Abstract
AIM: To investigate the influence of remote ischemic preconditioning (RIPC) on anastomotic integrity.
METHODS: Sixty male Wistar rats were randomized to six groups. The control group (n = 10) had an end-to-end ileal anastomosis without RIPC. The preconditioned groups (n = 34) varied in time of ischemia and time of reperfusion. One group received the amino acid L-arginine before constructing the anastomosis (n = 9). On postoperative day 4, the rats were re-laparotomized, and bursting pressure, hydroxyproline concentration, intra-abdominal adhesions, and a histological score concerning the mucosal ischemic injury were collected. The data are given as median (range).
RESULTS: On postoperative day 4, median bursting pressure was 124 mmHg (60-146 mmHg) in the control group. The experimental groups did not show a statistically significant difference (P > 0.05). Regarding the hydroxyproline concentration, we did not find any significant variation in the experimental groups. We detected significantly less mucosal injury in the RIPC groups. Furthermore, we assessed more extensive intra-abdominal adhesions in the preconditioned groups than in the control group.
CONCLUSION: RIPC directly before performing small bowel anastomosis does not affect anastomotic stability in the early period, as seen in ischemic preconditioning.
Keywords: Anastomotic healing, Hydroxyproline, Bursting pressure, Mucosal injury index, Wound healing, Remote ischemic preconditioning
INTRODUCTION
Wound healing in intestinal anastomoses is dependent on operative technique, the underlying medical condition, medical treatment, and individual, often unknown factors[1,2]. Primarily, the stability of an intestinal anastomosis is regulated by the suture holding capacity, due to a high activity of collagenases that peaks in the first 48-72 h. Over time, the suture holding capacity decreases, whereas the tissue strength increases because of upregulated collagen synthesis and remodeling[3]. Despite optimal treatment, anastomotic insufficiency occurs, which causes significantly higher morbidity and mortality[4-6]. The underlying medical condition and individual genetic factors are different in each case. Accordingly, investigational interest is focused on fundamentals in gastrointestinal wound healing[1,2] and on innovative perioperative management to improve anastomotic healing, especially in patients at risk.
Ischemic preconditioning (IPC) in general was first described by Murry in 1986, when a delay in cell death in the myocardium after preconditioning was described[7]. Concerning IPC in the intestine, Hotter et al[8] have found an increased NO level, which was the first report of such a feature. Several local and systemic effects of IPC have been described, including decreased bacterial translocation[9], mucosal injury[9], epithelial apoptosis[10], and on the other hand, an improvement in microvascular perfusion and oxygenation[11,12] after ischemia-reperfusion injury (IRI). Our group previously has shown that IPC of the superior mesenteric artery improves stability of intestinal anastomoses[13].
Contrary to IPC, remote ischemic preconditioning (RIPC) is induced by temporary occlusion of different arteries, which are not responsible for direct blood supply of the organ of interest. Less is known about the influence of RIPC on the gastrointestinal tract, and especially on its impact on anastomotic healing in the small intestine. RIPC was first reported in the literature in 1993 when Przyklenk et al[14] discussed the effect of temporary coronary occlusion on virgin myocardium not affected by the artificial ischemia. Subsequent studies have all suggested an increased tolerance to IRI in different tissue types[15-18]. To the best of our knowledge, Colak et al[19] are the only group that has investigated the effect of RIPC on intestinal anastomosis. They have performed an anastomosis in the descending colon after branching off the superior mesenteric artery in an IR setting. No beneficial effect was found concerning IRI-induced delay of anastomotic healing. Neither in IPC nor RIPC are the optimal IR intervals defined.
The aim of the present study was to investigate the effect of RIPC, directly before anastomotic construction, in the early period (postoperative day 4) when suture holding capacity has already decreased and tissue strength has gained more importance. We intentionally did not perform an IRI interval because we claim that an anastomosis itself is a temporary ischemic injury. Moreover, we were interested in the implication of L-arginine, as an NO progenitor and semi-essential proteinogenic amino acid, on intestinal wound healing because of its reported importance in this process[20,21].
MATERIALS AND METHODS
Subjects
The local Ethics Committee at the University of Freiburg approved all animal experiments. Male Wistar rats (Charles River, Sulzfeld) weighing 219-350 g were used for all experiments. The animals were housed two per cage, fed standard chow and given water ad libitum. Twelve hours before anesthesia, rats were fasting but had free access to water. Postoperatively, rats also had free access to water but were fed stepwise following a specified increasing amount of chow to prevent postoperative ileus.
Experimental design
Randomization (closed envelopes) took place after a preoperative acclimatization under laboratory conditions of 5-7 d. Rats were assigned to one of the six groups. Each group consisted of at least nine (9-14) Wistar rats (Table 1): the RIPC 5/20 (n = 9) group was characterized by 5 min of intraoperative ischemia followed by 20 min of reperfusion, before constructing an anastomosis. This was compared with the RIPC 5/30 (n = 11), RIPC 10/20 (n = 14) and RIPC 10/30 (n = 10) groups. In the control group (CO) and the L-arginine group, the same anastomosis was created without preconditioning.
Table 1.
