Abstract
Glial cells in the gut represent the morphological and functional equivalent of astrocytes and microglia in the central nervous system (CNS). In recent years, the role of enteric glial cells (EGCs) has extended from that of simple nutritive support for enteric neurons to that of being pivotal participants in the regulation of inflammatory events in the gut. Similar to the CNS astrocytes, the EGCs physiologically express the S100B protein that exerts either trophic or toxic effects depending on its concentration in the extracellular milieu. In the CNS, S100B overexpression is responsible for the initiation of a gliotic reaction by the release of pro-inflammatory mediators, which may have a deleterious effect on neighboring cells. S100B-mediated pro-inflammatory effects are not limited to the brain: S100B overexpression is associated with the onset and maintenance of inflammation in the human gut too. In this review we describe the major features of EGCs and S100B protein occurring in intestinal inflammation deriving from such.
Keywords: Enteric glial cells, Nitric oxide, Intestinal diseases
INTRODUCTION
Intestinal tissues are innervated by a complex and extensive component known as the enteric nervous system (ENS)[1]. The ENS is characterized by the presence of neurons and enteric glial cells (EGCs) which are arranged into interconnected ganglia distributed between 2 major plexuses, and they control several gut functions[2,3]. During the course of time, the traditional view of EGCs has changed from being a mere mechanical support for surrounding neurons to that of a more articulate and complex nature, since they are actively involved in the regulation of homeostasis, motility and inflammatory processes within the gut[4,5].
Similar to the astrocytes in the central nervous system (CNS), the EGCs release several signaling molecules[4,5]. Among these, great importance has been given to the better comprehension of the specific glial-derived S100B protein[6-8]. This protein is a small, diffusible neurotrophin that is situated in the cytoplasm and/or the nucleus of both nervous and non-nervous tissues[9,10]. In the brain, S100B has been considered a “Janus face” neurotrophin[11,12] because it exerts opposite actions depending on its concentration in the extracellular milieu: it has a pro-proliferative and neurogenic effect on astroglia and on serotonergic neurons at nanomolar concentrations, as well as a neurodegenerative function[12] at micromolar concentrations, determining mysregulated glial cell proliferation and amplifying neuroinflammation. Similar to the brain, recent studies have suggested the involvement of S100B in inflammatory processes occurring in the gut, highlighting the importance of EGCs as key regulators of gut homeostasis[13-15].
In this review, we will focus on the role of EGCs and the S100B protein and we will take them into consideration by looking at both experimental animal models and some human diseases for which evidence exists, and in particular their involvement in inflammatory conditions of the human gut where the role of S100B appears to be prominent.
ENTERIC NERVOUS SYSTEM
The gut is characterized by a sequence, starting from the serosa, as follows: subserosa, longitudinal muscle, myenteric plexus, circular muscle, submucosal plexus, muscularis mucosae and mucosa[2]. The myenteric and submucosal plexuses are characterized by the presence of ganglia which, in turn, contain enteric neurons and EGCs in a ratio of 1:7[16]. In the ENS, the enteric ganglia are involved in basic gut functions, such as the regulation of peristalsis, secretion and blood flow and the modulation of the immune/inflammatory processes[17-19]. Several neurotransmitters are involved in the control of all these intestinal functions, such as vasoactive intestinal peptide and nitric oxide (NO)[20,21]. In particular, NO is produced by the biosynthetic enzyme neuronal NO synthase (NOS), which is expressed in myenteric neurons, and by the inducible form of NOS (iNOS), which is expressed in EGCs[15].
