Abstract
Drug-related stimuli appear to contribute to the persistence of drug seeking and relapse. Behavioral momentum theory is a framework for understanding how the discriminative-stimulus context in which operant behavior occurs governs the persistence of that behavior. The theory suggests that both resistance to change and relapse are governed by the Pavlovian stimulus-reinforcer relation between a stimulus context and all sources of reinforcement obtained in that context. The present experiment examined the role of the Pavlovian stimulus-reinforcer relation in reinstatement of ethanol seeking of rats by including added response-independent non-drug reinforcement in the self-administration context. Although rates of ethanol-maintained responding were lower in a context with added non-drug reinforcement than a context with ethanol alone, relative resistance to extinction and relative reinstatement were greater in the context previously associated with the non-drug reinforcer. Thus, both relative resistance to extinction and relative relapse of ethanol seeking depended upon the Pavlovian stimulus-reinforcer relation between a context and all sources of reinforcement in that context. These findings suggest that in order to understand how drug-related contexts contribute to relapse, it may be necessary to consider not only the history of drug reinforcement in a context, but also the wide variety of other reinforcers obtained in such contexts.
Keywords: drug cues, behavioral momentum theory, resistance to change, alternative non-drug reinforcement, reinstatement, relapse, ethanol, self-administration, rat
Introduction
Drug addiction is characterized by persistent drug seeking and relapse after periods of abstinence (American Psychiatric Association 2004; National Institute on Drug Abuse 2007). Drug-related stimuli appear to contribute to persistent drug seeking and relapse, and play an important role in modern approaches to addiction (see Robinson and Berridge 2008; Taylor et al 2009 for reviews). Drug taking is operant behavior maintained by its consequences and is therefore modulated by discriminative stimuli in the presence of which it is reinforced. Thus, we have suggested that behavioral momentum theory (e.g., Nevin and Grace 2000)--which describes how discriminative-stimulus contexts modulate the persistence of operant behavior--might be useful for understanding the effects of drug-related stimuli on the persistence of drug seeking (e.g., Shahan and Burke 2004; Quick and Shahan 2009).
Behavioral momentum theory suggests that baseline response frequency and response persistence under changing conditions (i.e., resistance to change) are two separate aspects of operant behavior. The frequency of behavior is governed by the contingent relation between a response and the reinforcer it produces, but resistance to change is governed by the Pavlovian relation between the discriminative-stimulus context in which the behavior occurs and all sources of reinforcement in that context. Thus, despite the fact that non-contingent reinforcers or reinforcers contingent on an alternative response tend to decrease response rates, they have been shown to increase resistance to change across a range of species from fish to humans (e.g. Mace et al. 1990; Nevin et al. 1990; Grimes and Shull 2001; Ahearn et al. 2003; Igaki and Sakagami 2004). Such effects of the contextual Pavlovian stimulus–reinforcer relation on response persistence are akin to other suggested motivational or incentive effects of Pavlovian stimuli on operant behavior (e.g., Rescorla and Solomon 1967; Bindra 1972; Stewart et al. 1984; Robinson and Berridge 1993).
Shahan and Burke (2004) have demonstrated effects of the Pavlovian stimulus-reinforcer relation on the persistence of drug self-administration. They showed that although an alternative source of response-independent non-drug reinforcement (i.e., food) in a context decreased ethanol-maintained responding of rats, relative resistance to extinction increased in that context. Thus, as suggested by behavioral momentum theory, the relative persistence of ethanol-maintained responding appears to be governed by the Pavlovian relation between the discriminative context in which the behavior is occurring and all sources of reinforcement (drug and non-drug) in that context.
Recently, Podlesnik and Shahan (2009, 2010) have suggested that behavioral momentum theory can also be extended to relapse of extinguished operant behavior. In a number of experiments, they showed that relative relapse of extinguished food-maintained operant behavior in reinstatement, renewal, and resurgence paradigms is also governed by the Pavlovian stimulus-reinforcer relation. Specifically, extinguished food-maintained responding previously occurring in a discriminative-stimulus context previously associated with more food reinforcement resulted in greater relative resistance to extinction and greater relative relapse in that context.