Observations during the planned re-laparotomy on postoperative day 4
| Group | n | Dehiscence | Abscess | Hemorrhage | Ileus | Expelled |
| Control | 10 | 1 | 0 | 1 | 1 | 3 |
| RIPC 5/20 | 9 | 1 | 1 | 0 | 0 | 1 |
| RIPC 5/30 | 11 | 1 | 1 | 0 | 0 | 1 |
| RIPC 10/20 | 14 | 0 | 0 | 0 | 0 | 0 |
| RIPC 10/30 | 10 | 0 | 0 | 0 | 0 | 0 |
| Arginine | 9 | 0 | 1 | 0 | 0 | 1 |
RIPC: Remote ischemic preconditioning
Operative procedure
Rats were operated upon by the same investigator under sterile laboratory conditions. After induction of anesthesia with isoflurane (4% isoflurane in 3 L/min oxygen) in an acrylic glass box, narcosis was maintained, after transferring the rats to an operating table, through a mask (1.5% isoflurane in 3 L/min oxygen). A 26 G silicon venous catheter was placed into the tail vein. A catheter was used for continuous infusion (9 mL/h per kg body weight) of an iso-osmolar electrolyte solution (Jonosteril; Fresenius, Bad Homburg, Germany: 137 mmol/L Na+, 4 mmol/L K+, 1.65 mmol/L Ca2+, 1.25 mmol/L Mg2+, 110 mmol/L Cl-, 18 mmol/L CHCOO-, pH 5.0-7.0; osmolarity 291 mosm/L). After anesthesia was established, the abdominal coat was shaved and disinfected with polyvidone (Betaisadonna; Mundipharma, Limburg, Germany). A 4-5-cm midline incision in the lower half of the abdomen was performed to accomplish optimal exposition.
In animals assigned to one of the four RIPC groups, the infrarenal aorta was prepared. Clamping off the infrarenal aorta directly above the bifurcation was achieved with an atraumatic microsurgical clamp (Medicon, Germany), following the different intervals of IR, as mentioned above. Intervals were similar to our previously published paper on IPC. In the control group, the infrarenal aorta was prepared but not branched off. The arginine group received 200 μg/kg L-arginine (L-Arginin-Hydrochlorid; Fresenius) intraoperatively without preparing the abdominal aorta.
Afterwards, an approximately 1-cm segment, about 15 cm oral to the ileocecal valve was resected. Ileal continuity was restored by eight inverting interrupted sutures (Prolene 8/0; Ethicon, Germany). We used a silicon catheter (Heidelberger Verlängerung; Braun, Melsungen, Germany; diameter 5 mm) to standardize and simplify the suture technique. The silicon catheter was inserted into both lumina of the small intestine. At first, front-wall sutures were made, then we turned around the anastomosis to perform the back-wall sutures, removing the catheter just before the last two sutures. The distance between the single sutures and the stitches to the resection margin was 1-2 mm. The abdominal cavity was closed using a two-layer technique: musculoperitoneal layer (Monocryl 4/0 SHplus; Ethicon), and fasciocutaneous layer (Vicryl 4/0 SHplus; Ethicon).
On postoperative day 4, the rats were re-laparotomized and sacrificed by cardiac puncture with injection of a lethal dose of potassium. The abdomen was opened by a complete midline incision combined with a transverse incision to gain maximum exposure. Careful exploration was carried out to look for signs of inflammation, adhesions, anastomotic insufficiency, and abscesses. Without dissecting the directly adjacent tissue around the anastomosis, an approximately 4-6-cm segment bearing the anastomosis was harvested for further analysis.
Bursting pressure
The bowel segment was water-tight and connected to an infusion pump (Perfusor fm; Braun) filled with iso-osmolar saline solution (0.9% NaCl; Braun) via a 14 G silicon catheter (Vasofix Safety; Braun) and to a digital pressure transducer (Codman ICP Express; Ethicon). Intraluminal pressure was increased by an infusion rate of 60 mL/h. Monitoring included the bursting pressure recorded just before sudden loss of tension and the site of rupture (mesenterial vs anti-mesenterial). Subsequently, the bowel wall was released from all adhering tissue and the complete suture line was excised within a total length of the bowel of 1 cm. The anastomosis was opened at the mesenterial site and gently washed using a saline solution (0.9% NaCl, Braun). The anastomosis was divided at the anti-mesenterial site into two parts of the same length for paraffin embedding and measurement of hydroxyproline concentration. The former was achieved by immediately fixing the specimen in 4% phosphate-buffered formaldehyde (pH 7.3), and the latter by preservation of the anastomotic strip in an Eppendorf tube at -80°C until spectrophotometric measurement.
Hydroxyproline concentration
The specimens used for hydroxyproline concentration measurement were desiccated in an oven (Heraeus Electronic UT5042EK, Germany) until a constant dry weight was achieved. Hydroxyproline concentration was determined by using the Chloramine-T spectrophotometric method as previously described by Reddy et al[22]. The practice is based on alkaline hydrolysis of the tissue homogenate and the consecutive measurement of free hydroxyproline. Before measurement, Chloramine-T was used to oxidize the free hydroxyproline in a pyrrole. Adding Ehrlich’s reagent resulted in a chromophore that could be recorded at 550 nm. Data finally were calculated to express the results as micrograms of hydroxyproline per gram dry weight of tissue.
Histological evaluation
After fixation in formalin and embedding in paraffin, histological sections were stained with hematoxylin-eosin. Mucosal injury, inflammation and hyperemia/hemorrhage were assessed and graded in a blinded manner by two pathologists. Pathologists used the injury scale (Table 2) as described by Chiu et al[23].
Table 2.
