Abstract
AIM: To study the prognostic factors for intrahepatic cholangiocarcinoma (ICC) and evaluate the impact of chronic hepatitis B virus (HBV) infection on survival rate of ICC patients.
METHODS: A total of 155 ICC patients who underwent macroscopic curative resections (R0 and R1) were enrolled in this retrospective study and divided into group A with HBV infection and group B without HBV infection according to their chronic HBV infection, represented by positive hepatitis B surface antigen (HBsAg) in serum or in liver tissue. Clinicopathological characteristics and survival rate of the patients were evaluated.
RESULTS: All patients underwent anatomical resection. Their 1- and 3-year survival rates were 60.6% and 32.1%, respectively. Multivariate analyses revealed that HBV infection, hepatolithiasis, microscopic satellite lesion, and lymphatic metastasis were the independent prognostic factors for the survival rate of ICC patients. The median disease-free survival time of the patients was 5.0 mo. The number of tumors, microscopic satellite lesion, and vascular invasion were the independent prognostic factors for the disease-free survival rate of the patients. The prognostic factors affecting the survival rate of ICC patients with HBV infection and those without HBV infection were not completely consistent. Alkaline phosphatase > 119 U/L, microscopic satellite lesion, vascular invasion, and lymphatic metastasis were the independent factors for the patients with HBV infection, while r-glutamyltransferase > 64 U/L, microscopic satellite lesion, and poor tumor differentiation were the independent factors for the patients without HBV infection.
CONCLUSION: HBV infection is a valuable clinical factor for predicting tumor invasiveness and clinical outcome of ICC patients. ICC patients with HBV infection should be distinguished from those without HBV infection because they have different clinicopathological characteristics, prognostic factors and outcomes after surgical resection.
Keywords: Intrahepatic cholangiocarcinoma, Hepatitis B virus, Survival, Prognosis
INTRODUCTION
Cholangiocarcinoma originates from the extrahepatic bile duct, hilar bifurcation, and intrahepatic duct. Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic tumor after hepatocellular carcinoma (HCC). Primary sclerosingcholangitis (PSC)[1,2], liver fluke infestation (particularly endemic Opisthorcis viverrini)[3], and hepatolithiasis are the known risk factors for ICC[4,5]. Recent evidence suggests that hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is also an important risk factor for ICC[5-10]. Our previous study also demonstrated that the incidence of HBV infection is significantly higher in ICC patients than in those without cancer (48.6% vs 6.6%) and chronic HBV infection is the most important independent risk factor for ICC in Chinese[11].
The prognosis of ICC patients is poorer than that of HCC patients, mainly due to frequent lymphatic involvement, periductal invasion, poor encapsulation, or difficulty of early diagnosis. These characteristics are more prominent in ICC patients with seronegative hepatitis B surface antigen (HBsAg) than in those with seropositive HBsAg[11], indicating that ICC patients with HBV infection have a more favorable prognosis than those without HBV infection. Given a higher incidence of microvascular invasion, poor tumor differentiation, and liver function in seropositive-HBsAg ICC patients compared with seronegative-HBsAg ICC patients, the real difference in prognosis is still unclear.
In the present study, the prognostic factors for ICC and the impact of chronic HBV infection on the survival rate of ICC patients were studied.
MATERIALS AND METHODS
Patients
A total of 209 patients underwent surgical dissection for ICC (the diagnosis of ICC was confirmed by pathology) at three departments of hepatobiliary surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Shanghai, China) in January 2005 - December 2007. Of the 209 patients, 195 underwent macroscopic curative resection (R0 and R1), 14 underwent only laparotomy and biopsy because of advanced lesions, such as peritoneal seeding. Of the 195 patients, there were 155 patients with information on survival and 40 lost their follow-up due to death, loss of contact, or other unknown reasons. Finally, 155 patients were included in the study. The prognostic factors influencing their survival rate and tumor recurrence were analyzed. The study was approved by the local ethics committee.
Clinicopathological investigations
Demographic and clinicopathological information was obtained from medical records of the patients including age, gender, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), r-glutamyltransferase (r-GT), alkaline phosphatase (ALP), α-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), HBV infection, number of tumors, and tumor location, size, capsule formation, histologic type, differentiation and recurrence, as well as vascular invasion, microscopic satellite lesion, lymphatic and extrahepatic metastasis, surgical procedures, postoperative complications.
Statistical analysis
Patients were screened for carcinoembryonic antigen (CEA), AFP, CA19-9 and CT scan every 3-6 mo after operation. When recurrence was suspected, magnetic resonance imaging (MRI) or PET images were taken for confirmation. Disease-free survival was measured from the date of surgery to the date of recurrence. Survival was measured from the date of surgery. Follow-up of patients was continued until death or April 10, 2010.
Statistical analysis was performed using the SPSS, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Overall and disease-free survival rates were calculated using the Kaplan-Meier method. Prognostic factors for the patients were evaluated using the univariate Kaplan-Meier method and compared with the log-rank test. Multivariate regression analysis was performed using the Cox proportional hazards model to identify the independent prognostic factors for the survival rate of patients and tumor recurrence. Variables to be entered into the multivariate analysis were selected on the basis of the results of univariate analysis (P < 0.1). χ2 test was used for the comparison of categorical variables and t-test was employed for the comparison of discrete variables between the patients with HBV infection and those without HBV infection. P < 0.05 was considered statistically significant.
RESULTS
General characteristics of the patients and surgical procedures
One hundred and fifty-five ICC patients (102 men and 53 women with a male/female ratio of 1.92/1) were enrolled in this study. Their mean age was 54.97 ± 10.65 years (range, 27-76 years). Of the 87 patients with chronic HBV infection, 14 were positive for HBsAg in liver tissue, 29 were positive for HBsAg in serum, and 44 were positive for HBsAg both in serum and liver tissue. Of the 155 patients with anatomical en bloc resections, 111 (71.6%) underwent segmentectomy or bisegmentectomy or trisegmentectomy, 30 (19.4%) left hepatectomy, 2 (1.3%) left extended hemihepatectomy, and 14 (9.0%) right hemihepatectomy, 5 (3.2%) concomitant caudate segmentectomy, 6 (3.9%) common bile duct exploration for cholelithiasis or thrombus resection, and 3 (1.9%) Roux-en-Y cholangiojejunostomy. If the tumor invaded its adjacent organs grossly in the operative field, combined resection was performed to achieve complete removal of the tumor. An additional 21 combined resections of other organs were performed in 16 patients (10.3%) as shown in Table 1. Surgical complications occurred in 6 patients, including biliary leakage in 2, subphrenic infection in 1, liver abscess in 2, and bleeding in 1, respectively.