Given that relapse is an important aspect of addiction, the present experiment examined whether all sources of reinforcement previously experienced in a discriminative context contribute to relapse of drug seeking in that context. Using a procedure similar to that of Shahan and Burke (2004), we examined whether added response-independent non-drug reinforcement increases relative reinstatement of extinguished ethanol self-administration of rats.
Methods
Subjects
Five male Long-Evans rats with similar previous histories of ethanol self-administration were used. Rats were maintained at approximately 80% of their adult free-feeding weights via post-session feedings and were housed individually with free access to water in a temperature-controlled room (12:12 h light cycle; lights on 07:00 h). Experimental sessions occurred daily at the same time. Care and use of these rats was approved by the Utah State University Institutional Animal Care and Use Committee.
Apparatus
Four Med-Associates modular operant chambers (30 cm × 24 cm × 21 cm) housed in sound-attenuating cubicles were used. Operant chambers consisted of two Plexiglas and two aluminum walls on opposite sides. A retractable lever and associated lamp were centered on one wall with a receptacle (5 cm × 5 cm) to the right side within which 3-s of access to 0.1-ml dipper deliveries of ethanol solution were provided. A receptacle (5 cm × 5 cm) for delivering food pellets was centered on the opposite wall. All receptacles could be lit during reinforcer delivery, and each chamber was equipped with a house light and speaker for producing a range of tones.
Procedure
As a result of their previous history of ethanol self-administration, the rats did not require ethanol self-administration training. In the previous training, the rats were trained to self-administer a 10% (v/v) ethanol solution according to a modified sucrose fading procedure (Samson 1986) as described by Shahan and Burke (2004). Presses to the center lever produced ethanol deliveries on a two-component multiple schedule arranging two discriminative-stimulus contexts that alternated within daily sessions. Discriminative stimuli associated with the two components consisted of a steady tone and houselight or a pulsing tone and houselight (0.5-s on/off). Components were 60-s long and were separated by 30-s inter-component blackouts during which the lever was inoperable. Sessions consisted of 15 presentations of each component. In one component (i.e., EtOH component), lever presses produced ethanol deliveries on a variable-interval 15-s schedule. In the other component (i.e., EtOH+Food component), lever presses produced ethanol deliveries on a variable-interval 15-s schedule and additional response-independent food pellets (45 mg) were delivered according to a variable-time 120-s schedule. The stimuli associated with the two components were counterbalanced across subjects. The first component type of the session was randomly chosen and strict alternation followed thereafter. Exposure to these baseline conditions lasted 20 sessions for all rats.
Next, lever pressing was extinguished in both components. During extinction, the multiple-schedule component stimuli continued to alternate as during baseline, but both food and ethanol deliveries were discontinued. Ethanol dipper trays were loaded with ethanol solution outside the operant chamber as they were during baseline. Extinction continued for a minimum of 10 sessions and until response rates for individual rats were below 10% of baseline. Reinstatement was then examined for 5 days by delivering response-independent dippers of the ethanol solution at 2-s and 8-s into the first two components of the session. The first component presented during the first day of reinstatement was counterbalanced across rats and alternated across days of reinstatement.
