Lysosomes serve multiple degradative functions that are potentiated by a profound luminal acid pH generated by an electrogenic proton pump. This proton transport requires charge compensation, and chloride has long been assumed to be the primary counter ion. In this Journal Club, we review the recent conflicting literature surrounding the identity of the putative lysosomal chloride counter ion pathway, with a focus on both ClC-7 and CFTR conductances. We propose that the discrepant conclusions within the literature can be largely accounted for by differences in the methodologies used to assay organellar pH. As part of our analysis, we include a comparison of techniques used to measure organellar pH, highlighting their respective strengths and limitations.
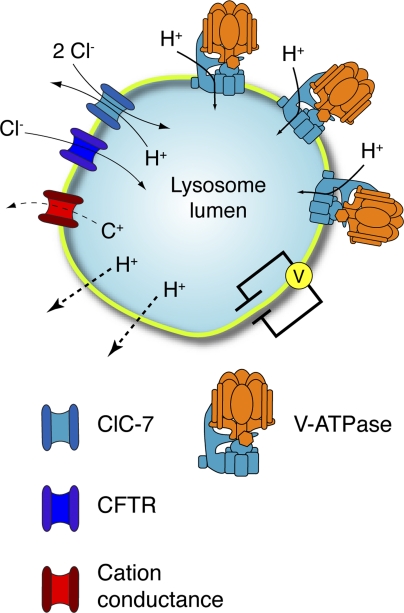
The endocytic pathway is important for multiple processes including the regulation, recycling, and degradation of material from the plasma membrane and other organelles (Doherty and McMahon, 2009; Sorkin and von Zastrow, 2009). Lysosomes, the final compartment in this pathway, contain hydrolases that facilitate the decomposition of proteins, lipids, and polysaccharides. These enzymes are active in acidic conditions, requiring the organelle to maintain an optimal luminal pH between 4 and 5 (Pillay et al., 2002). Lysosomal acidification is achieved by activity of the vacuolar-type ATPase (V-ATPase), a multi-subunit protein complex that uses the energy derived from ATP hydrolysis to transport protons across the lysosomal membrane into the lumen of the organelle (Forgac, 2007). Because the translocation of protons is rheogenic, it tends to generate an electrical potential across the membrane that, if left uncompensated, limits the ability of the V-ATPase to continue pumping and reach a sufficiently acidic pH. To alleviate this restraint to proton accumulation, counter ion pathways involving either the influx of anions or the efflux of cations, or a combination of both, must be functioning in conjunction with the V-ATPase to dissipate the development of a restrictive electrical gradient (Fig. 1).
Figure 1.
Determinants of lysosomal pH. Lysosomal acidification is dependent on V-ATPase, a large multimeric enzyme complex that transforms the energy of ATP hydrolysis into the movement of protons across the lysosome membrane. Electrogenic proton transport creates an electrical gradient that must be dissipated to establish the substantial chemical proton gradient. Electroneutrality can be maintained through the parallel influx of anions alongside protons. ClC-7, a chloride proton antiporter, and CFTR have been proposed to constitute the counter ion pathways in the lysosome membrane, as described in the text. The efflux of cations (C+) through distinct channels or transporters can also occur. Parallel proton leak pathways (dotted lines) are also known to exist and require continued V-ATPase activity to maintain a steady-state pH. Acidification kinetics are also contingent on the luminal buffering power (not depicted).
The identity of the counter ions involved in lysosomal acidification remains unclear; however, chloride influx has been proposed to play a major role in neutralizing the lumen-positive charge generated by the V-ATPase (Kornak et al., 2001; Di et al., 2006; Graves et al., 2008; Deriy et al., 2009). Members of the CLC family of chloride transporters mediate conductive Cl− transport in the endocytic pathway and are therefore attractive prospective counter ion pathways to neutralize the entry of protons. ClC-3, ClC-4, ClC-5, and ClC-6 are found in earlier compartments of the endocytic pathway (Jentsch, 2008), whereas ClC-7 localizes to lysosomes (Kornak et al., 2001). The CFTR, a cAMP-regulated chloride channel, has similarly been proposed to serve as a counter ion permeation pathway. Indeed, persistent lung inflammation associated with cystic fibrosis (CF) has been proposed to result, in part, from the failure of alveolar macrophages expressing mutant CFTR to correctly acidify their degradative compartments, causing an inability to resolve infection (Di et al., 2006; Deriy et al., 2009).