EGCs: both protective and destructive cells in the gut
EGCs are small cells with a “star-like” appearance[16] containing intracellular arrays of 10 nm filaments made up of glial fibrillary acidic protein (GFAP)[16,22-24]. This cell population was first described by Dogiel[25] using methylene blue staining on full thickness preparations. At present, the S100B protein and GFAP are commonly used as specific markers in order to identify EGCs[22,26]. More recently, other markers have been proposed for the identification of glial cells in the human gut, especially in whole-mount preparations: Sox8/9/10, a specific nuclear marker[16]. EGCs release a wide range of factors accounting for the development, survival and differentiation of peripheral neurons[27]. Traditionally, EGCs have been considered as a mechanical support for enteric neurons, but, in recent years, this restrictive view has changed to one of a more articulate and complex nature, since it has been described that EGCs are involved in the maintenance of intestinal homeostasis[28-30]. Indeed, EGCs control intestinal epithelial barrier (IEB) functions, as demonstrated in animal studies in which the ablation of enteroglial network enhances intestinal vascular permeability together with an increase in IEB paracellular permeability[31-34]. Furthermore, in vitro data has shown that EGCs partially decrease IEB permeability via the release of S-nitrosoglutathione and the regulation of zonulin-1 and occludin expression[35,36]. Although the function of glial mediators still have to be identified, it is conceivable that they could be actively involved in the EGCs-mediated effects on barrier functions.
Besides the well documented ‘protective role’, EGCs are activated by means of inflammatory insults and they may directly contribute to an inflammatory condition working as an antigen presenting cell-type promoting a variegate release of cytokine synthesis[13-15,35-37] in the gut milieu. Therefore, EGCs may act as “receptors” for cytokines and they themselves produce interleukin-6 (IL-6) and IL-1b[38,39]. Moreover, ECGs express iNOS and L-arginine, the machinery for the time-delayed and micromolar release of NO, one of the most important signaling molecules involved in host-immune defense against viruses and bacteria as well as a well-known pro-inflammatory mediator[15,40,41].
EGC-SELECTIVELY EXPRESSED PROTEINS
GFAP
Mature EGCs are rich in the intermediate filament protein, GFAP[42,43]. Its expression is modulated by glial cell differentiation, inflammation and injury[42], indicating that the level of GFAP accords with the functional state of glial cells.
In animals, two classes of glial cells can be distinguished, namely the GFAP positive (+) and GFAP negative (-) groups, as demonstrated by von Boyen et al[8]. In the same study, it was suggested that pro-inflammatory cytokines control GFAP+ enteric glia, which, in turn, are involved in the modulation of the integrity of the bowel during inflammation[8].
In humans, GFAP expression is altered in the mucosa of patients with inflammatory bowel diseases (IBD), as well as ulcerative colitis (UC) and Crohn’s disease[33].
S100B protein
S100B belongs to the S100 protein family that includes more than 20 EF-hand Ca2+-Zn2+ binding proteins[9,10,44-47]. S100B is the homodimer of β subunit[48]. In the brain, S100B in nanomolar concentrations promotes neuronal survival, neurite outgrowth[49] and it stimulates astrocytic proliferation[50], increasing the intracellular free Ca2+ levels in vitro[51]. On the other hand, micromolar amounts of S100B protein have been observed in several neuropathologies such as Alzheimer’s disease and Down’s syndrome[52,53].
In the human gut, among S100 proteins, only the S100B protein is specifically and physiologically expressed by EGCs[13-15], while other members, such as S100A8, S100A9 and S100A12 are found in phagocytes and in intestinal epithelial cells in patients affected by IBD[54,55].
Recent findings have demonstrated that aberrant expression and the release of S100B correlate with the gut inflammatory status[13,14]. Interestingly, the search for a specific S100B signaling receptor has demonstrated that this protein may accumulate at the RAGE (receptor for advanced glycation end products) site only in micromolar concentrations[14,56-59]. Such interaction leads to mitogen-activated protein kinase (MAPK) phosphorylation and consequent nuclear factor-κB (NF-κB) activation[13] which, in turn, leads to the transcription of different cytokines and iNOS protein. Thus, S100B can be considered as an easily diffusible pro-inflammatory cytokine which gains access to the extracellular space especially at immune-inflammatory reaction sites in the gut[13-15,60,61].