Results
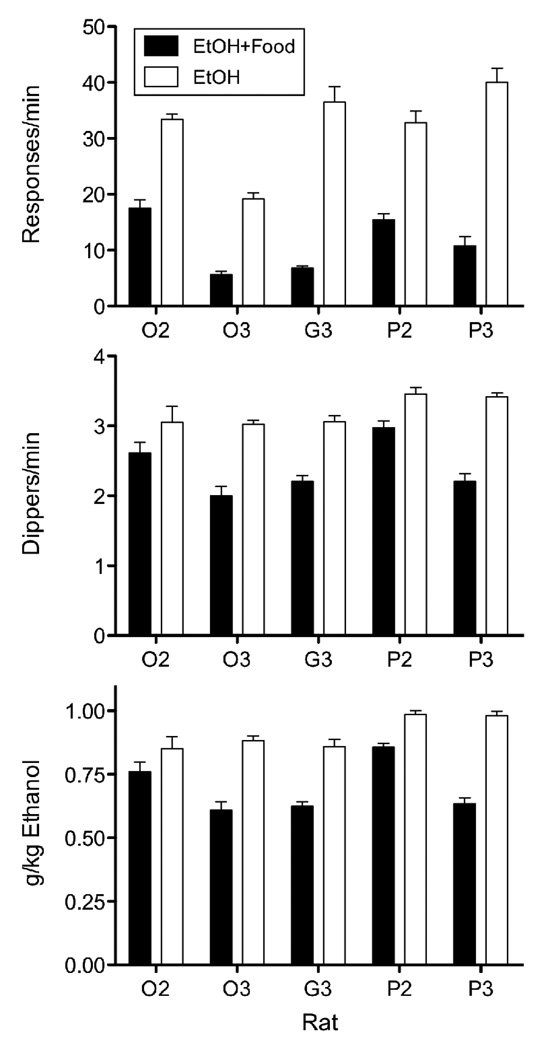
Figure 1 shows absolute response rates (i.e., responses/min), dipper rates, and g/kg ethanol consumed in the two components of the multiple schedule for individual rats. Response and reinforcer rates were calculated based on the time in each component minus time during dipper (3-s) and food (.04-s) deliveries. The top panel of Fig. 1 shows that absolute response rates were lower in EtOH+Food component than in the EtOH component [t(4) = −6.14, p < .01]. The middle panel shows that fewer dippers were earned [t(4) = −5.33, p < .01], and accordingly, the bottom panel shows that less ethanol was consumed in the EtOH+Food component than in the EtOH component [t(4) = −4.60, p < .05].
Figure 1.
Absolute baseline response rates (top panel), ethanol dipper rates (middle panel), and g/kg ethanol (bottom panel) for each rat, in the component previously associated with added response-independent food (EtOH+Food) and the component with ethanol alone (EtOH). Data are means (± SD) for individual rats from the last five sessions of baseline.
An initial 2 (component) × 10 (extinction session) repeated-measures ANOVA conducted on absolute response rates during extinction revealed a significant main effect of component [F(1,4) = 19.20, p < .05], extinction session [F(9,36) = 20.31, p < .001], and a significant interaction [F(9,36) = 27.09, p < .001]. Thus, absolute response rates decreased across sessions in both components, with responding in the EtOH+food component remaining suppressed compared to the EtOH component. Absolute response rates did not differ significantly in the last extinction session [t(4) = −1.75, NS]. An additional 2 (component) × 5 (session) repeated-measures ANOVA revealed a significant main effect of reinstatement session [F(4,16) = 5.45, p < .01], but no significant main effect of component [F(1,4) = 2.08, NS] or component × session interaction [F(4,16) = 2.24, NS]. Thus, despite the suppressed response rates in the component with the added food during baseline and extinction, response rates were similar in the two components during reinstatement. Regardless, it is important to note that from the perspective of behavioral momentum absolute response rate is not an appropriate measure of resistance to extinction or relapse. Responding during extinction and relapse must be considered relative to absolute response rates during the pre-extinction baseline (see Nevin and Grace 2000; Podlesnik and Shahan 2009). Baker et al. (1991) have similarly suggested that baseline rates during reinstatement should be normalized with respect to absolute baseline rates. Thus, we focus our analysis on relative resistance to extinction and relative relapse as measured by responding as a proportion of baseline rates.