Mucosal injury scale from Chiu et al[23]
| Grade | Definition |
| 0 | Normal mucosal villi |
| 1 | Development of subepithelial Gruenhagen’s space at the apex of the villus, often with capillary congestion |
| 2 | Extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria |
| 3 | Massive epithelial lifting down the sides of villi, possibly with few denuded tips |
| 4 | Denuded villi with lamina propria and dilated capillaries exposed, possibly with increased cellularity of lamina propria |
| 5 | Digestion and disintegration of the lamina propria, hemorrhage and ulceration |
Statistical analysis
All data are expressed as median and range. Overall significance was proved using the Kruskal-Wallis test. Subsequent comparison of subgroups was done using the Mann-Whitney U test; P < 0.05 was assumed to be significant. SPSS for Windows version 14.0.2 (Chicago, IL, USA) was used.
RESULTS
General observations
One rat died intraoperatively in the RIPC 10/20 group because of acute mesenterial bleeding. One anastomosis in the RIPC 10/20 group had to be redone because of a technical issue. Two rats in the RIPC group died on postoperative day 3: one had advanced peritonitis caused by bacterial translocation in ileus without anastomotic dehiscence; and the other had ileus without macroscopic signs of peritonitis.
The findings during planned re-laparotomy on postoperative day 4 are summarized in Table 1. There were three insufficient anastomoses. Further observations were ileus, mesenterial hemorrhage, and three abscesses. Two of the three abscesses were adjacent to the anastomosis without macroscopic signs of insufficiency, whereas one was distant from the anastomosis. These nine rats were excluded from further examination (hydroxyproline concentration, bursting pressure and histology). We observed that intra-abdominal adhesions, especially near the anastomosis, were more pronounced in the RIPC groups than in the control or the arginine group.
Bursting pressure
Control vs RIPC groups: During bursting pressure measurement, the site of rupture was equally allocated to both the mesenterial and anti-mesenterial site along the suture line, and never occurred distant to the anastomosis. Median bursting pressure in the control group was 124 mm Hg (60-146 mmHg). Similar bursting pressure (P > 0.05) was documented in all of the RIPC groups: 125 mmHg (65-144 mmHg) in the RIPC 5/20 group; 125 mmHg (65-144 mmHg) in the RIPC 5/30 group; 130 mmHg (52-175 mmHg) in the RIPC 10/20; and 117 mmHg (41-162 mmHg) in the RIPC 10/30 group (Figure 1).
Figure 1.
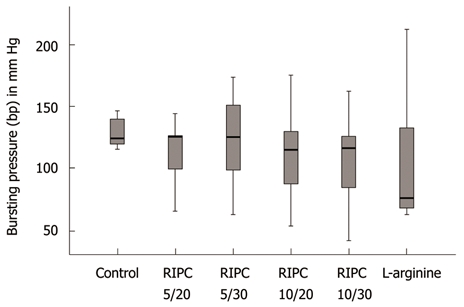
Bursting pressure after different remote ischemic preconditioning settings and after arginine application expressed as box plot. There was no significant overall difference between the groups (P > 0.05). RIPC: Remote ischemic preconditioning.
Control/RIPC vs arginine: The arginine group showed a median bursting pressure of 90 mmHg (65-212 mmHg) and was not different to the control and RIPC groups (Figure 1).
Hydroxyproline levels
Hydroxyproline was measured to establish a relationship with the collagen content in the anastomotic region. Median hydroxyproline concentration in the control group was 86 μg/g dry weight (36-160 μg/g). Hydroxyproline concentration in the RIPC groups was not significantly different from the control group (P > 0.05): 58 μg/g (30-158 μg/g) in the RIPC 5/20 group; 78 μg/g (19-227 μg/g) in the RIPC 5/30 group; 99 μg/g (40-250 μg/g) in the RIPC 10/20 group; and 108 μg/g (57-198 μg/g) in the RIPC 10/30 group. The arginine group also showed no significant disparity: 78 μg/g (21-130 μg/g).
Histological examination
The above described histological score for mucosal damage according to Chiu et al[23] was applied to evaluate the structural damage due to the creation of the anastomosis. Different grades of damage (0-3) were seen in all groups at the suture line. Maximum damage was seen up to grade 3, which means massive epithelial lifting down the sides of the villi, with possibly a few denuded tips. Mucosal damage was observed in the first few villi on both sides of the anastomotic line. Twelve rats (27%) in the RIPC groups did not show any mucosal damage (grade 0); and five in the RIPC 10/30 group, four in the RIPC 5/20 group, and three in the RIPC 5/30 group. The control group had only one rat (10%) with grade 0 damage (Figure 2).
Figure 2.
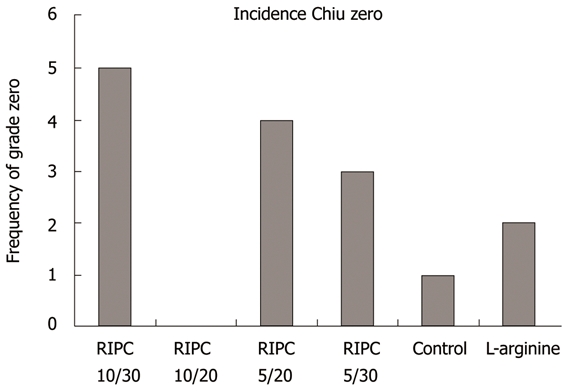
The histological mucosal damage score by Chiu[23] showed significantly more low-grade alterations in the remote ischemic preconditioning groups (Remote ischemic preconditioning, 27% vs control, 10%). RIPC: Remote ischemic preconditioning.