EGCs AND S100B IN GUT INFLAMMATION
In humans, recent and increasing data has demonstrated that EGCs and S100B protein are directly involved in gut inflammatory diseases[13,14,33]. Previous investigations described abnormalities of the enteroglial network in patients with Crohn’s disease and UC[33]. More recently, glial abnormalities have been confirmed by 2 separate studies carried out by our group[13,14]. In particular, we demonstrated that, in patients with celiac disease (CD)[13] and UC[14], EGCs participate in the modulation of mucosal NO production via S100B overexpression and release. Indeed, in patients with CD, we demonstrated that S100B plays an active role in NO-dependent inflammation[13]. In particular, increased S100B protein expression and release were observed in the duodenal mucosa of patients with untreated CD, compared to healthy controls (Figure 1A)[13]. Very interestingly, S100B upregulation was accompanied by enhanced iNOS protein expression and consequent NO release, both representing crucial features in CD[62].
Figure 1.
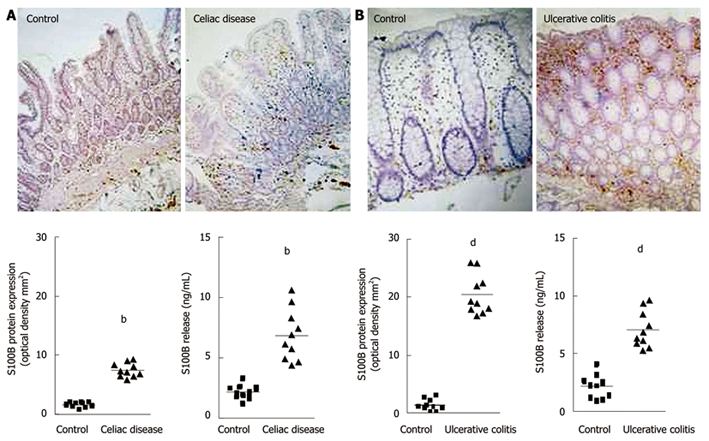
Changes in S100B protein expression during intestinal inflammation. A: Celiac disease[13]. Immunohistochemistry shows stronger S100B immunopositivity in the duodenal mucosa of patients affected by celiac disease, compared with healthy controls (original magnification, × 100). The graphs represent S100B protein expression (left) and release (right) in healthy controls and patients with celiac disease (bP < 0.01); B: Ulcerative colitis[14]. Immunohistochemistry shows stronger S100B immunopositivity in the rectal submucosa of patients with ulcerative colitis, compared with healthy controls (original magnification, × 100). The graphs represent S100B protein expression (left) and release (right) in healthy controls and patients with ulcerative colitis (dP < 0.01).
The relationship between S100B and NO production was confirmed by the demonstration that the administration of exogenous S100B protein to non-inflamed duodenal biopsy specimens from healthy controls, resulted in both iNOS protein expression and NO release, indicating that micromolar concentrations of this protein are able to participate in the inflammatory response of even “healthy duodenum”[13]. Besides NO production, exogenous S100B mediates a significant increase in lipid peroxidation associated with a marked increase in phosphorylated-p38 MAPK protein expression and with the activation of NF-κB, in accordance with the previously mentioned studies[13].
Taken together, these observations represented the first data in humans suggesting that via S100B upregulation, EGCs directly participate in NO-dependent inflammation occurring in CD, and they paved the way by supposing that EGCs are part of complex immuno-regulatory effectors since they establish a strategic first defense line against foreign antigens. By means of EGC proliferation, changes in enteroglial architecture have been reported also in patients with IBD[33,63]. Several studies have shown that EGC markers are differentially altered in Crohn’s disease and UC with a decrease in EGC density in Crohn’s disease and a gliosis-like phenomenon in UC[33,63].