Table 1.
Surgical procedures performed for patients
| Operation | n | Combination resection | n |
| Partial hepatectomy | 111 | Abdominal wall focus resection | 1 |
| Left hemihepatectomy | 30 | Diaphragm wedge resection | 8 |
| Right hemihepatectomy | 14 | Right adrenalectomy | 2 |
| Left extended hemihepatectomy | 2 | Omentumectomy | 7 |
| Caudate segmentectomy | 5 | Gallbladder removal | 3 |
Survival and recurrence
The cumulative 1- and 3-year survival rates were 60.6% and 32.1%, respectively, for the ICC patients (Figure 1). The median survival time was 17.0 mo and 35 patients survived more than 3 years. Univariate analysis demonstrated that absence of HBV infection, hepatolithiasis, r-GT > 64 U/L, ALP > 119 U/L, CA19-9 > 37 U/mL, multiple tumors, tumor size ≥ 5 cm and location, microscopic satellite lesion, lymphatic metastasis, and extrahepatic metastasis were the significant prognostic factors for the poor survival rates of ICC patients. Cox regression analyses revealed that HBV infection (hazard ratio: 4.075), hepatolithiasis (hazard ratio: 4.254), microscopic satellite lesion (hazard ratio: 9.418), and lymphatic metastasis (hazard ratio: 7.078) were the significant factors for overall survival rates of ICC patients. Sex, age, AST, ALT, TBIL, AFP, cirrhosis, liver schistosomiasis, capsule formation, tumor differentiation, vascular invasion, and CK19 were not significantly correlated with the overall survival rate of ICC patients after hepatic resection (Table 2).
Figure 1.
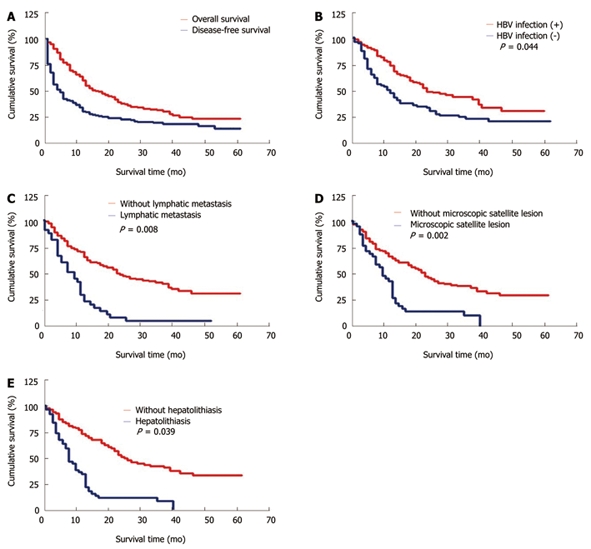
Overall and disease-free survival rates of patients with intrahepatic cholangiocarcinoma after surgical resection (A), higher survival rate of intrahepatic cholangiocarcinoma patients with hepatitis B virus infection than that of those without hepatitis B virus infection (B), significantly poorer survival rate of intrahepatic cholangiocarcinoma patients with lymphatic metastasis than that of those without lymphatic metastasis (C), significantly poorer survival rate of intrahepatic cholangiocarcinoma patients with microscopic satellite lesion than that of those without microscopic satellite lesion (D), and significantly poorer survival rate of intrahepatic cholangiocarcinoma patients with hepatolithiasis than that of those without hepatolithiasis (E).
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival rate of intrahepatic cholangiocarcinoma patients included in this study
| Factor | n |
Survival rate (%) |
P value |
Hazard ratio | 95% CI | ||
| 1-yr | 3-yr | Univariate analysis | Multivariate analyses | ||||
| Age (yr) | |||||||
| > 65 | 28 | 71.4 | 33.7 | ||||
| ≤ 65 | 127 | 58.3 | 31.8 | 0.590 | NA | NA | NA |
| Sex | |||||||
| Female | 53 | 64.2 | 29.4 | ||||
| Male | 102 | 58.8 | 33.7 | 0.973 | NA | NA | NA |
| HBV infection | |||||||
| No | 68 | 45.6 | 20.5 | ||||
| Yes | 87 | 72.4 | 41.8 | 0.003 | 0.044 | 4.075 | 0.418-0.987 |
| Cirrhosis | |||||||
| Yes | 45 | 62.6 | 32.7 | ||||
| No | 110 | 60 | 31.9 | 0.964 | NA | NA | NA |
| Hepatolithiasis | |||||||
| Yes | 12 | 16.7 | < 0.01 | ||||
| No | 143 | 64.3 | 34.4 | 0.001 | 0.039 | 4.254 | 1.040-4.614 |
| Liver schistosomiasis | |||||||
| Yes | 7 | 57.1 | 14.3 | ||||
| No | 148 | 60.8 | 33 | 0.063 | 0.612 | 0.257 | 0.532-2.920 |
| ALT | |||||||
| ≤ 42 U/L | 113 | 60.2 | 32.9 | ||||
| > 42 U/L | 42 | 61.9 | 29.8 | 0.956 | NA | NA | NA |
| AST | |||||||
| ≤ 37 U/L | 102 | 58.8 | 32.7 | ||||
| > 37 U/L | 53 | 64.2 | 30.5 | 0.949 | NA | NA | NA |
| TBIL | |||||||
| ≤ 20 μmol/L | 119 | 60.5 | 30.7 | ||||
| > 20 μmol/L | 36 | 61.1 | 37.5 | 0.355 | NA | NA | NA |
| r-GT | |||||||
| ≤ 64 U/L | 68 | 67.6 | 44.7 | ||||
| > 64 U/L | 87 | 55.2 | 22.4 | 0.003 | 0.102 | 2.667 | 0.918-2.547 |
| ALP | |||||||
| ≤ 119 U/L | 89 | 71.9 | 44.5 | ||||
| > 119 U/L | 66 | 45.5 | 15.8 | < 0.001 | 0.386 | 0.75 | 0.758-2.047 |
| AFP | |||||||
| ≤ 20 μg/L | 125 | 58.4 | 32.8 | ||||
| > 20 μg/L | 30 | 70 | 29.3 | 0.788 | NA | NA | NA |
| CA19-9 | |||||||
| ≤ 37 U/mL | 66 | 66.7 | 41.7 | ||||
| > 37 U/mL | 89 | 56.2 | 24.7 | 0.035 | 0.534 | 0.388 | 0.555-1.356 |
| Tumor number | |||||||
| Single | 137 | 63.5 | 36 | ||||
| Multiple | 18 | 38.9 | 11.1 | 0.007 | 0.188 | 1.734 | 0.811-2.913 |
| Tumor size | |||||||
| < 5 cm | 58 | 74.1 | 42.1 | ||||
| ≥ 5 cm | 97 | 52.6 | 26.1 | 0.014 | 0.563 | 0.335 | 0.737-1.754 |
| Tumor location | |||||||