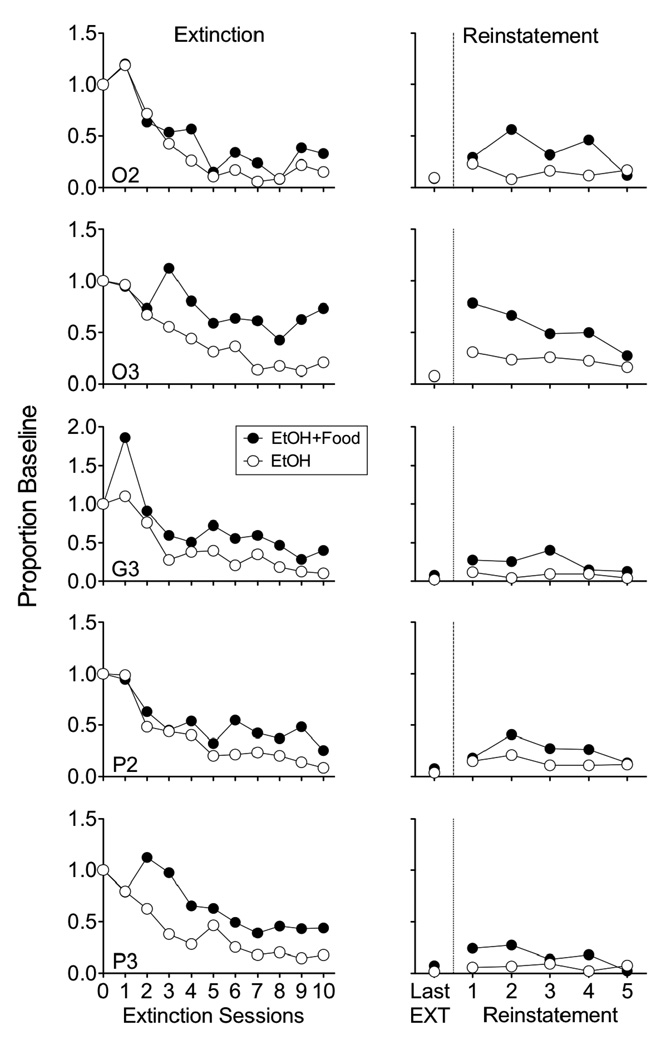
Accordingly, the left column of Figure 2 shows a resistance to change analysis of responding on the ethanol lever during the first 10 extinction sessions as a proportion of baseline response rates. Relative resistance to extinction was greater in the EtOH+Food component than in the EtOH component for all rats. A 2 (component) × 10 (extinction session) repeated-measures ANOVA conducted on log-transformed data (appropriate for comparing rates of change) reveals a significant main effect of component [F(1,4) = 116.09, p < .001], extinction session [F(9,36) = 13.26, p < .001], and a significant interaction [F(9,36) = 6.17, p < .001]. An alternative analysis based on deriving slopes of resistance to extinction functions with linear regression for each rat in the two components and then comparing those slopes also suggests that resistance to extinction was greater in the EtOH+Food component [t(4) = 7.48, p < .01].
Figure 2.
The left column shows a resistance to change analysis of responding during the first 10 days of extinction in the component previously associated with added response-independent food (EtOH+Food) and the component with ethanol alone (EtOH). The right column shows responding during the last session of extinction and during 5 days of reinstatement produced by response-independent ethanol deliveries in both components. Data are presented for individual rats.
The right column of Figure 2 shows responding on the last day of extinction and during the 5 days of reinstatement by free alcohol deliveries in both components. Relative response rates in the two components were not significantly different in the last session of extinction [t(4) = 1.92, NS]. For every rat, relative response rates during reinstatement were greater in the EtOH+Food component than the EtOH component. A 2 (component) × 5 (session) repeated-measures ANOVA revealed a significant main effect of component [F(1,4) = 24.09, p < .01], session [F(4,16) = 6.43, p < .01], and component × session interaction [F(4,16) = 4.39, p < .05]. Thus, relative reinstatement was greater in the EtOH+Food component than in the EtOH component.