In support of these observations, it has recently been confirmed that EGCs directly participate in the chronic mucosal inflammation of patients with UC[14]. In fact, S100B immunoreactivity significantly increased in the rectal mucosa of these patients when compared to the mucosal S100B expression in healthy controls (Figure 1B)[14]. This upregulation was associated with the specific stimulation of iNOS and consequent abnormal mucosal NO production, both representing characteristic features of UC[64,65].
In addition, via iNOS expression, exogenous S100B induces a significant and concentration-dependent increase in NO production in the human rectal mucosa of healthy controls via RAGE interaction[14], confirming the ability of EGCs to modulate NO production and the specificity of S100B protein-mediated responses in the human gut.
Further confirming that EGCs are part of the complex system of immunoregulatory effectors in the gut, it has been shown that the addition of pro-inflammatory stimuli to rectal mucosal tissue led to EGC activation, again via RAGE involvement[14] as demonstrated by both S100B upregulation and enhanced NO production. These findings indicate that EGCs are able to recognize inflammatory stimuli and that once activated, they produce and release S100B up to micromolar concentrations, thereby contributing to NO production in the human gut.
CONCLUSION
ECGs as a target for new drugs aimed at inflammatory gut disorder management
In summary, emerging evidence now indicates that EGCs actively participate in the modulation of inflammatory responses in the human gut. Targeting their hyperactivation in the gut in inflammatory disorders may represent a novel approach to diminish tissue damage and to counteract the lack of long-term effectiveness of classical immunosuppressant agents.
Additional studies investigating the relationship between EGCs and immune cells are warranted in order to carry out an in-depth examination of the role of glial cells and glia-derived factors in the modulation of immune/inflammatory responses in the human gut. Preliminary data indicates that EGCs-derived S100B is able to affect peripheral blood and intestinal mucosal immune cell responses via RAGE[66].
The application of this approach may help the future evaluation of the relationships between EGCs and immune cells in order to better understand the pathophysiology of intestinal inflammation and to establish new therapeutic approaches towards the treatment of gut inflammatory disorders.
Acknowledgments
We would like to thank Mrs Thérèse Marshall for her help with the preparation of the manuscript.
Footnotes
Supported by research funds from the Italian Ministry of University and Research (COFIN Projects No. 2004062155 to GS and RC)
Peer reviewer: Wojciech Blonski, MD, PhD, University of Pennsylvania, GI Research-Ground Centrex, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
References
- 1.Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 1:55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 2.Furness JB. The Enteric Nervous System. Oxford: Blackwell Publishing; 2006. [Google Scholar]
- 3.Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- 4.Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 5.Rühl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17:777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 6.Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002;172:69–80. doi: 10.1385/1-59259-183-3:069. [DOI] [PubMed] [Google Scholar]
- 7.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 8.von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489–498. [PubMed] [Google Scholar]
- 10.Zimmer DB, Van Eldik LJ. Tissue distribution of rat S100 alpha and S100 beta and S100-binding proteins. Am J Physiol. 1987;252:C285–C289. doi: 10.1152/ajpcell.1987.252.3.C285. [DOI] [PubMed] [Google Scholar]
- 11.Fanò G, Biocca S, Fulle S, Mariggiò MA, Belia S, Calissano P. The S-100: a protein family in search of a function. Prog Neurobiol. 1995;46:71–82. doi: 10.1016/0301-0082(94)00062-m. [DOI] [PubMed] [Google Scholar]