| Left lobe | 55 | 52.7 | 32.2 | ||||
| Right lobe | 90 | 70 | 35.8 | ||||
| Both lobes | 10 | 20 | < 0.01 | 0.008 | 0.314 | 1.015 | 0.962-1.128 |
| Microscopic satellite lesion | |||||||
| Yes | 39 | 43.6 | 11.5 | ||||
| No | 116 | 66.4 | 38.9 | < 0.001 | 0.002 | 9.418 | 1.287-3.140 |
| Capsule formation | |||||||
| Yes | 17 | 64.7 | 45.8 | ||||
| No | 138 | 60.1 | 30.5 | 0.251 | NA | NA | NA |
| Tumor differentiation | |||||||
| Well to moderately | 118 | 61.9 | 33.3 | ||||
| Poorly | 37 | 56.8 | 29.5 | 0.785 | NA | NA | NA |
| Vascular invasion | |||||||
| Yes | 35 | 51.4 | 22.2 | ||||
| No | 120 | 63.3 | 36.3 | 0.211 | NA | NA | NA |
| Lymphatic metastasis | |||||||
| Yes | 32 | 28.1 | 3.1 | ||||
| No | 123 | 69.1 | 40.8 | < 0.001 | 0.008 | 7.078 | 1.193-3.203 |
| Extrahepatic metastasis | |||||||
| Yes | 10 | 20 | < 0.01 | ||||
| No | 145 | 63.4 | 33.7 | 0.001 | 0.225 | 1.474 | 0.743-3.541 |
| CK19 (n = 153)1 | |||||||
| Yes | 139 | 59.7 | 30.5 | ||||
| No | 14 | 71.4 | 45.9 | 0.178 | NA | NA | NA |
Number of tumors. HBV: Hepatitis B virus; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AFP: α-fetoprotein; ALP: Alkaline phosphatase; r-GT: R-glutamyltransferase; CA 19-9: Carbohydrate antigen 19-9; CK19: Cytokeratin 19; NA: Not available.
Tumor recurrence occurred in 108 patients. The disease-free survival rate was 31.1% and 20.3%, respectively, for the ICC patients 1 and 3 years after operation with a median disease-free survival time of 5.0 mo (Figure 1). Univariate analysis showed that hepatolithiasis, r-GT > 64 U/L, ALP > 119 U/L, CA19-9 > 37 U/mL, multiple tumors, microscopic satellite lesion, vascular invasion, lymph node metastasis, and positive CK19 were the significant risk factors for tumor recurrence in ICC patients. Multivariate analysis demonstrated that the number of tumors (95.0% CI = 1.194-3.863), microscopic satellite lesion (95.0% CI = 1.106-2.745), and vascular invasion (95.0% CI = 1.072-2.812) were the independent prognostic factors for disease-free survival rate of ICC patients (Table 3). The most common tumor recurrence sites were the remnant liver and regional lymph nodes. The treatment modalities for recurrent tumors included repeated operation (n = 9), transplantation (n = 2), radiation therapy (n = 7), radiofrequency ablation (n = 9), microwave coagulation (n = 3), percutaneous ethanol injection therapy (n = 23), and transarterial chemoembolization (n = 93).
Table 3.
Univariate and multivariate analyses of prognostic factors for disease-free survival rate of intrahepatic cholangiocarcinoma patients included in this study
| Factor | n |
Survival rate (%) |
P value |
Hazard ratio | 95% CI | ||
| 1-yr | 3-yr | Univariate analysis | Multivariate analysis | ||||
| Age (yr) | |||||||
| > 65 | 20 | 45 | 25 | ||||
| ≤ 65 | 112 | 28.6 | 19.4 | 0.243 | NA | NA | NA |
| Sex | |||||||
| Female | 43 | 34.9 | 20.7 | ||||
| Male | 89 | 29.2 | 20.1 | 0.679 | NA | NA | NA |
| HBV infection | |||||||
| Yes | 71 | 39.4 | 23.5 | ||||
| No | 61 | 21.3 | 16.4 | 0.087 | 0.351 | 0.869 | 0.524-1.258 |
| Cirrhosis | |||||||
| Yes | 39 | 25.6 | 25.6 | ||||
| No | 93 | 33.3 | 18 | 0.606 | NA | NA | NA |
| Hepatolithiasis | |||||||
| Yes | 7 | 14.3 | < 0.01 | ||||
| No | 135 | 32 | 21.4 | 0.041 | 0.130 | 2.291 | 0.807-5.299 |
| Liver schistosomiasis | |||||||
| Yes | 6 | < 0.01 | < 0.01 | ||||
| No | 126 | 32.5 | 21.2 | 0.307 | NA | NA | NA |
| r-GT | |||||||
| ≤ 64 U/L | 59 | 44.1 | 28.3 | ||||
| > 64 U/L | 73 | 20.5 | 13.7 | 0.005 | 0.289 | 1.124 | 0.797-2.140 |
| ALP | |||||||
| ≤ 119 U/L | 79 | 41.8 | 27.7 | ||||
| > 119 U/L | 53 | 15.1 | 9.4 | 0.001 | 0.589 | 0.293 | 0.713-1.813 |
| AFP | |||||||
| ≤ 20 μg/L | 104 | 34.6 | 21.8 | ||||
| > 20 μg/L | 28 | 17.9 | 14.3 | 0.212 | NA | NA | NA |
| CA19-9 | |||||||
| ≤ 37 U/mL | 55 | 40 | 27.3 | ||||
| > 37 U/mL | 77 | 24.7 | 15.3 | 0.048 | 0.720 | 0.129 | 0.707-1.652 |
| Tumor number | |||||||
| single | 115 | 35.7 | 23.2 | ||||
| multiple | 17 | < 0.01 | < 0.01 | < 0.001 | 0.011 | 6.515 | 1.194-3.863 |
| Tumor size | |||||||
| < 5 cm | 48 | 39.6 | 24.4 | ||||
| ≥ 5 cm | 84 | 26.2 | 17.7 | 0.135 | NA | NA | NA |
| Tumor location | |||||||
| Left lobe | 43 | 37.2 | 20.9 | ||||
| Right lobe | 81 | 30.9 | 21.9 | ||||
| Both lobes | 8 | < 0.01 | < 0.01 | 0.105 | NA | NA | NA |
| Microscopic satellite lesion | |||||||