Although a role for these chloride transporters in lysosomal acidification is both reasonable and appealing, there are conflicting reports in the literature regarding the contribution of ClC-7 and CFTR. Here, we will discuss the results of recent studies addressing this contentious area and propose potential explanations for the discrepancies, with a focus on the methodology used in the individual studies to measure organellar pH.
Key results: chloride conductances and lysosome acidification
Deriy et al. (2009) recently reported that acidification of lysosomes is impaired in CFTR knockout and mutant mice, and they suggested that this defect may contribute to the lung inflammation associated with CF. Phagosomes play a critical role in the innate immune response and undergo a similar acidification to lysosomes. Because of their large size, phagosomes are readily amenable to microscopic analysis, and their maturation pathway serves as a model of lysosomal acidification. Deriy et al. (2009) examined the role of CFTR in acidification by treating wild-type mouse alveolar macrophages with the CFTR inhibitor CFTRinh-172, while measuring pH by confocal microscopy using phagocytic targets (yeast particles) labeled with a pH-sensitive dye. In their experiments, the inhibition of CFTR increased the phagosomal pH from 5.75–6.0 to 7.25–7.5. These results argue that CFTR is important for the acidification of phagosomes. Moreover, the authors reported an acidification defect in lysosomes from cftr−/− mice when compared with wild-type mice (pH 6.91 ± 0.05 vs. pH 5.16 ± 0.06, respectively). Remarkably, this difference was observed only in alveolar macrophages, but not in peritoneal macrophages or blood monocytes from the same mice. Modest acidification defects were also observed in lysosomes of alveolar macrophages from mice with the most common CF-causing mutations found in humans, ΔF508 (pH 6.11 ± 0.06) and G551D (pH 5.91 ± 0.04). These findings are supported by previous work from Nelson’s group in alveolar macrophages (Di et al., 2006) and the work of others in respiratory epithelial cells (Teichgräber et al., 2008).
These findings, however, are not universally consistent. A study by Haggie and Verkman (2007) contradicted these observations and suggested instead that CFTR is not a significant source of counter ions in lysosomal or phagosomal acidification. The authors reported no change in lysosomal or phagosomal acidification between untreated and CFTRinh-172–treated J774A.1 cells (a mouse macrophage-like cell line) and primary mouse or human alveolar macrophages. They also observed no differences between wild-type and CFTR-deficient (ΔF508-CFTR) mouse alveolar macrophages. Other groups corroborated these observations (Steinberg et al., 2010), and additional studies by Haggie and Verkman showed that CFTR is similarly dispensable for lysosomal acidification in respiratory epithelial cells (Haggie and Verkman, 2009). Jointly, these data argue against CFTR as the principal contributing counter ion pathway in lysosomal acidification and indicate that the lung inflammation that accompanies CF is unlikely to be the result of impaired lysosomal acidification in macrophages expressing mutant CFTR. This conclusion is diametrically opposite to that reached by Deriy et al. (2009).
A similar and concurrent conflict exists in the literature regarding the role of ClC-7 as a putative source of counter ions in lysosomal acidification. Graves et al. (2008) described ClC-7 as the primary source of anion influx in lysosomes and suggested that this pathway is important for lysosomal acidification. These authors confirmed that ClC-7, like other endosomal CLC family members, is an antiporter with 2 Cl−/1 H+ stoichiometry (Picollo and Pusch, 2005; Scheel et al., 2005; Graves et al., 2008; Neagoe et al., 2010). To examine whether it plays a role in acidification, they depleted ClC-7 in HeLa cells using siRNA and then used LysoTracker, a fluorophore that partitions into acidic intracellular compartments where it becomes trapped by protonation, to assess lysosomal pH. A significant decrease in dye accumulation was observed in cells treated with ClC-7 siRNA when compared with cells treated with control (scrambled siRNA-treated or untreated) HeLa cells, arguing that ClC-7 is necessary for lysosomal acidification.