DISCUSSION
Our research group has focused on fundamental aspects of gastrointestinal wound healing[13,24,25], because anastomotic insufficiency still occurs and results in high morbidity and mortality[4-6,26,27]. We previously have presented data on the influence of IPC on the stability of small intestine anastomoses. IPC has a beneficial effect on wound healing in the gastrointestinal tract[13]. In the present study, we are the first to present experimental data on the effect of RIPC of the infrarenal aorta on anastomotic healing in the small bowel, with RIPC being performed directly before anastomotic construction.
RIPC has been shown to have consistently favorable effects in several other organs, such as heart, lungs, brain and kidney. Mostly, and contrary to our data, the effect has been measured by a reduction in IRI[15-18,28-31]. However, in most publications, the best practice intervals for IR in IPC and RIPC are not clear. It may be that there are differences according to which organ is being studied. Colak et al have performed left colonic anastomoses after temporary closure of the superior mesenteric artery (SMA). Their experimental setting consisted of four groups: a control group without preconditioning and without IRI, a remote IRI group in which the anastomosis was executed after a temporary closure of the SMA for 40 min; a preconditioned IRI group with two cycles of preconditioning (5 min closure of SMA) before IRI; and a preconditioned group without IRI. IRI significantly impaired anastomotic healing in terms of a decreased bursting pressure in the non-preconditioned and preconditioned groups. Furthermore, they observed the lowest bursting pressure in the preconditioned group without IRI on postoperative day 7[19]. The data are contrary to our findings because we worked out a non-inferiority of RIPC vs the control group. It could be that re-laparotomy on postoperative day 7, and the fact that they performed colonic anastomoses, are responsible for their findings, but on the other hand, there also could have been a delayed negative effect of remote preconditioning in general. Colonic anastomoses lose 70% of their initial strength and approach 75% of normal strength at 4 mo, whereas small intestine anastomoses primarily lose less strength and reach their original state at 4 wk[32].
In our current experimental model, we could not demonstrate a superiority of RIPC over non-preconditioned rats, as expressed by bursting pressure and hydroxyproline concentration. Also, in our non-remote preconditioned model, hydroxyproline levels were not significantly higher than in the control group, whereas the bursting pressure was. In the literature, a positive but also a negative correlation between anastomotic stability and hydroxyproline concentration has been reported[33,34]. Two of the studies have demonstrated the influence of pentoxifylline[35] and doxycycline[33] on intestinal anastomoses, thus indicating further factors that are involved in early anastomotic healing (e.g. other extracellular matrix molecules). Ahrendt et al have subdivided the total protein content of a tissue sample bearing an anastomosis into collagenase-digestible protein (CDP) and non-collagenous protein[36]. Although they have primarily focused on the impact of sepsis on collagen synthesis, an alteration of non-collagenous protein synthesis after construction of an anastomosis is ascertainable[36]. On the other hand, hydroxyproline concentration measurements do not distinguish between collagen subtypes (especially types I and III), which are known to influence wound stability in subject to their ratio[36-41]. IPC may result in a higher collagen type I /III ratio or in a higher degree of crosslinking. Moreover, hydroxyproline measurement just reflects the total amount of collagen, but does not discern between pre-existing structural collagen and newly synthesized collagen[36]. We suggest that anastomotic stability is not strictly correlated to the amount of collagen, but rather to the quality of collagen and other extracellular proteins that have not yet been identified to have an influence on anastomotic healing.
The physiological process of anastomotic healing is potentially disturbed by hypoperfusion, tension, hypovolemia, infection, drugs, malnutrition and immunodeficiency[42-48]. Tension resulting in hypoperfusion, or hypoperfusion itself at the region of the anastomosis, primarily has to be avoided by the surgeon via adequate mobilization of the oral and aboral segment, and foresighted transsection to maintain sufficient perfusion. Jönsson et al[3] have shown that collagen synthesis is dependent on tissue oxygenation, thus indicating disturbed anastomotic healing in cases of insufficient blood supply at the site of transsection. Moreover, collagenase activity simultaneously is elevated in hypoxia[43]. Against the background of the above-mentioned facts, any improvements in (micro)circulation could be beneficial to gastrointestinal wound healing in general. Although we did not find an impact on anastomotic stability in our RIPC model, the trend towards a lower grade of mucosal injury could be shown.
Even if significant improvements in bursting pressure and/or hydroxyproline concentration are not visible on postoperative day 4, a beneficial effect in the later phase cannot be excluded. The effects of preconditioning in general are subdivided into an early and a delayed response. Furthermore, subdivision into a humoral, neural and systemic pathway has been described previously[15,49]. The early response leads to a release of trigger substances such as adenosine, bradykinin, norepinephrine, endocannabinoids, calcitonin gene-related peptide (CGRP), and opioids at the site of ischemia. Via membrane bound receptors and subsequent intracellular signaling, rapid protection in terms of avoiding necrosis and apoptosis is induced locally. Early protection lasts for 2-3 h but reappears approximately 24 h after preconditioning, and is dependent on de novo synthesis of molecules such as inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2) and heat shock proteins (HSPs)[15]. In RIPC, the early local protection is lacking because the site of preconditioning is distant to the region of interest. After the washout (reperfusion), mediators in an RIPC setup also reach the site of the anastomosis/IRI, but this secondary local effect seems to be attenuated compared with an IPC setting[50]. The systemic effect of RIPC resulting in transcription of anti-apoptotic and anti-inflammatory proteins seems to be similar to IPC[29,51]. Considering the attenuated local effect, it may be speculated that a positive effect of RIPC on intestinal wound healing becomes evident in a later phase due to newly synthesized molecules.