| Yes | 34 | 11.8 | 8.8 | ||||
| No | 98 | 37.8 | 24.2 | 0.002 | 0.017 | 5.736 | 1.106-2.745 |
| Histological inflammation | |||||||
| Yes | 34 | 47.3 | 20.6 | ||||
| No | 98 | 25.5 | 20.3 | 0.332 | NA | NA | NA |
| Capsule formation | |||||||
| Yes | 14 | 42.9 | 21.4 | ||||
| No | 118 | 29.7 | 20.1 | 0.683 | NA | NA | NA |
| Tumor differentiation | |||||||
| Well to moderately | 98 | 33.7 | 21.1 | ||||
| Poorly | 34 | 23.5 | 17.6 | 0.647 | NA | NA | NA |
| Vascular invasion | |||||||
| Yes | 31 | 16.1 | 9.7 | ||||
| No | 101 | 35.6 | 23.5 | 0.005 | 0.025 | 5.030 | 1.072-2.812 |
| Lymphatic metastasis | |||||||
| Yes | 109 | 4.3 | < 0.01 | ||||
| No | 23 | 36.7 | 24.5 | < 0.001 | 0.182 | 1.781 | 0.834-2.597 |
| Extrahepatic metastasis | |||||||
| Yes | 8 | < 0.01 | < 0.01 | ||||
| No | 124 | 33.1 | 21.6 | 0.059 | 0.609 | 0.261 | 0.551-2.764 |
| CK19 staining (n = 130)1 | |||||||
| Positive | 13 | 27.4 | 17.9 | ||||
| Negative | 117 | 61.5 | 34.6 | 0.038 | 0.522 | 0.410 | 0.597-2.762 |
Number of available data. AFP: α-fetoprotein; ALP: Alkaline phosphatase; r-GT: R-glutamyltransferase; CA 19-9: Carbohydrate antigen 19-9; CK19: Cytokeratin19; NA: Not available.
Prognostic factors for ICC patients according to their HBV infection
The clinicopathological characteristics of ICC patients with HBV infection and those without HBV infection were compared to further interpret the influence of chronic HBV infection on their survival rate. Univariate analysis showed that the following variables were significantly different between the patients with HBV infection and those without HBV infection, including gender, AST, AFP, CA19-9, inflammation of liver tissue, cirrhosis, hepatolithiasis, tumor capsule formation, tumor differentiation, lymphatic metastasis, and positive immunohistochemical staining of CK19. Although perineural infiltration was not significantly different between them, it occurred more frequently in ICC patients without HBV infection than in those with HBV infection (Table 4).
Table 4.
Clinicopathological features of intrahepatic cholangiocarcinoma patients according to their hepatitis B virus infection
|
HBV infection |
P value | ||
| Yes (n = 87) | No (n = 68) | ||
| Gender (M/F) | 64/23 | 38/30 | 0.021 |
| Age (> 65 yr) (%) | 17 (19.54) | 13 (14.94) | 0.763 |
| Hepatolithiasis (%) | 2 (2.30) | 10 (14.71) | 0.004 |
| Hepatic schistosomiasis (%) | 2 (2.30) | 5 (7.35) | 0.133 |
| ALT (> 42 U/L) (%) | 27 (34.03) | 15 (22.06) | 0.212 |
| AST (> 37 U/L) (%) | 38 (43.68) | 15(22.06) | 0.005 |
| TBIL (> 20 μmol/L) (%) | 20 (22.99) | 16 (23.53) | 0.937 |
| r-GT (> 64 U/L) (%) | 49 (56.32) | 38 (55.88) | 0.956 |
| ALP(> 119 U/L) (%) | 33 (37.93) | 33 (48.53) | 0.185 |
| AFP (> 20 μg/L) (%) | 25 (28.74) | 5 (7.35) | 0.001 |
| CA19-9 (> 37 U/mL) (%) | 44 (50.57) | 45 (66.18) | 0.051 |
| CA19-9 (> 200 U/mL) (%) | 20 (22.99) | 32 (47.06) | 0.002 |
| Tumor location (%) | 0.520 | ||
| Left lobe | 28 (32.18) | 27 (39.71) | |
| Right lobe | 54 (62.07) | 36 (52.94) | |
| Both lobes | 5 (5.75) | 5 (7.35) | |
| Tumor size | |||
| < 5 cm | 38 (43.68) | 20 (29.41) | 0.069 |
| ≥ 5 cm | 49 (56.32) | 48 (70.59) | |
| Tumor number (%) | 0.958 | ||
| Single | 77 (88.51) | 60 (88.24) | |
| Multiple | 10 (11.49) | 8 (11.76) | |
| Histological inflammation (%) | 35 (40.23) | 4 (11.76) | < 0.001 |
| Cirrhosis (%) | 40 (45.98) | 5 (7.35) | < 0.001 |
| Capsule formation (%) | 15 (17.24) | 2 (2.41) | 0.005 |
| Tumor differentiation (%) | 0.036 | ||
| Well | 0 (< 0.01) | 5 (7.35) | |
| Moderately | 66 (75.86) | 47 (69.12) | |
| Poorly | 21 (24.14) | 16 (23.53) | |
| Vascular invasion (%) | 22 (25.29) | 13 (19.12) | 0.362 |
| Perineural infiltration (%) | 0 (< 0.01) | 3 (4.41) | 0.082 |
| Microscopic satellite lesion (%) | 23 (26.44) | 16 (23.53) | 0.679 |
| Lymphatic metastasis (%) | 12 (13.79) | 20 (29.41) | 0.017 |
| Extrahepatic metastasis (%) | 5 (5.75) | 5 (7.35) | 0.749 |
| Immunohistochemical examinations | |||
| CK18 positive staining (%) (n = 135)1 | 78 (89.66) | 57 (86.36) | 0.531 |
| CK19 positive staining (%) (n = 139)1 | 75 (86.21) | 64 (96.97) | 0.022 |
Number of available data. HBV: Hepatitis B virus; M: Male; F: Female; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AFP: α-fetoprotein; ALP: Alkaline phosphatase; r-GT: R-glutamyltransferase; CA19-9: Carbohydrate antigen 19-9; CK: Cytokeratin.