- 12.Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- 13.Esposito G, Cirillo C, Sarnelli G, De Filippis D, D'Armiento FP, Rocco A, Nardone G, Petruzzelli R, Grosso M, Izzo P, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–925. doi: 10.1053/j.gastro.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Calì G, D'Armiento FP, Rocco A, Nardone G, et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil. 2009;21:1209–e112. doi: 10.1111/j.1365-2982.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- 15.Cirillo C, Sarnelli G, Mango A, Esposito I and Cuomo R. Effect of pro-inflammatory stimuli on cellular activation and nitric oxide production in primary cultures of human enteric glia. Gastroenterology. 2009;136:A4. [Google Scholar]
- 16.Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Rühl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol. 2008;509:356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- 17.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 18.Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Minneap) 1999;34:31–32, 35-38, 41-42 passim. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 19.Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Grider JR. Regulation of excitatory neural input to longitudinal intestinal muscle by myenteric interneurons. Am J Physiol. 1998;275:G973–G978. doi: 10.1152/ajpgi.1998.275.5.G973. [DOI] [PubMed] [Google Scholar]
- 21.Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71–78. doi: 10.1002/1097-0185(20010101)262:1<71::AID-AR1012>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Elfvin LG, Björklund H, Dahl D, Seiger A. Neurofilament-like and glial fibrillary acidic protein-like immunoreactivities in rat and guinea-pig sympathetic ganglia in situ and after perturbation. Cell Tissue Res. 1987;250:79–86. doi: 10.1007/BF00214657. [DOI] [PubMed] [Google Scholar]
- 23.Endo Y, Kobayashi S. A scanning electron microscope study on the autonomic groundplexus in the lamina propria mucosae of the guinea-pig small intestine. Arch Histol Jpn. 1987;50:243–250. doi: 10.1679/aohc.50.243. [DOI] [PubMed] [Google Scholar]
- 24.Mestres P, Diener M, Rummel W. Electron microscopy of the mucosal plexus of the rat colon. Acta Anat (Basel) 1992;143:275–282. doi: 10.1159/000147262. [DOI] [PubMed] [Google Scholar]
- 25.Dogiel AS. Uber den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Saugethiere. Z Naturforsch B. 1899;5:130–158. [Google Scholar]
- 26.Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- 27.Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Bassotti G, Villanacci V, Antonelli E, Morelli A, Salerni B. Enteric glial cells: new players in gastrointestinal motility? Lab Invest. 2007;87:628–632. doi: 10.1038/labinvest.3700564. [DOI] [PubMed] [Google Scholar]
- 29.Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035–4041. doi: 10.3748/wjg.v13.i30.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Landeghem L, Mahé MM, Teusan R, Léger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics. 2009;10:507. doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aubé AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630–637. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 33.Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA. 2001;98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Neunlist M, Van Landeghem L, Bourreille A, Savidge T. Neuro-glial crosstalk in inflammatory bowel disease. J Intern Med. 2008;263:577–583. doi: 10.1111/j.1365-2796.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 36.Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest. 2007;87:731–736. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- 37.Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- 38.Murakami M, Ohta T, Ito S. Lipopolysaccharides enhance the action of bradykinin in enteric neurons via secretion of interleukin-1beta from enteric glial cells. J Neurosci Res. 2009;87:2095–2104. doi: 10.1002/jnr.22036. [DOI] [PubMed] [Google Scholar]
- 39.Rühl A, Franzke S, Collins SM, Stremmel W. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1163–G1171. doi: 10.1152/ajpgi.2001.280.6.G1163. [DOI] [PubMed] [Google Scholar]
- 40.Aoki E, Semba R, Kashiwamata S. Evidence for the presence of L-arginine in the glial components of the peripheral nervous system. Brain Res. 1991;559:159–162. doi: 10.1016/0006-8993(91)90300-k. [DOI] [PubMed] [Google Scholar]