These results were contested by Jentsch and his colleagues, who generated ClC-7–deficient mice (Clcn7−/−). These animals die within 7 wk of birth and display lysosomal storage disease, osteopetrosis, and growth retardation (Kornak et al., 2001; Kasper et al., 2005). The occurrence of lysosomal storage defects suggested abnormalities in pH homeostasis and prompted these authors to measure the lysosomal pH in multiple cell types. However, no lysosomal acidification defect was observed either in vitro or in vivo (Kasper et al., 2005; Lange et al., 2006; Weinert et al., 2010). Based on the collected evidence, Jentsch and colleagues concluded that ClC-7 is therefore not involved in lysosomal acidification, and that altered pH is not a factor in the pathology of Clcn7−/− mice (Weinert et al., 2010).
Interpretation: methodological considerations
Although the recent output of data addressing lysosome acidification has been abundant in amount and impressive in quality, it has left the reader with starkly conflicting results. What accounts for the reported discrepancies? We propose that one possibility lies in the methodology used to assay organellar pH—the principal phenotype measured in these studies. In what follows, we include a comparison of the methods used to assay luminal pH in the different studies, highlighting their strengths and weaknesses to clarify how technical limitations of some of these methods may have affected their key conclusions.
In their study of ClC-7, Graves et al. (2008) used an acidotropic dye to estimate lysosomal pH. The dye can traverse biological membranes and accumulate indiscriminately in acidic intracellular organelles, thereby providing an indirect and qualitative measurement of pH. An integrated estimate of the cellular fluorescence is often reported as a surrogate of lysosomal pH; however, the detailed relationship between the total fluorescence of acidotropic dyes and pH is usually not known and, importantly, is affected by the size, number, and nature of the contents of the acidic organelles. Unfortunately, Graves et al. (2008) did not provide these parameters, making the reported differences difficult to interpret. It is noteworthy also that their data relied on seemingly incomplete knockdown of ClC-7 using a single siRNA, and that the control siRNA used by these authors induced a significant lysosome alkalinization.
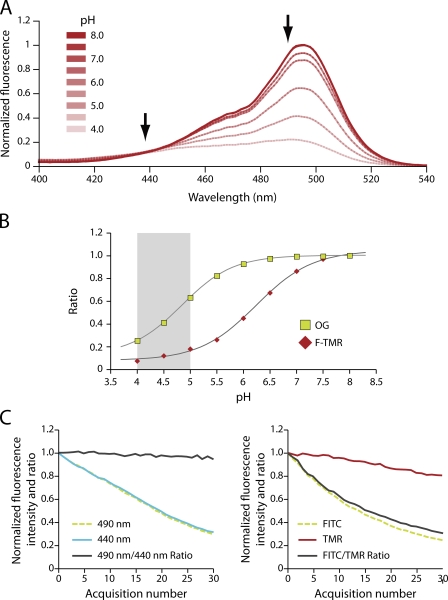
Because of their simplicity, acidotropic dyes represent an attractive option for qualitative pilot studies. More precise, quantitative measurements of organellar pH, however, can be obtained using probes that emit fluorescence in a manner that is dictated by their state of protonation (Fig. 2 A). A variety of these probes are available, including conjugates of fluorescein and of its derivative, Oregon Green. These dyes, commonly used for pH measurements of endocytic compartments, have a considerable added advantage: they are amenable to ratiometric determinations, which are insensitive to changes in fluorescence introduced by parameters other than pH, such as focal plane, thickness of the optical slice, and photobleaching. As weak acids, these pH sensors have an inherent pKa at which their pH sensitivity is most dynamic. The choice of sensor should thus reflect the anticipated pH of the compartment under investigation. In the case of the lysosome, an appropriate dye would optimally have a pKa between 4 and 5, the range of pH values consistently measured in lysosomes across the literature (for examples, see Christensen et al., 2002; Trombetta et al., 2003; Lange et al., 2006; Poët et al., 2006; Tabeta et al., 2006). Oregon Green and fluorescein have pKa values of 4.8 and 6.4, respectively (Fig. 2 B). Thus, the former is a more appropriate choice for the lysosome, although reproducible measurements should be obtainable with fluorescein.
Figure 2.