The potential prognostic factors affecting the survival rate of the patients with HBV infection and those without HBV infection were compared to further clarify the difference in prognostic factors affecting their survival rate. The prognostic factors for the patients with HBV infection and those without HBV infection were not completely consistent (Tables 5 and Table 6). Univariate analysis demonstrated that ALP > 119 U/L, microscopic satellite lesion, tumor size ≥ 5 cm, lymphatic metastasis, and vascular invasion were the significant poor prognostic factors for the survival rate of patients with HBV infection (Table 5), while r-GT > 64 U/L, CA19-9 > 37 U/mL, hepatolithiasis, microscopic satellite lesion, lymphatic and extrahepatic metastasis were the significant poor prognostic factors for the survival rate of those without HBV infection (Table 6). Cox regression analysis demonstrated that ALP > 119 U/L(hazard ratio: 4.800), microscopic satellite lesion (hazard ratio: 12.066), lymphatic metastasis (hazard ratio: 7.887), and vascular invasion (hazard ratio: 4.167) were the independent poor prognostic factors for patients with HBV infection (Table 5, Figure 2), while r-GT > 64 U/L (hazard ratio: 4.157), microscopic satellite lesion (hazard ratio: 5.965), and poor tumor differentiation (hazard ratio: 5.844) were the independent poor prognostic factors for those with out HBV infection (Table 6, Figure 3).
Table 5.
Univariate and multivariate analyses of prognostic factors for survival rate of intrahepatic cholangiocarcinoma patients with hepatitis B virus infection
| Factor | n |
HBV infection |
P value |
Hazard ratio | 95% CI | ||
| 1-yr (%) | 3-yr (%) | Univariate analysis | Multivariate analyses | ||||
| r-GT | |||||||
| ≤ 64 U/L | 38 | 76.3 | 52.1 | ||||
| > 64 U/L | 49 | 69.4 | 34.1 | 0.223 | NA | NA | NA |
| ALP | |||||||
| ≤ 119 U/L | 54 | 85.2 | 53.2 | ||||
| > 119 U/L | 33 | 60.6 | 24.2 | 0.001 | 0.028 | 4.800 | 1.075-3.662 |
| CA19-9 | |||||||
| ≤ 37 U/mL | 43 | 69.8 | 41.0 | ||||
| > 37 U/mL | 44 | 75.0 | 42.5 | 0.575 | NA | NA | NA |
| Tumor number | |||||||
| single | 77 | 75.3 | 44.9 | ||||
| multiple | 10 | 50.0 | 20.0 | 0.066 | 0.058 | 3.599 | 0.972-5.587 |
| Tumor size | |||||||
| < 5 cm | 38 | 86.8 | 52.0 | ||||
| ≥ 5 cm | 49 | 61.2 | 33.7 | 0.049 | 0.655 | 0.199 | 0.436-1.686 |
| Tumor location | |||||||
| Left lobe | 28 | 64.3 | 42.9 | ||||
| Right lobe | 54 | 79.6 | 45.5 | ||||
| Both lobes | 5 | 40.0 | < 0.01 | 0.065 | 0.933 | 0.007 | 0.899-1.122 |
| Microscopic satellite lesion | |||||||
| Yes | 23 | 52.2 | 21.7 | ||||
| No | 64 | 79.7 | 48.8 | 0.001 | 0.001 | 12.066 | 1.648-6.014 |
| Capsule formation | |||||||
| Yes | 15 | 60.0 | 45.7 | ||||
| No | 72 | 75.0 | 41.0 | 0.979 | NA | NA | NA |
| Tumor differentiation | |||||||
| Well to moderately | 66 | 71.2 | 40.3 | ||||
| Poorly | 21 | 76.2 | 47.1 | 0.354 | NA | NA | NA |
| Vascular invasion | |||||||
| Yes | 22 | 50.0 | 17.0 | ||||
| No | 65 | 80.0 | 50.1 | 0.007 | 0.041 | 4.167 | 1.030-4.388 |
| Lymphatic metastasis | |||||||
| Yes | 12 | 33.3 | < 0.01 | ||||
| No | 75 | 78.7 | 48.5 | < 0.001 | 0.005 | 7.887 | 1.408-6.848 |
| Extrahepatic metastasis | |||||||
| Yes | 5 | 40.0 | < 0.01 | ||||
| No | 82 | 74.4 | 43.1 | 0.099 | 0.857 | 0.032 | 0.251-3.154 |
NA: Not applicable; ALP: Alkaline phosphatase; r-GT: R-glutamyltransferase; CA 19-9: Carbohydrate antigen 19-9.
Table 6.
Univariate and multivariate analyses of prognostic factors for survival of intrahepatic cholangiocarcinoma patients without hepatitis B virus infection.