In our study, one group received 200 μg/kg L-arginine to test whether increased availability of an NO progenitor (L-arginine) and/or proteinogenic amino acid influenced the anastomotic stability. Compared to promising results after oral application[20], we did not find any positive results compared to the control group. Whether a dose-dependent effect of arginine on anastomotic healing plays an important role remains to be proven. Thornton and colleagues have pointed out a possible dose-dependent effect of NO on collagen synthesis in sepsis[52]. Stechmiller et al[20] have summed up that NO is of paramount importance in the early phase (inflammation stage) of wound healing, but disturbs it in the proliferation phase[53]. Infusion of a substrate regarding the applied dose therefore is not as productive as mimicking preconditioning via direct mediators (pharmacological preconditioning)[54]. In general, mimicking the effect of (remote) ischemic preconditioning could be promising because it could easily be implicated in clinical routine, and could avoid any harm to the site of preconditioning (e.g. vascular dissection in preconditioning)[54].
The partly massive adhesions around the anastomosis, but also the whole abdominal cavity in the RIPC groups, are highly relevant for surgeons. Preparation on postoperative day 4 was significantly more difficult and technically demanding in the RIPC groups. There were two rats in the RIPC 10/20 group that died due to ileus. Although adhesions are not constant, re-laparotomy on postoperative day 4 could be problematic. Concerning these adhesions, re-laparotomy in the later phase (e.g. postoperative day 7, as mentioned above) in our experimental setting could shed light on the dynamics of these adhesions. Otherwise, it is known that the primary sealing of the anastomosis emanates from the serosal layer, therefore, the adhesions could imply better primary sealing of the anastomosis.
The most interesting issue is the tendency to a lower degree of mucosal injury in the RIPC groups. This could in part be a mark of improved microcirculation, at least at the mucosal level. Additionally, the mucosal injury index of Chiu[23] (Table 2) is definitely affected by the stitching technique during surgery. Only strictly extramucosal stitches avoid partial mucosal necrosis; transmural stitches would have resulted in a higher degree of mucosal injury. In our highly standardized anastomotic technique, we paid attention to a strictly correct suture technique in a single surgical design. Figure 3 shows a sample of two different Chiu grades. Early or improved mucosal repair is important for the formation of an antimicrobial barrier, and furthermore, for untroubled anastomotic healing at a submucosal level. Mucosal integrity itself is definitely not responsible for anastomotic stability, but aseptic milieu at the submucosa accelerates wound healing and avoids disturbance caused by inflammation due to infection. This could be useful for patients at risk (e.g. genetic alteration of collagen synthesis) in whom collagen synthesis and/or remodeling takes longer, or for patients suffering from an immunodeficiency who are susceptible to infection.
Figure 3.
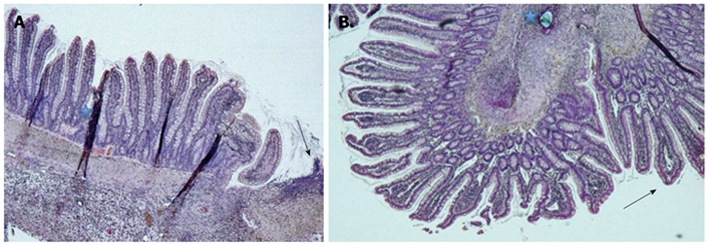
Histological view. A: Chiu grade 0 with intact epithelium and a few villi along one side of the anastomosis (HE staining; magnification 5 ×), Region of the anastomosis (arrow) and staining artefacts (*); B: Chiu grade 2 with a pronounced subepithelial space in the villi next to the inverted anastomosis (arrow) and a suture hole (*) (HE staining; magnification 5 ×).
Given the fact that oxygenation at the site of anastomosis is of paramount importance, angiogenesis and especially drugs that boost or restrict the expression of pro-angiogenic proteins [e.g. vascular endothelial growth factor; (VEGF)] are of note. Ishii et al[55] have demonstrated an increased bursting pressure and hydroxyproline content on postoperative day 4 in colonic anastomosis. Another interesting approach is VEGF gene therapy, as reported by Enestvedt et al[56]. After esophagogastrectomy and gastric tube formation, a VEGF plasmid vector is injected at the site of anastomosis. Subjects treated with VEGF transfection resulted in an increased bursting pressure and neovascularization[56]. They assumed a strong correlation between the number of microvessels and bursting pressure, which supports the enormous importance of oxygenation in anastomotic healing. In contrast, anti-angiogenic agents impair anastomotic integrity if applied shortly before surgery. It may be that (R)IPC can prevent the negative effect of agents such as bevacizumab[57]. Pro-angiogenic agents could improve gastrointestinal wound healing in the absence of anti-angiogenic drugs, especially in patients with life-time steroid and/or combined immunosuppressive therapy.
