LH, Pan HF, Li WX, Li XP, Xu JH, Ye DQ. Study on clinical features and complications with systemic lupus erythematosus (SLE) activity in Chinese Han population. Clin Rheumatol. 2009;28(11):1301–7. doi: 10.1007/s10067-009-1240-x. [DOI] [PubMed] [Google Scholar]
- 45.Lafyatis R, York M, Marshak-Rothstein A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum. 2006;54(10):3068–70. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 46.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]