| Factor | n |
No HBV infection |
P value |
Hazard ratio | 95% CI | ||
| 1-yr (%) | 3-yr (%) | Univariate analysis | Multivariate analyses | ||||
| Hepatolithiasis | |||||||
| Yes | 10 | 10.0 | < 0.01 | ||||
| No | 58 | 51.7 | 24.1 | 0.013 | 0.084 | 2.979 | 0.900-5.223 |
| r-GT | |||||||
| ≤ 64 U/L | 30 | 56.7 | 36.4 | ||||
| > 64 U/L | 38 | 36.8 | 7.9 | 0.001 | 0.041 | 4.157 | 1.035-5.614 |
| ALP | |||||||
| ≤ 119 U/L | 35 | 51.4 | 31.4 | ||||
| > 119 U/L | 33 | 39.4 | 9.1 | 0.017 | 0.407 | 0.688 | 0.316-1.595 |
| CA19-9 | |||||||
| ≤ 37 U/mL | 23 | 60.9 | 43.5 | ||||
| > 37 U/mL | 45 | 37.8 | 8.9 | 0.001 | 0.173 | 1.858 | 0.806-3.311 |
| Tumor number | |||||||
| Single | 60 | 48.3 | 23.2 | ||||
| Multiple | 8 | 25.0 | < 0.01 | 0.050 | 0.392 | 0.734 | 0.609-3.540 |
| Tumor size | |||||||
| < 5 cm | 20 | 50.0 | 24.0 | ||||
| ≥ 5 cm | 48 | 43.8 | 18.8 | 0.308 | NA | NA | NA |
| Tumor location | |||||||
| Left lobe | 27 | 40.7 | 21.6 | ||||
| Right lobe | 36 | 55.6 | 22.2 | ||||
| Both lobes | 5 | < 0.01 | < 0.01 | 0.137 | NA | NA | NA |
| Microscopic satellite lesion | |||||||
| Yes | 16 | 31.2 | < 0.01 | ||||
| No | 52 | 50.0 | 26.9 | 0.003 | 0.015 | 5.956 | 1.183-4.657 |
| Tumor differentiation | |||||||
| Well to moderately | 52 | 50.0 | 24.9 | ||||
| Poorly | 16 | 31.2 | 6.2 | 0.076 | 0.016 | 5.844 | 1.172-4.569 |
| Vascular invasion | |||||||
| Yes | 13 | 53.8 | 30.8 | ||||
| No | 55 | 43.6 | 18.0 | 0.459 | NA | NA | NA |
| Lymphatic metastasis | |||||||
| Yes | 20 | 25.0 | 5.0 | ||||
| No | 48 | 54.2 | 26.9 | 0.015 | 0.430 | 0.624 | 0.683-2.452 |
| Extrahepatic metastasis | |||||||
| Yes | 5 | < 0.01 | < 0.01 | ||||
| No | 63 | 49.2 | 22.1 | < 0.001 | 0.065 | 3.403 | 0.933-9.940 |
NA: Not applicable; ALP: Alkaline phosphatase; r-GT: R-glutamyltransferase; CA 19-9: Carbohydrate antigen 19-9.
Figure 2.

Adjusted survival curves according to the independent prognostic factors by multivariate analysis (Cox model) for intrahepatic cholangiocarcinoma with hepatitis B virus infection after resection. A: Alkaline phosphatase (ALP); B: Microscopic satellite lesion; C: Vascular invasion; D: Lymphatic metastasis.
Figure 3.
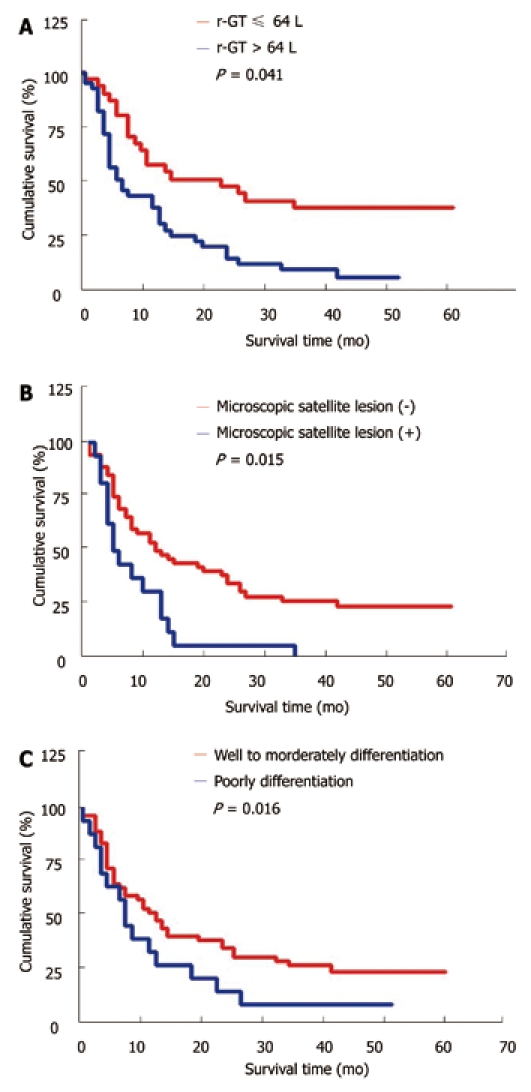
Adjusted survival curves according to the independent prognostic factors by multivariate analysis (Cox model) for intrahepatic cholangiocarcinoma without hepatitis B virus infection after resection. A: R-glutamyltransferase (r-GT); B: Microscopic satellite lesion; C: Tumor differentiation.
DISCUSSION
Although a number studies are available on the correlation between chronic HBV infection and ICC[5-11], the impact of HBV infection on the survival rate of ICC patients remains unclear. In the present study, HBV infection was found to be a favorable prognostic factor for the patients with ICC after resection, thus ICC patients with HBV infection should be distinguished from those without HBV infection. First, ICC patients with HBV infection and those without HBV infection are different in their clinicopathological characteristics. It was reported that the number of male ICC patients with HBV infection is more, with a younger age, a higher abnormal liver function and a higher serum AFP level, a worse histological inflammation and cirrhosis, a poorer tumor differentiation and encapsulation, a lower serum CA19-9 level, and a lower frequency of lymphatic metastasis and positive CK19 than those of ICC patients without HBV infection[11]. Second, ICC patients with HBV infection have a more favorable outcome after surgical resection than those without HBV infection. Third, the prognostic factors for the survival rate of ICC patients with and those without HBV infection are different.
Surgical resection remains the curable procedure for ICC. However, the prognosis of ICC after surgery is poor because of its high recurrence rate. The median survival time of ICC patients after operation is 11.0-37.4 mo[12-18]. The overall 1- and 3-year survival rates of ICC patients after operation are 46.3%-73.3% and 23.0%-55.0%, respectively[14,15,19-23], which are consistent with the findings in our study. It was reported that the preoperative CA19-9 level, vascular invasion, perineural invasion, lymph node metastasis, intrahepatic metastasis, the number and differentiation of tumors are the significant prognostic factors for the overall survival rate of ICC patients[13,15-19]. In the present study, several clinicopathological factors that significantly influence the survival rate of ICC patients and tumor recurrence were investigated, and univariate analysis showed that absence of HBV infection, hepatolithiasis, high CA19-9 or ALP or r-GT level before operation, multiple tumors, tumor location, microscopic satellite lesion, lymphatic and extrahepatic metastasis, tumor size greater than 5 cm in diameter, were the significantly poor prognostic factors for the survival rate of ICC patients, Multivariate analysis revealed that the presence of HBV infection, hepatolithiasis, lymph node metastasis, microscopic satellite lesion were independent prognostic factor on survival. However, tumor differentiation, vascular invasion, tumor capsule formation were not found to be the significant prognostic factors for the survival rate of ICC patients.