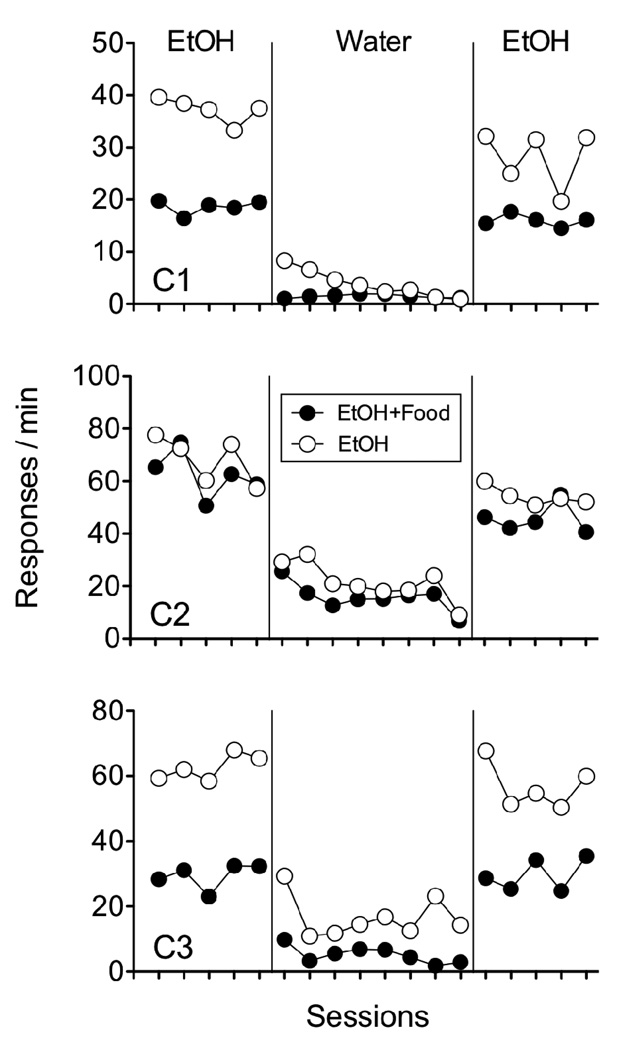
One potential limitation of the present experimental design was that it did not allow confirmation that ethanol was the functional reinforcer in the solution. To examine this possibility, 3 additional rats with identical histories of alcohol self-administration training and similar histories of multiple schedule performance experienced 15 sessions of the same baseline. Distilled water then replaced the ethanol solution in the dipper reservoirs for 8 sessions, followed by a return to 10% ethanol for an additional 5 sessions. Food deliveries continued in the EtOH+Food component across all three conditions. As shown in Figure 3, response rates were lower in the EtOH+Food component than in the EtOH component. When ethanol was removed from the solution, responding decreased substantially in both components, but increased again when ethanol was reintroduced. Thus, the ethanol in the solution does appear to have been functioning as a reinforcer.
Figure 3.
The left portion of the figures shows response rates on the alcohol lever in the component associated with added response-independent food (EtOH+Food) and the component with ethanol alone (EtOH) during the last 5 sessions of baseline when 10% ethanol was available. The middle portion of the figures shows response rates in both components when ethanol was removed from the solution. The right portion shows 5 sessions immediately following the water control in which 10% ethanol was again available. Data are presented for individual rats.
Discussion
Consistent with previous findings (see Carroll 1996), an added source of non-drug reinforcement decreased baseline drug-maintained behavior in the present experiment. Nonetheless, as in Shahan and Burke (2004), relative resistance to extinction of ethanol-maintained behavior was greater in a discriminative-stimulus context previously associated with an added source of non-drug reinforcement. The present experiment extends this finding by showing that an added source of non-drug reinforcement also increases relative reinstatement of ethanol-maintained responding in the context. Thus, like resistance to extinction, relative relapse of ethanol seeking appears to depend upon all sources of reinforcement previously experienced in the presence of a discriminative-stimulus context. This finding is predicted by behavioral momentum theory, and is consistent with the suggestion that the effects of the Pavlovian stimulus-reinforcer on resistance to extinction of operant responding may be extended to relative propensity to relapse after extinction (Podlesnik and Shahan 2009, 2010). Further extensions of the methods and theoretical framework of behavioral momentum theory to drug self-administration may provide additional insights into the persistence of drug seeking and relapse in the presence of drug-related contexts.