The informative value of the current study could be influenced by the small groups and the wide data range, but the data are equally distributed within the range of each group. Therefore, it remains to be proven if larger groups would have led to distinctive findings. Moreover, the group size calculation was based on a presumed high difference in primary outcome measure between the groups indicating a potentially high clinical relevance. As mentioned above, we sacrificed the rats on postoperative day 4 to investigate early anastomotic healing and to match the results with our IPC study. Before postoperative day 4, anastomotic stability is more influenced by suture holding capacity than by tissue regeneration due to wound healing. Postoperative day 4 is a vulnerable phase in anastomotic healing because suture holding capacity has already decreased and collagen synthesis increases and just overcomes collagenolysis. In fact, increasing the stability of anastomoses on postoperative day 4 would be of great clinical relevance. Given the fact that RIPC results in a commensurate effect, we cannot exclude a positive impact in a later phase of anastomotic healing. Optimum intervals of ischemia and reperfusion are not exactly established, therefore, we adopted the intervals from our previous study. It is not clear whether these are suitable or whether ideal intervals in RIPC differ from those in IPC, and moreover in different effector organs. Our current experiment is a highly mechanistic model, and it is not know whether it can be transferred into clinical routine, but it is easy to repeat and it is one small piece of experimental research on the fundamentals of gastrointestinal wound healing.
In conclusion, RIPC of the infrarenal aorta does not seem to have an influence on anastomotic stability, as was shown for IPC of the SMA. However, according to our previous results with IPC, mucosal injury seems to be less in the RIPC groups, thus indicating improved mucosal microcirculation at the anastomotic region. The observed relevant intra-abdominal adhesions around the anastomosis support the presumption of a gaugeable effect of RIPC on intestinal anastomoses. Further studies, especially in respect to the mucosal and submucosal microcirculation at the anastomotic region, will be of interest to verify the potential implementation of RIPC in intestinal wound healing.
COMMENTS
Background
Wound healing is a widely researched topic. Many experimental studies of wound healing, especially in the skin, have been published. The healing of an intestinal anastomosis in phases is similar to wound healing in the skin. The dehiscence of an anastomosis results in higher morbidity and mortality. To avoid anastomotic dehiscence, the best surgical technique is a prerequisite. Even though an optimal surgical technique for dehiscence is used, it is necessary to research the fundamentals of gastrointestinal wound healing to improve and innovate perioperative management for lowering the rate of anastomotic dehiscence.
Research frontiers
To study the fundamentals of gastrointestinal wound healing, ischemic preconditioning (IPC) and remote ischemic preconditioning (RIPC) were used. Other studies have investigated the effect of different volume regimens perioperatively, and the application of additive medication before or after installation of an anastomosis.
Innovations and breakthroughs
The Surgical Metabolic and Anastomotic Research Team has shown that IPC improves the stability of small-intestinal anastomoses. Other researchers who have used RIPC have not found an advantage in colonic anastomoses. Examination of mucosal injury in anastomoses is an innovation but its consequences are not clear.
Applications
Preconditioning in general could probably be transferred to clinical routine although there are risks, but RIPC has shown no benefit in terms of increasing anastomotic stability. The reduction of mucosal injury in this setting should be confirmed by in vivo microscopy.
Terminology
In preconditioning, ischemia is induced by temporarily branching off an artery, followed by an interval of reperfusion in order to minimize the IR injury of subsequent prolonged ischemia. The ischemia following the preconditioning in our setting is the creation of an anastomosis. In (direct) IPC, the branched off artery supplies the region of interest, and in that case, it would be the later constructed anastomosis. In RIPC, the clamped artery does not supply the region of interest but other organs/regions.
Peer review
In this experimental work, the authors examined the effect of RIPC on small-intestinal anastomosis healing and the paper is interesting.
Acknowledgments
The authors would like to thank Mrs. Olivia Sick for supporting the present study in statistical issues.
Footnotes
Supported by Science Fund of the Department for General and Visceral Surgery at the University of Freiburg
Peer reviewer: Paola De Nardi, MD, Department of Surgery, Scientific Institute San Raffaele Hospital, Via Olgettina 60, Milan, 20132, Italy
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
References
- 1.Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26:131–136. doi: 10.1002/micr.20197. [DOI] [PubMed] [Google Scholar]
- 2.Enestvedt CK, Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part II. Microsurgery. 2006;26:137–143. doi: 10.1002/micr.20198. [DOI] [PubMed] [Google Scholar]
- 3.Jönsson K, Jiborn H, Zederfeldt B. Breaking strength of small intestinal anastomoses. Am J Surg. 1983;145:800–803. doi: 10.1016/0002-9610(83)90144-7. [DOI] [PubMed] [Google Scholar]
