Abstract
We have identified a means by which agonist-evoked responses of nicotinic receptors can be conditionally eliminated. Modification of α7L119C mutants by the sulfhydryl reagent 2-aminoethyl methanethiosulfonate (MTSEA) reduces responses to acetylcholine (ACh) by more than 97%, whereas corresponding mutations in muscle-type receptors produce effects that depend on the specific subunits mutated and ACh concentration. We coexpressed α7L119C subunits with pseudo wild-type α7C116S subunits, as well as ACh-insensitive α7Y188F subunits with wild-type α7 subunits in Xenopus laevis oocytes using varying ratios of cRNA. When mutant α7 cRNA was coinjected at a 5:1 ratio with wild-type cRNA, net charge responses to 300 µM ACh were retained by α7L119C-containing mutants after MTSEA modification and by the ACh-insensitive Y188F-containing mutants, even though the expected number of ACh-sensitive wild-type binding sites would on average be fewer than two per receptor. Responses of muscle-type receptors with one MTSEA-sensitive subunit were reduced at low ACh concentrations, but much less of an effect was observed when ACh concentrations were high (1 mM), indicating that saturation of a single binding site with agonist can evoke strong activation of nicotinic ACh receptors. Single-channel patch clamp analysis revealed that the burst durations of fetal wild-type and α1β1γδL121C receptors were equivalent until the α1β1γδL121C mutants were exposed to MTSEA, after which the majority (81%) of bursts were brief (≤2 ms). The longest duration events of the receptors modified at only one binding site were similar to the long bursts of native receptors traditionally associated with the activation of receptors with two sites containing bound agonists.
INTRODUCTION
Modern understanding of synaptic ion channels began with the isolation (Karlin and Cowburn, 1973) and subsequent cloning of nicotinic acetylcholine (ACh) receptors (nAChRs) (Numa et al., 1983). Based on the presence of primary binding site elements, including a pair of vicinal cysteines, 10 different nAChR subunits have been identified in vertebrates as α subunits (α1–α10). Non–α subunits, which demonstrably contain the required elements for forming the complementary surface of an agonist binding site, are γ, δ, and ε in muscle-type receptors and β2 and β4 in neuronal receptors.
The nAChR ligand binding domain is formed by the interface of two protein subunits; the primary surface is formed by an α subunit, which contains several other key elements in addition to the adjacent cysteines of a subdomain identified as the C-loop (Sine, 2002). The distinction between α and non–α subunits relates to a key dichotomy in several of the subfamilies of Cys-loop receptors between types that function as pentamers of an identical α subunit (homomeric receptors) and those that require both α and non–α subunits in each pentameric complex (heteromeric receptors). In the subfamily of mammalian nAChRs, homomeric receptors, such as α7, are considered ancestral to heteromeric combinations and can be activated by both ACh and the precursor/metabolite, choline (Papke et al., 1996). Subunits like α7 are able to contribute to five agonist binding sites at both primary and complementary interface surfaces (Palma et al., 1996), whereas heteromeric receptors, which require specialized non–α subunits for the complementary side of the binding site, are limited to two agonist binding sites.
ACh is a nearly perfect molecule for fast and transient synaptic signaling at sites like the neuromuscular junction, as it is rapidly released and efficiently hydrolyzed. However, nicotinic signaling appears to be fundamentally different in the brain, where rhythmic ACh release occurs diffusely, rather than at focused synaptic sites, and a primary role of nAChRs in the brain is to modulate neuronal excitability and release of other neurotransmitters (Descarries et al., 1997; Dani and Bertrand, 2007). As a result of esterases, extracellular concentrations of diffusely released ACh are expected to be low. Although choline is ubiquitous in the brain and body, concentrations are still well below the EC50 for acute activation of α7 (Papke and Porter Papke, 2002), even under conditions of trauma when choline concentrations reach 100 µM (Jope and Gu, 1991; Scremin and Jenden, 1991). In addition, responses of α7 receptors to high ACh concentrations are very limited (Papke et al., 2000; Papke and Porter Papke, 2002), which raises the question of whether α7 nAChR may function effectively under conditions of low fractional occupancy of the agonist binding sites. Single-channel studies of muscle-type nAChR have associated brief openings observed at low agonist concentrations with mono-liganded receptors (Colquhoun and Sakmann, 1981, 1985; Takeda and Trautmann, 1984; Labarca et al., 1985), supporting the hypothesis that the brief openings characteristic of α7 may also arise from the binding of single agonist molecules.
In this paper, we investigate the functional significance of the multiple agonist binding sites in heteromeric muscle-type and homomeric α7 forms of nAChR using the L119C mutation (α7 numbering), which is located on the complementary face of the agonist binding site across from the C-loop (see Fig. 1 A), together with sulfhydryl modification at that site to achieve varying levels of conditional binding site modification (Papke et al., 2011). We also use ACh-insensitive α7Y188F subunits (Horenstein et al., 2007) coexpressed with wild-type α7 subunits in different ratios. Our data are consistent with previous reports that heteromeric muscle-type receptors and homomeric Cys-loop receptors can activate with levels of submaximal agonist occupancy (Jackson, 1984, 1986; Colquhoun and Sakmann, 1985; Amin and Weiss, 1996; Mott et al., 2001; Beato et al., 2004; Rayes et al., 2009; Jha and Auerbach, 2010). Our data offer the additional insight that strong activation of muscle-type and α7 nAChR may be achieved under conditions of agonist saturation at individual binding sites.