In Asia, intrahepatic duct stone (IHDS) is one of the factors highly related with ICC[24]. Since Sanes and MacCallum[25] reported two cases of hepatolithiasis-related cholangiocarcinoma discovered incidentally at autopsy for the first time in 1942, the correlation between IHDS with ICC has been reported in case series from all over the world[4,5]. Our previous study also demonstrated that the incidence of IHDS is significantly higher in ICC patients than in non cancer patients (7.8% vs 1.1%), and IHDS is an independent risk factor for the development of ICC in Chinese (OR = 11.020, 95%,CI = 4.238-28.657)[11], which are consistent with the findings in the current study. IHDS was also found to be a negative prognostic factor affecting the survival rate of ICC patients in this study. Our explanation is that it is more difficult to diagnose early ICC with IHDS than to diagnose ICC without IHDS, and the recurrence of early ICC with IHDS is higher than that of ICC without IHDS.
It was reported that a high preoperative CA19-9 level (> 37 U/mL) greatly influences the overall survival rate of ICC patients after hepatic resection[26]. It has been demonstrated that the preoperative CA19-9 level is an indication of ICC in patients without primary sclerosing cholangitis, and the serum CA19-9 level is related to tumor burden[27]. In our study, the median survival time of ICC patients with their preoperative CA19-9 level ≤ 37 U/mL was significantly longer than that of those with their preoperative CA19-9 level > 37 U/mL (23 mo vs 13 mo).
It has been shown that lymph node metastasis is a significant factor for the poor prognosis of ICC patients[12,14,15,17,21]. The presence of lymph node metastasis is correlated with other poor prognosis factors, such as gross type of tumor, poorly or undifferentiated tumor, vascular invasion, and perineural invasion. In the current study, multivariate analysis showed that lymph node metastasis was correlated with both the overall and disease-free survival rates of ICC patients. The median survival time of ICC patients without lymph node metastasis was significantly longer than that of those with lymph metastasis (23 mo vs 8 mo).
CK19 belongs to type 1 cytokeratin with a molecular weight of 40-56 kDa[28] and is normally expressed in ductal epithelium (bile ducts, pancreas, and renal collecting tubules) and in mucosa of the gastrointestinal (GI) tract[29]. Most adenocarcinomas of the GI tract including cholangiocarcinoma are CK19 positive[30]. It was reported that CK19, as a prognostic marker, plays a role in the pathogenesis of papillary thyroid carcinoma[31], hepatocellular carcinoma[32-33] and colorectal adenocarcinoma[34]. For example, CK19 expressing HCCs had a higher rate of recurrence (hazard ratio 12.5) after transplantation[35]. It has been shown that the expression level of CK19 in HCC patients increases with a worse prognosis of HCC patients and a faster recurrence of it after surgical treatment[36-39], indicating that CK19 is a useful prognostic marker for HCC. However, the role of CK19 as a prognostic marker in ICC has not been explored. In the current study, CK19 was expressed more frequently in ICC patients in the absence of HBV infection and the tumor disease-free survival rate of patients with CK19 expressing ICC was also lower after curative resection.
Recent studies showed that HBV infection is an important risk factor for the development of ICC[5-11]. In the current study, 73 ICC patients (47.1%) were positive for serum HBsAg. Interestingly, 14 out of the 155 ICC patients were positive for HBsAg only in liver tissue, indicating that occult HBV infection is also a risk factor for the development of ICC as for HCC. In the current study, HBV infection was significantly correlated with some important clinicopathological factors, such as hepatolithiasis, high preoperative CA19-9 level, capsule formation, lymph node metastasis, perineural invasion, and positive CK19 (Table 4), which is consistent with the findings in our previous study[11], indicating that the absence of HBV infection may be a predictor for the invasiveness of ICC and the poor survival rate of ICC patients. In the current study, the outcome of ICC patients with HBV infection was better than that of those without HBV infection after curative resection. The median survival time of patients without HBV infection was significantly shorter than that of those with HBV infection (11 mo vs 23 mo), the prognostic factors affecting the survival rates of ICC patients with HBV infection and those without HBV infection were not completely consistent. Univariate analysis demonstrated that high preoperative ALP level, microscopic satellite lesion, tumor size greater than 5cm in diameter, lymph node metastasis, and vascular invasion were the poor prognostic factors for the survival rate of ICC patients with HBV infection (Table 5), while high preoperative r-GT or CA19-9 level, hepatolithiasis, microscopic satellite lesion, lymph node and extrahepatic metastasis were the poor prognostic factors for the survival rate of those without HBV infection (Table 6). Cox regression analysis demonstrated that high preoperative ALP level (hazard ratio: 4.800), microscopic satellite lesion (hazard ratio: 12.066), lymphatic metastasis (hazard ratio: 7.887), and vascular invasion (hazard ratio: 4.167) were the independent prognostic factors for ICC patients with HBV infection (Table 5), while high preoperative r-GT level (hazard ratio: 4.157), microscopic satellite lesion (hazard ratio: 5.965), and poor tumor differentiation (hazard ratio: 5.844) were the independent prognostic factors for those without HBV infection (Table 6), indicating that ICC patients with HBV infection should be distinguished from those without HBV infection.
It has been shown that vascular invasion or poor differentiation of tumor is a negative prognosis factor for ICC patients[40], in this study, however, vascular invasion or differentiation of tumor was not a significant predictor for the overall survival rate of ICC patients. We hypothesize that compared ICC without HBV infection, ICC with HBV infection are associated with more vascular invasion and poor differentiation. While HBV infection is a favorable prognostic factor for survival, this may decrease the influence of vascular invasion or tumor differentiation on overall survival. Vascular invasion and poor differentiation were independent negative prognostic factors in ICC with HBV infection and in ICC without HBV infection, respectively (Tables 5 and 6). The result may indirectly provide support for our hypothesis.
In conclusion, absence of HBV infection, hepatolithiasis, microscopic satellite lesion, and lymphatic metastasis are the independent predictors for a dismal prognosis of ICC patients. HBV infection is a valuable clinical factor for the invasiveness of tumor and the clinical outcome of ICC patients. ICC patients with HBV infection should be distinguished from those without HBV infection.
COMMENTS
Background
Although the correlation between chronic Hepatitis B virus (HBV) infection and intrahepatic cholangiocarcinoma (ICC) has been documented, the impact of HBV infection on the survival rate of ICC patients remains unclear.