- 4.Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587–592. doi: 10.1111/j.1463-1318.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 5.Iancu C, Mocan LC, Todea-Iancu D, Mocan T, Acalovschi I, Ionescu D, Zaharie FV, Osian G, Puia CI, Muntean V. Host-related predictive factors for anastomotic leakage following large bowel resections for colorectal cancer. J Gastrointestin Liver Dis. 2008;17:299–303. [PubMed] [Google Scholar]
- 6.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–1154. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 7.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 8.Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27–32. doi: 10.1006/bbrc.1996.0692. [DOI] [PubMed] [Google Scholar]
- 9.Aksöyek S, Cinel I, Avlan D, Cinel L, Oztürk C, Gürbüz P, Nayci A, Oral U. Intestinal ischemic preconditioning protects the intestine and reduces bacterial translocation. Shock. 2002;18:476–480. doi: 10.1097/00024382-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Cinel I, Avlan D, Cinel L, Polat G, Atici S, Mavioglu I, Serinol H, Aksoyek S, Oral U. Ischemic preconditioning reduces intestinal epithelial apoptosis in rats. Shock. 2003;19:588–592. doi: 10.1097/01.shk.0000055817.40894.84. [DOI] [PubMed] [Google Scholar]
- 11.Mallick IH, Yang W, Winslet MC, Seifalian AM. Protective effects of ischemic preconditioning on the intestinal mucosal microcirculation following ischemia-reperfusion of the intestine. Microcirculation. 2005;12:615–625. doi: 10.1080/10739680500301631. [DOI] [PubMed] [Google Scholar]
- 12.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischaemic preconditioning improves microvascular perfusion and oxygenation following reperfusion injury of the intestine. Br J Surg. 2005;92:1169–1176. doi: 10.1002/bjs.4988. [DOI] [PubMed] [Google Scholar]
- 13.Marjanovic G, Jüttner E, zur Hausen A, Theodor Hopt U, Obermaier R. Ischemic preconditioning improves stability of intestinal anastomoses in rats. Int J Colorectal Dis. 2009;24:975–981. doi: 10.1007/s00384-009-0696-0. [DOI] [PubMed] [Google Scholar]
- 14.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 15.Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: a novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84:445–458. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- 16.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Review and meta-analysis of randomized controlled clinical trials of remote ischemic preconditioning in cardiovascular surgery. Am J Cardiol. 2008;102:1487–1488. doi: 10.1016/j.amjcard.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 18.Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection 'outside the box'--the evolving paradigm of remote preconditioning. Basic Res Cardiol. 2003;98:149–157. doi: 10.1007/s00395-003-0406-y. [DOI] [PubMed] [Google Scholar]
- 19.Colak T, Turkmenoglu O, Dag A, Polat A, Comelekoglu U, Bagdatoglu O, Polat G, Kanik A, Akca T, Aydin S. The effect of remote ischemic preconditioning on healing of colonic anastomoses. J Surg Res. 2007;143:200–205. doi: 10.1016/j.jss.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20:52–61. doi: 10.1177/011542650502000152. [DOI] [PubMed] [Google Scholar]
- 21.Schulz G, Stechmiller J. Wound healing and nitric oxide production: too little or too much may impair healing and cause chronic wounds. Int J Low Extrem Wounds. 2006;5:6–8. doi: 10.1177/1534734606286633. [DOI] [PubMed] [Google Scholar]
- 22.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 23.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 24.Marjanovic G, Villain C, Timme S, zur Hausen A, Hoeppner J, Makowiec F, Holzner P, Hopt UT, Obermaier R. Colloid vs. crystalloid infusions in gastrointestinal surgery and their different impact on the healing of intestinal anastomoses. Int J Colorectal Dis. 2010;25:491–498. doi: 10.1007/s00384-009-0854-4. [DOI] [PubMed] [Google Scholar]
- 25.Marjanovic G, Villain C, Juettner E, zur Hausen A, Hoeppner J, Hopt UT, Drognitz O, Obermaier R. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg. 2009;249:181–185. doi: 10.1097/SLA.0b013e31818b73dc. [DOI] [PubMed] [Google Scholar]
- 26.Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980;281:411–444. doi: 10.1136/bmj.281.6237.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanellos I, Blouhos K, Demetriades H, Pramateftakis MG, Mantzoros I, Zacharakis E, Betsis D. The failed intraperitoneal colon anastomosis after colon resection. Tech Coloproctol. 2004;8 Suppl 1:s53–s55. doi: 10.1007/s10151-004-0111-3. [DOI] [PubMed] [Google Scholar]
- 28.Walsh SR, Tang T, Sadat U, Dutka DP, Gaunt ME. Cardioprotection by remote ischaemic preconditioning. Br J Anaesth. 2007;99:611–616. doi: 10.1093/bja/aem273. [DOI] [PubMed] [Google Scholar]
- 29.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150:304–330. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Birch SE, He R, Tawadros P, Szaszi K, Kapus A, Rotstein OD. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg. 2010;251:292–299. doi: 10.1097/SLA.0b013e3181bfda8c. [DOI] [PubMed] [Google Scholar]
- 31.Shahid M, Tauseef M, Sharma KK, Fahim M. Brief femoral artery ischaemia provides protection against myocardial ischaemia-reperfusion injury in rats: the possible mechanisms. Exp Physiol. 2008;93:954–968. doi: 10.1113/expphysiol.2007.041442. [DOI] [PubMed] [Google Scholar]
- 32.Marjanovic G, Hopt UT. [Physiology of anastomotic healing] Chirurg. 2011;82:41–47. doi: 10.1007/s00104-010-1898-2. [DOI] [PubMed] [Google Scholar]