Ratiometric pH measurements. (A) pH sensitivity of the excitation spectra of Oregon Green (OG)-labeled dextran between pH 4.0 and 8.0. The arrows indicate the wavelengths used to construct a ratiometric pH titration curve. (B) In vitro pH titration of OG dextran (green squares) and fluorescein-TMR (F-TMR) dextran (red diamonds). The normalized excitation fluorescence intensity ratio of 490:440 nm and 490:550 nm are plotted for the OG dextran and F-TMR dextran, respectively. The gray bar indicates the range of recently reported lysosome pH values (Christensen et al., 2002; Trombetta et al., 2003; Kasper et al., 2005; Lange et al., 2006; Poët et al., 2006; Tabeta et al., 2006; Haggie and Verkman, 2007). (C) Macrophage lysosomes were loaded with either OG dextran (left) or F-TMR dextran (right), and their pH clamped at pH 7.4 using ionophores before repeated illumination of the sample. For the OG dextran, the normalized fluorescence intensity of the 490-nm (dotted green) and the 440-nm (blue) channels are shown along with the 490:440 nm ratio (black). F-TMR was imaged in both the FITC (dotted green) and TMR (red) channels, with the FITC/TMR ratio given by the black line. The latter is unstable even in conditions of constant pH because of the differential photobleaching of the FITC and TMR. This is in contrast to the intramolecular ratio of OG that remains uniform.
A recent study used epifluorescence ratiometric imaging of Oregon Green dextran to assess the contribution of ClC-7 to lysosomal acidification. Weinert et al. (2010) used a standard pulse–chase protocol to load the lysosomal compartment via the physiological endosome maturation pathway. By measuring fluorescence emission at 535 nm after sequential excitation at 488 nm, a pH-sensitive wavelength, and 440 nm, a pH-insensitive wavelength (Fig. 2 A), they calculated a ratio that is a reliable index of the luminal pH. Such ratiometric data were then converted to absolute pH levels by generating calibration curves, such as the one illustrated in Fig. 2 B, obtained by clamping the pH in situ at desired values using ionophore-containing solutions. Clearly, this approach provides a more precise and robust measure of organellar pH than that obtained with the acidotropic fluorophores like LysoTracker. We therefore regard the recent findings of Jentsch’s group (Weinert et al., 2010) as being more reliable than those reported by Graves et al. (2008).
An alternative to single-fluorophore ratiometric imaging is the engineered ratiometric pH sensor, where a pH-sensitive dye is paired with another pH-insensitive fluorophore such as tetramethylrhodamine (TMR). Many laboratories prefer this strategy, as it does not require additional microscopy hardware to capture fluorescence in the pH-insensitive domain of the fluorophore’s excitation or emission spectra. Moreover, the signal of the reference dye, TMR in the example above, is strong. Unfortunately, serial acquisitions often used to obtain temporal profiles of organellar acidification are susceptible to artifact caused by the differential bleaching of the two fluorophores: changes in the fluorescence intensity ratio often occur independently of pH changes, as the signal of one dye is preferentially diminished by repeated illumination (Fig. 2 C). In the case of ratiometric imaging using a single fluorophore, this risk is obviated (Fig. 2 C). Notably, quantitative intracellular pH measurements represent one case where the use of epifluorescence imaging is advantageous over confocal laser scanning, which can produce significant photobleaching, and is more sensitive to motion artifact as a result of its inherently thin optical sectioning.
Some of the preceding considerations are relevant to the conflicting literature regarding the role of CFTR in lysosomal acidification. Deriy et al. (2009) assayed lysosomal acidification using fluorescein as the pH sensor, normalized against the TMR colabel. Their images were acquired by laser scanning confocal microscopy. In contrast, Haggie and Verkman (2007) relied on wide-field detection and used Oregon Green, a dye with a more suitable pKa, as a pH sensor. Although the differences in methodology used by the two groups are not drastic, we think the approach of Haggie and Verkman to be more appropriate and therefore their results to be more convincing than those of Deriy et al. Indeed, other recent studies have also failed to validate a role for CFTR as the counter ion pathway in lysosome acidification (Lamothe and Valvano, 2008; Barriere et al., 2009; Steinberg et al., 2010), casting doubt on the conclusions of Deriy et al.