- 41.Nagahama M, Semba R, Tsuzuki M, Aoki E. L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. Biol Signals Recept. 2001;10:336–340. doi: 10.1159/000046901. [DOI] [PubMed] [Google Scholar]
- 42.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 43.Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Martinez T, Perez-Piñera P, Díaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633–638. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- 45.Iwanaga T, Takahashi Y, Fujita T. Immunohistochemistry of neuron-specific and glia-specific proteins. Arch Histol Cytol. 1989;52 Suppl:13–24. doi: 10.1679/aohc.52.suppl_13. [DOI] [PubMed] [Google Scholar]
- 46.Sugimura K, Haimoto H, Nagura H, Kato K, Takahashi A. Immunohistochemical differential distribution of S-100 alpha and S-100 beta in the peripheral nervous system of the rat. Muscle Nerve. 1989;12:929–935. doi: 10.1002/mus.880121109. [DOI] [PubMed] [Google Scholar]
- 47.Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 48.Baudier J, Glasser N, Gerard D. Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100B (beta beta) protein: Zn2+ regulates Ca2+ binding on S100B protein. J Biol Chem. 1986;261:8192–8203. [PubMed] [Google Scholar]
- 49.Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R. Biomarker s of glial cell proliferation and differentiation in culture. Front Biosci (Schol Ed) 2010;2:558–570. doi: 10.2741/s85. [DOI] [PubMed] [Google Scholar]
- 50.Zimmer DB, Chaplin J, Baldwin A, Rast M. S100-mediated signal transduction in the nervous system and neurological diseases. Cell Mol Biol (Noisy-le-grand) 2005;51:201–214. [PubMed] [Google Scholar]
- 51.Chow SK, Yu D, Macdonald CL, Buibas M, Silva GA. Amyloid β-peptide directly induces spontaneous calcium transients, delayed intercellular calcium waves and gliosis in rat cortical astrocytes. ASN Neuro. 2010;2:e00026. doi: 10.1042/AN20090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Eldik LJ, Griffin WS. S100 beta expression in Alzheimer's disease: relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223:398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 54.Leach ST, Yang Z, Messina I, Song C, Geczy CL, Cunningham AM, Day AS. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321–1331. doi: 10.1080/00365520701416709. [DOI] [PubMed] [Google Scholar]
- 55.Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids. 2009;36:381–389. doi: 10.1007/s00726-008-0097-7. [DOI] [PubMed] [Google Scholar]
- 56.Adami C, Bianchi R, Pula G, Donato R. S100B-stimulated NO production by BV-2 microglia is independent of RAGE transducing activity but dependent on RAGE extracellular domain. Biochim Biophys Acta. 2004;1742:169–177. doi: 10.1016/j.bbamcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia. 2001;33:131–142. [PubMed] [Google Scholar]
- 61.Petrova TV, Hu J, Van Eldik LJ. Modulation of glial activation by astrocyte-derived protein S100B: differential responses of astrocyte and microglial cultures. Brain Res. 2000;853:74–80. doi: 10.1016/s0006-8993(99)02251-9. [DOI] [PubMed] [Google Scholar]
- 62.Daniels I, Cavill D, Murray IA, Long RG. Elevated expression of iNOS mRNA and protein in coeliac disease. Clin Chim Acta. 2005;356:134–142. doi: 10.1016/j.cccn.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 63.Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn's disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189–202. doi: 10.1046/j.1365-2982.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 64.Linehan JD, Kolios G, Valatas V, Robertson DA, Westwick J. Effect of corticosteroids on nitric oxide production in inflammatory bowel disease: are leukocytes the site of action? Am J Physiol Gastrointest Liver Physiol. 2005;288:G261–G267. doi: 10.1152/ajpgi.00336.2004. [DOI] [PubMed] [Google Scholar]
- 65.Menchén L, Colón AL, Madrigal JL, Beltrán L, Botella S, Lizasoain I, Leza JC, Moro MA, Menchén P, Cos E, et al. Activity of inducible and neuronal nitric oxide synthases in colonic mucosa predicts progression of ulcerative colitis. Am J Gastroenterol. 2004;99:1756–1764. doi: 10.1111/j.1572-0241.2004.40065.x. [DOI] [PubMed] [Google Scholar]
- 66.Cirillo C, Sarnelli G, Mango A, Esposito I, Grosso M, Cuomo R. Enteric glia stimulates inflammation-induced responses in human intestine and interacts with immune cells via S100B protein. Gastroenterology. 2009;136:A271–A272. [Google Scholar]