Drug-related stimuli play an important role in contemporary approaches to addiction and are widely thought to contribute to the persistence of drug seeking and relapse (see Robinson and Berridge 2008; Taylor et al. 2009 for reviews). The present findings suggest that in order to understand how drug-related contexts contribute to relapse, it may be necessary to consider not only the history of drug reinforcement experienced within a context, but also the wide variety of other reinforcers obtained in such contexts. Although drug and non-drug reinforcers share the same neurobiological mechanisms (e.g., Kelley and Berridge 2002), much remains to be learned about the combined effects of drug and non-drug reinforcers on the persistence of drug seeking and relapse, as mediated through the incentive-motivational effects of drug-related stimuli (cf. Panlilio et al. 1998; Lombas et al. 2008).
Acknowledgements
This research was funded by NIH grant R01AA016786 (TAS). The authors thank Michael Sirrine and Carrie Adams for their help conducting this experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahearn WH, Clark KM, Gardenier NC, Chung BI, Dube WV. Persistence of stereotyped behavior: examining the effects of external reinforcers. J Appl Behav Anal. 2003;36:439–448. doi: 10.1901/jaba.2003.36-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. pp. 191–197. [Google Scholar]
- Baker AG, Steinwald H, Bouton ME. Contextual conditioning and reinstatement of extinguished instrumental conditioning. Q J Exp Psychol. 1991;43B:199–218. [Google Scholar]
- Bindra D. A unified account of classical conditioning and operant training. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton; 1972. pp. 453–481. [Google Scholar]
- Carroll ME. Reducing drug abuse by enriching the environment with alternative nondrug reinforcers. In: Green L, Kagel JH, editors. Advances in Behavioral Economics Volume 3: Substance Use and Abuse. New Jersey: Ablex Publishing Corporation; 1996. pp. 37–68. [Google Scholar]
- Grimes JA, Shull RL. Response-independent milk delivery enhances persistence of pellet reinforced lever pressing by rats. J Exp Anal Behav. 2001;76:179–194. doi: 10.1901/jeab.2001.76-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T, Sakagami T. Resistance to change in goldfish. Behav Process. 2004;66:139–152. doi: 10.1016/j.beproc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombas AS, Kearns DN, Weiss SJ. Differential effects of a food-based conditioned inhibitor on food- or cocaine-seeking behavior. Learn Motiv. 2008;39:323–333. doi: 10.1016/j.lmot.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace FC, Lalli JS, Shea MC, Lalli EP, West BJ, Roberts M, Nevin JA. The momentum of human behavior in a natural setting. J Exp Anal Behav. 1990;54:163–172. doi: 10.1901/jeab.1990.54-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Drug abuse and addiction. [Accessed 11/8/2007];Drugs, Brains, and Behavior: The Science of Addiction. NIH Publication No 07-5605. 2007 Available at http://www.drugabuse.gov/scienceofaddiction/sciofaddiction.pdf.
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behav Brain Sci. 2000;23:73–130. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Tota ME, Torquato RD, Shull RL. Alternative reinforcement increases resistance to change: Pavlovian or operant contingencies? J Exp Anal Behav. 1990;53:359–379. doi: 10.1901/jeab.1990.53-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Motivational effects of compounding discriminative stimuli associated with food and cocaine. Psychopharmacology. 1998;136:70–74. doi: 10.1007/s002130050540. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Shahan TA. Behavioral momentum and relapse of extinguished operant responding. Learn Behav. 2009;37:357–364. doi: 10.3758/LB.37.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Shahan TA. Extinction, relapse, and behavioral momentum. Behav Process. 2010;84:400–411. doi: 10.1016/j.beproc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick SL, Shahan TA. Behavioral momentum of cocaine self-administration: effects of frequency of reinforcement on resistance to extinction. Behav Pharm. 2009;20:337–345. doi: 10.1097/FBP.0b013e32832f01a8. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil Trans R Soc B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Burke KA. Ethanol-maintained responding of rats is more resistant to change in a context with added non-drug reinforcement. Behav Pharmacol. 2004;15:279–285. doi: 10.1097/01.fbp.0000135706.93950.1a. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]