Concluding remarks
It is clear that the interpretation of the conflicting literature addressing the counter ion pathway for organellar acidification requires a critical appraisal of the methodology used to measure pH. When the most suitable and stringent methodology is applied, the results suggest that neither ClC-7 (Weinert et al., 2010) nor CFTR (Haggie and Verkman, 2007) is essential for lysosomal acidification. Moreover, Weinert et al. (2010) found that isolated lysosomes—purified from wild-type and Clcn7−/− mice alike—acidified normally in nominally Cl−-free buffer. This rather unexpected result points to the possibility that a cation counterflux may provide a neutralizing counter ion instead of, or in addition to, the parallel transport of chloride (Fig. 1). The latter conclusion was given credence by experiments where the effects of pH were measured after the luminal cation concentration was manipulated; replacement of luminal sodium and potassium by a large (poorly permeant) organic cation reduced the rate at which lysosomes accumulate protons (Steinberg et al., 2010).
The nature of such cation conductance(s) awaits explicit identification; however, candidates include endolysosomal calcium channels, such as the mucolipin members of the transient receptor potential superfamily, as well as the two-pore channels, the source of nicotinic acid adenine dinucleotide phosphate–mediated calcium mobilization (discussed in Scott and Gruenberg, 2011, and references therein). In fact, a recent study suggests that loss of calcium efflux by mucolipin-3 knockdown results in defective lysosome acidification (Lelouvier and Puertollano, 2011). It is equally important to consider that in order for cations to serve as acidification counter ions, they must be present in sufficient quantities within the lysosomal lumen (Steinberg et al., 2010). To this end, lysosomes continuously receive inorganic cations internalized by fluid-phase endocytosis and delivered through the endosome maturation program. This cation source does not preclude the existence of as yet unidentified electroneutral cation transport systems.
If chloride is not required for acidification, what is the need for specialized counter ion transporters like ClC-7? Notably, recent studies suggest that, rather than supporting the uptake of H+, chloride ions may use the H+ gradient to accumulate inside lysosomes at concentrations that may exceed that of the cytosol (Jentsch, 2008; Weinert et al., 2010; Scott and Gruenberg, 2011). What addsecbitional physiological roles chloride serves within the lysosome have yet to be precisely established, but undoubtedly represent an exciting and active area of research in intracellular physiology.
Acknowledgments
We would like to thank Sergio Grinstein for his critical reading and thoughtful suggestions regarding this manuscript.
B.E. Steinberg is supported by MD/PhD studentships from the Canadian Institutes of Health Research and the McLaughlin Centre for Molecular Medicine.
Sergio Grinstein served as faculty advisor.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- CF
- cystic fibrosis
- TMR
- tetramethylrhodamine
- V-ATPase
- vacuolar-type ATPase
References
- Barriere H., Bagdany M., Bossard F., Okiyoneda T., Wojewodka G., Gruenert D., Radzioch D., Lukacs G.L. 2009. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol. Biol. Cell. 20:3125–3141 10.1091/mbc.E09-01-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.A., Myers J.T., Swanson J.A. 2002. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 115:599–607 [DOI] [PubMed] [Google Scholar]
- Deriy L.V., Gomez E.A., Zhang G., Beacham D.W., Hopson J.A., Gallan A.J., Shevchenko P.D., Bindokas V.P., Nelson D.J. 2009. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J. Biol. Chem. 284:35926–35938 10.1074/jbc.M109.057372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di A., Brown M.E., Deriy L.V., Li C., Szeto F.L., Chen Y., Huang P., Tong J., Naren A.P., Bindokas V., et al. 2006. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 8:933–944 10.1038/ncb1456 [DOI] [PubMed] [Google Scholar]
- Doherty G.J., McMahon H.T. 2009. Mechanisms of endocytosis. Annu. Rev. Biochem. 78:857–902 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- Forgac M. 2007. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8:917–929 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- Graves A.R., Curran P.K., Smith C.L., Mindell J.A. 2008. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature. 453:788–792 10.1038/nature06907 [DOI] [PubMed] [Google Scholar]