- 33.Siemonsma MA, de Hingh IH, de Man BM, Lomme RM, Verhofstad AA, Hendriks T. Doxycycline improves wound strength after intestinal anastomosis in the rat. Surgery. 2003;133:268–276. doi: 10.1067/msy.2003.27. [DOI] [PubMed] [Google Scholar]
- 34.Posma LA, Bleichrodt RP, van Goor H, Hendriks T. Transient profound mesenteric ischemia strongly affects the strength of intestinal anastomoses in the rat. Dis Colon Rectum. 2007;50:1070–1079. doi: 10.1007/s10350-006-0822-9. [DOI] [PubMed] [Google Scholar]
- 35.Tireli GA, Salman T, Ozbey H, Abbasoglu L, Toker G, Celik A. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003;19:88–90. doi: 10.1007/s00383-002-0741-3. [DOI] [PubMed] [Google Scholar]
- 36.Ahrendt GM, Tantry US, Barbul A. Intra-abdominal sepsis impairs colonic reparative collagen synthesis. Am J Surg. 1996;171:102–107; discussion 107-108. doi: 10.1016/S0002-9610(99)80082-8. [DOI] [PubMed] [Google Scholar]
- 37.Stumpf M, Cao W, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. Increased distribution of collagen type III and reduced expression of matrix metalloproteinase 1 in patients with diverticular disease. Int J Colorectal Dis. 2001;16:271–275. doi: 10.1007/s003840100310. [DOI] [PubMed] [Google Scholar]
- 38.Stumpf M, Klinge U, Wilms A, Zabrocki R, Rosch R, Junge K, Krones C, Schumpelick V. Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery. 2005;137:229–234. doi: 10.1016/j.surg.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Junge K, Klinge U, Rosch R, Lynen P, Binnebosel M, Conze J, Mertens PR, Schwab R, Schumpelick V. Improved collagen type I/III ratio at the interface of gentamicin-supplemented polyvinylidenfluoride mesh materials. Langenbecks Arch Surg. 2007;392:465–471. doi: 10.1007/s00423-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 40.Brasken P, Lehto M, Renvall S. Changes in the connective tissue composition of the submucosal layer of colonic anastomosis. An immunohistologic study in rats. Acta Chir Scand. 1989;155:413–419. [PubMed] [Google Scholar]
- 41.Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res. 2000;32:43–48. doi: 10.1159/000008740. [DOI] [PubMed] [Google Scholar]
- 42.Mandai R, Eguchi Y, Tanaka M, Sai Y, Nosaka S. Effects of profound hemodilution on small-intestinal wound healing in rabbits. J Surg Res. 2001;99:107–113. doi: 10.1006/jsre.2001.6164. [DOI] [PubMed] [Google Scholar]
- 43.Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 44.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25:61–68. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 45.Wagner OJ, Egger B. [Influential factors in anastomosis healing] Swiss Surg. 2003;9:105–113. doi: 10.1024/1023-9332.9.3.105. [DOI] [PubMed] [Google Scholar]
- 46.Mantzoros I, Kanellos I, Demetriades H, Christoforidis E, Kanellos D, Pramateftakis MG, Zaraboukas T, Betsis D. Effects of steroid on the healing of colonic anastomoses in the rat. Tech Coloproctol. 2004;8 Suppl 1:s180–s183. doi: 10.1007/s10151-004-0150-9. [DOI] [PubMed] [Google Scholar]
- 47.Kube R, Mroczkowski P, Steinert R, Sahm M, Schmidt U, Gastinger I, Lippert H. [Anastomotic leakage following bowel resections for colon cancer: multivariate analysis of risk factors] Chirurg. 2009;80:1153–1159. doi: 10.1007/s00104-009-1725-9. [DOI] [PubMed] [Google Scholar]
- 48.Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am. 1997;77:549–573. doi: 10.1016/s0039-6109(05)70568-5. [DOI] [PubMed] [Google Scholar]
- 49.Riksen NP, Smits P, Rongen GA. Ischaemic preconditioning: from molecular characterisation to clinical application--part I. Neth J Med. 2004;62:353–363. [PubMed] [Google Scholar]
- 50.Gurcun U, Discigil B, Boga M, Ozkisacik E, Badak MI, Yenisey C, Kurtoglu T, Meteoglu I. Is remote preconditioning as effective as direct ischemic preconditioning in preventing spinal cord ischemic injury? J Surg Res. 2006;135:385–393. doi: 10.1016/j.jss.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 52.Thornton FJ, Ahrendt GM, Schäffer MR, Tantry US, Barbul A. Sepsis impairs anastomotic collagen gene expression and synthesis: a possible role for nitric oxide. J Surg Res. 1997;69:81–86. doi: 10.1006/jsre.1997.5034. [DOI] [PubMed] [Google Scholar]
- 53.Stechmiller JK, Langkamp-Henken B, Childress B, Herrlinger-Garcia KA, Hudgens J, Tian L, Percival SS, Steele R. Arginine supplementation does not enhance serum nitric oxide levels in elderly nursing home residents with pressure ulcers. Biol Res Nurs. 2005;6:289–299. doi: 10.1177/1099800405274732. [DOI] [PubMed] [Google Scholar]
- 54.Riksen NP, Smits P, Rongen GA. Ischaemic preconditioning: from molecular characterisation to clinical application--part II. Neth J Med. 2004;62:409–423. [PubMed] [Google Scholar]
- 55.Ishii M, Tanaka E, Imaizumi T, Sugio Y, Sekka T, Tanaka M, Yasuda M, Fukuyama N, Shinozaki Y, Hyodo K, et al. Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. Eur Surg Res. 2009;42:249–257. doi: 10.1159/000210671. [DOI] [PubMed] [Google Scholar]
- 56.Enestvedt CK, Hosack L, Winn SR, Diggs BS, Uchida B, O'Rourke RW, Jobe BA. VEGF gene therapy augments localized angiogenesis and promotes anastomotic wound healing: a pilot study in a clinically relevant animal model. J Gastrointest Surg. 2008;12:1762–1770; discussion 1771-1772. doi: 10.1007/s11605-008-0635-3. [DOI] [PubMed] [Google Scholar]
- 57.Deshaies I, Malka D, Soria JC, Massard C, Bahleda R, Elias D. Antiangiogenic agents and late anastomotic complications. J Surg Oncol. 2010;101:180–183. doi: 10.1002/jso.21447. [DOI] [PubMed] [Google Scholar]