Research frontiers
One hundred and fifty-five ICC patients who underwent macroscopic curative resections (R0 and R1) were classified according to their chronic HBV infection represented by positive hepatitis B surface antigen (HBsAg) in serum or in liver tissue. The clinicopathological characteristics and survival rate of these patients were evaluated.
Innovations and breakthroughs
Multivariate analyses revealed that HBV infection, hepatolithiasis, microscopic satellite lesion, and lymphatic metastasis were the independent prognostic factors for the survival rate of ICC patients. The prognostic factors affecting the survival rate of ICC patients with HBV infection and those without HBV infection were not completely consistent. Alkaline phosphatase (ALP) > 119 U/L, microscopic satellite lesion, vascular invasion, and lymphatic metastasis were the poorer prognoses of ICC patients with HBV infection, and r-glutamyltransferase (r-GT) > 64 U/L, microscopic satellite lesion, and poor tumor differentiation were the poorer prognoses of ICC patients without HBV infection.
Applications
HBV infection in ICC patients is a valuable clinical factor for predicting the invasiveness of tumor and the clinical outcome of ICC patients. ICC patients with HBV infection should be distinguished from those without HBV infection because they have different clinicopathological characteristics, prognostic factors and favorable outcomes after surgical resection.
Terminology
ICC is a fatal cancer of the biliary epithelium, arising from the intrahepatic bile ducts. Globally, ICC is the most common primary hepatic malignancy, after hepatocellular carcinoma (HCC). The incidence of ICC varies greatly in different areas of the world, and is related to the distribution of risk factors. HBV or hepatitis C virus, primary sclerosingcholangitis, liver fluke infestation particularly the endemic Opisthorcis viverrini, and hepatolithiasis are the known risk factors for ICC.
Peer review
This study showed that HBV infection, hepatolithiasis, microscopic satellite lesion, and lymphatic metastasis were the independent prognostic factors for the survival rate of ICC patients. HBV infection was a valuable clinical factor for the invasiveness of tumor and the clinical outcome of ICC patients and ICC patients with HBV infection should be distinguished from those without HBV infection, thus providing certain accurate data for the diagnosis of ICC.
Footnotes
Peer reviewers: Dr. Jonathan Koea, Hepatobiliary/Upper Gastrointestinal Unit, Department of Surgery, Auckland, New Zealand
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
References
- 1.De Groen PC. Cholangiocarcinoma in primary sclerosing cholangitis: who is at risk and how do we screen? Hepatology. 2000;31:247–248. doi: 10.1002/hep.510310137. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 3.Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962–970. doi: 10.1046/j.1365-2168.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2007;19:615–617. doi: 10.1097/MEG.0b013e328224b935. [DOI] [PubMed] [Google Scholar]
- 5.Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100:1765–1770. doi: 10.1038/sj.bjc.6605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632–635. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Wang H, Zhou D, Wang H, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–1531. doi: 10.1046/j.1365-2168.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 13.Miwa S, Miyagawa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, Kusama K, Soeda J, Ogawa S. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41:893–900. doi: 10.1007/s00535-006-1877-z. [DOI] [PubMed] [Google Scholar]
- 14.Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: univariate and multivariate analysis. Hepatogastroenterology. 2002;49:311–316. [PubMed] [Google Scholar]
- 15.Uenishi T, Yamazaki O, Yamamoto T, Hirohashi K, Tanaka H, Tanaka S, Hai S, Kubo S. Serosal invasion in TNM staging of mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2005;12:479–483. doi: 10.1007/s00534-005-1026-8. [DOI] [PubMed] [Google Scholar]
- 16.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 17.Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004;91:99–104. doi: 10.1002/bjs.4366. [DOI] [PubMed] [Google Scholar]
- 18.Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, Sui CJ, Yang JM. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–733. doi: 10.1007/s00268-005-7761-9. [DOI] [PubMed] [Google Scholar]
- 20.Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol. 2007;96:160–165. doi: 10.1002/jso.20792. [DOI] [PubMed] [Google Scholar]
- 21.Nakagohri T, Asano T, Kinoshita H, Kenmochi T, Urashima T, Miura F, Ochiai T. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–293. doi: 10.1007/s00268-002-6696-7. [DOI] [PubMed] [Google Scholar]
- 22.Yeh CN, Jan YY, Yeh TS, Hwang TL, Chen MF. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004;11:606–611. doi: 10.1245/ASO.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Jan YY, Yeh CN, Yeh TS, Hwang TL, Chen MF. Clinicopathological factors predicting long-term overall survival after hepatectomy for peripheral cholangiocarcinoma. World J Surg. 2005;29:894–898. doi: 10.1007/s00268-005-7763-7. [DOI] [PubMed] [Google Scholar]
- 24.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049–1055. doi: 10.1046/j.1440-1746.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanes S, Maccallum JD. Primary Carcinoma of the Liver: Cholangioma in Hepatolithiasis. Am J Pathol. 1942;18:675–687. [PMC free article] [PubMed] [Google Scholar]
- 26.Jan YY, Yeh CN, Yeh TS, Chen TC. Prognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:1779–1784. doi: 10.3748/wjg.v11.i12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 28.Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004;37:529–540. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding SJ, Li Y, Tan YX, Jiang MR, Tian B, Liu YK, Shao XX, Ye SL, Wu JR, Zeng R, et al. Freanalysis to clinical significance: overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis. Mol Cell Proteomics. 2004;3:73–81. doi: 10.1074/mcp.M300094-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001;14:338–342. doi: 10.1038/modpathol.3880312. [DOI] [PubMed] [Google Scholar]
- 32.Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, Tanaka S, Kaneda K, Hirohashi K. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhatavdekar JM, Patel DD, Chikhlikar PR, Shah NG, Vora HH, Ghosh N, Trivedi TI. Molecular markers are predictors of recurrence and survival in patients with Dukes B and Dukes C colorectal adenocarcinoma. Dis Colon Rectum. 2001;44:523–533. doi: 10.1007/BF02234324. [DOI] [PubMed] [Google Scholar]
- 35.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, et al. Risk factors contributing to early and late phase in trahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 36.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43:979–92. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Nagao T, Inoue S, Yoshimi F, Sodeyama M, Omori Y, Mizuta T, Kawano N, Morioka Y. Postoperative recurrence of hepatocellular carcinoma. Ann Surg. 1990;211:28–33. doi: 10.1097/00000658-199001000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, Tanaka S, Kaneda K, Hirohashi K. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, Chung JB. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]