- Haggie P.M., Verkman A.S. 2007. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J. Biol. Chem. 282:31422–31428 10.1074/jbc.M705296200 [DOI] [PubMed] [Google Scholar]
- Haggie P.M., Verkman A.S. 2009. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J. Biol. Chem. 284:7681–7686 10.1074/jbc.M809161200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. 2008. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43:3–36 10.1080/10409230701829110 [DOI] [PubMed] [Google Scholar]
- Kasper D., Planells-Cases R., Fuhrmann J.C., Scheel O., Zeitz O., Ruether K., Schmitt A., Poët M., Steinfeld R., Schweizer M., et al. 2005. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24:1079–1091 10.1038/sj.emboj.7600576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U., Kasper D., Bösl M.R., Kaiser E., Schweizer M., Schulz A., Friedrich W., Delling G., Jentsch T.J. 2001. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 104:205–215 10.1016/S0092-8674(01)00206-9 [DOI] [PubMed] [Google Scholar]
- Lamothe J., Valvano M.A. 2008. Burkholderia cenocepacia-induced delay of acidification and phagolysosomal fusion in cystic fibrosis transmembrane conductance regulator (CFTR)-defective macrophages. Microbiology. 154:3825–3834 10.1099/mic.0.2008/023200-0 [DOI] [PubMed] [Google Scholar]
- Lange P.F., Wartosch L., Jentsch T.J., Fuhrmann J.C. 2006. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 440:220–223 10.1038/nature04535 [DOI] [PubMed] [Google Scholar]
- Lelouvier B., Puertollano R. 2011. Mucolipin-3 regulates luminal calcium, acidification, and membrane fusion in the endosomal pathway. J. Biol. Chem. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagoe I., Stauber T., Fidzinski P., Bergsdorf E.Y., Jentsch T.J. 2010. The late endosomal ClC-6 mediates proton/chloride countertransport in heterologous plasma membrane expression. J. Biol. Chem. 285:21689–21697 10.1074/jbc.M110.125971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo A., Pusch M. 2005. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 436:420–423 10.1038/nature03720 [DOI] [PubMed] [Google Scholar]
- Pillay C.S., Elliott E., Dennison C. 2002. Endolysosomal proteolysis and its regulation. Biochem. J. 363:417–429 10.1042/0264-6021:3630417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poët M., Kornak U., Schweizer M., Zdebik A.A., Scheel O., Hoelter S., Wurst W., Schmitt A., Fuhrmann J.C., Planells-Cases R., et al. 2006. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. USA. 103:13854–13859 10.1073/pnas.0606137103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel O., Zdebik A.A., Lourdel S., Jentsch T.J. 2005. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 436:424–427 10.1038/nature03860 [DOI] [PubMed] [Google Scholar]
- Scott C.C., Gruenberg J. 2011. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays. 33:103–110 10.1002/bies.201000108 [DOI] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. 2009. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10:609–622 10.1038/nrm2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg B.E., Huynh K.K., Brodovitch A., Jabs S., Stauber T., Jentsch T.J., Grinstein S. 2010. A cation counterflux supports lysosomal acidification. J. Cell Biol. 189:1171–1186 10.1083/jcb.200911083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K., Hoebe K., Janssen E.M., Du X., Georgel P., Crozat K., Mudd S., Mann N., Sovath S., Goode J., et al. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156–164 10.1038/ni1297 [DOI] [PubMed] [Google Scholar]
- Teichgräber V., Ulrich M., Endlich N., Riethmüller J., Wilker B., De Oliveira-Munding C.C., van Heeckeren A.M., Barr M.L., von Kürthy G., Schmid K.W., et al. 2008. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 14:382–391 10.1038/nm1748 [DOI] [PubMed] [Google Scholar]
- Trombetta E.S., Ebersold M., Garrett W., Pypaert M., Mellman I. 2003. Activation of lysosomal function during dendritic cell maturation. Science. 299:1400–1403 10.1126/science.1080106 [DOI] [PubMed] [Google Scholar]
- Weinert S., Jabs S., Supanchart C., Schweizer M., Gimber N., Richter M., Rademann J., Stauber T., Kornak U., Jentsch T.J. 2010. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl- accumulation. Science. 328:1401–1403 10.1126/science.1188072 [DOI] [PubMed] [Google Scholar]