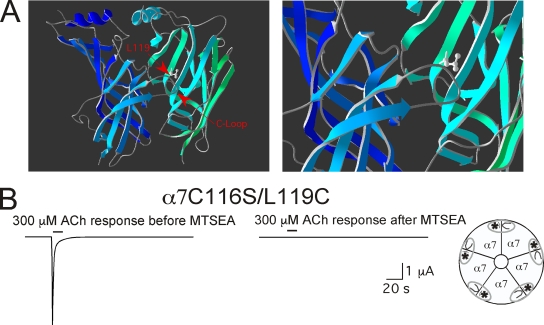
Figure 1.
Location of the L119 residue in a homology model of α7 (Celie et al., 2004). (A) The overview at left shows an α7-α7 homodimer and the location of the L119 residue in relation to the C-loop in the primary face of the agonist binding site. The image on the right shows the proximity at increased magnification. Images were created in Deep View (Swiss-PdbViewer; Guex and Peitsch, 1997) from the crystal structure model of the ACh binding protein (deposited in the Protein Data Bank under accession no. 1I9B; Brejc et al., 2001). (B) The effect of MTSEA treatment (2 mM for 60 s) on the ACh-evoked responses of oocytes expressing the α7L119C mutation in a cysteine-null (α7C116S) background. In this experiment, peak current responses to 300 µM ACh were reduced 99.4 ± 0.2%, and net charge was reduced by 99.7 ± 0.1% (n = 4). Responses to 3 mM ACh were reduced to a similar extent: 97.9 ± 0.3 and 95.9 ± 2.0% for peak current and net charge, respectively (n = 4).
MATERIALS AND METHODS
nAChR clones and mutants
Mouse muscle nAChR α1, β1, γ, and δ clones used for receptor expression in Xenopus laevis oocytes were obtained from J. Boulter (University of California, Las Angeles, Los Angeles, CA), and the mouse ε clone was provided by P. Gardner (University of Massachusetts Medical School, Worcester, MA). The mouse muscle cDNA clones in pRBG4 used for transfection of BOSC 23 cells were obtained from S. Sine (Mayo Clinic, Rochester, MN). The human α7 clones were provided by J. Lindstrom (University of Pennsylvania, Philadelphia, PA). Mutations to cDNA clones were introduced using the QuikChange kit from Agilent Technologies according to the manufacturer’s instructions. The mutations were confirmed with automated fluorescent sequencing.
Preparation of RNA for injection into Xenopus oocytes
After linearization and purification of cloned cDNAs, cRNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit (Invitrogen).
Expression in Xenopus oocytes
Mature (>9 cm) female Xenopus African frogs (Nasco) were used as the source of oocytes. The frogs were maintained in the Animal Care Services facility of the University of Florida, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Before surgery, frogs were anesthetized by placing each animal in a 1.5-g/liter solution of 3-aminobenzoic acid ethyl ester (Sigma-Aldrich) for 30 min. Oocytes were removed from an abdominal incision.
To remove the follicular cell layer, harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemical Corporation) for 2 h at room temperature in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, pH 7.6, and 12 g/liter tetracycline). Subsequently, stage 5 oocytes were isolated and injected with 50 nl (5–20 ng) each of the appropriate subunit cRNAs. Muscle-type receptor cRNAs were injected in the ratio of 2α:1β:1δ:1γ or 1ε. Recordings were made 1–7 d after injection. Although the absolute magnitude of the evoked current responses increased over time, the normalized values of the experimental responses did not vary significantly over time.
Cell culture and transient transfection of BOSC 23 cells
BOSC 23 cells obtained from American Type Culture Collection were cultured at 37°C, 5% CO2 in DMEM (Invitrogen) supplemented with 10% fetal bovine serum in the absence of antibiotics. Cells were discarded and fresh cells were thawed once 35 passages were reached. Cells were transiently transfected using Fugene 6 (Roche) according to the manufacturer’s instructions. 1 d before transfection, cells were plated onto 12-mm glass coverslips (Thermo Fischer Scientific) coated with poly-d-lysine (Sigma-Aldrich). 1 µg of mouse fetal muscle–type receptor cDNA (2α:1β:1γ:1δ) in pRBG4 with 0.8 µg of the cDNA encoding red fluorescent protein in pDsRed (Takara Bio Inc.) was added to each 35-mm dish containing cells and coverslips. Experiments were performed 48–72 h after transfection. The red fluorescent protein was used as a marker to identify successfully transfected cells.
Chemicals
2-aminoethyl methanethiosulfonate (MTSEA) was purchased from Toronto Research Chemicals Inc. All other chemicals for electrophysiology were obtained from Sigma-Aldrich. Fresh ACh and MTSEA stock solutions were made each day of experimentation. MTSEA stock solutions were made in water, kept on ice, and diluted just before experiments. 3-(4-hydroxy, 2-methoxybenzylidene) anabaseine (4OH-GTS-21) was provided by Taiho Pharmaceutical Co.
Two-electrode voltage clamp electrophysiology
Experiments were conducted using OpusXpress 6000A (Molecular Devices) (Stokes et al., 2004). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Oocytes were automatically perfused with bath solution, and agonist solutions were delivered from a 96-well plate. Both the voltage and current electrodes were filled with 3 M KCl. The agonist solutions were applied via disposable tips, which eliminated any possibility of cross-contamination. Drug applications alternated between ACh controls and experimental applications. Flow rates were set at 2 ml/min for experiments with α7 receptors and at 4 ml/min for other subtypes. Oocytes were voltage clamped at a holding potential of −60 mV. Data were collected at 50 Hz and filtered at 20 Hz. Unless otherwise indicated, drug applications were 12 s in duration followed by 181-s washout periods with α7 receptors and 8 s with 241-s washout periods for other subtypes.
Each oocyte received two initial control applications of ACh, an experimental drug application, and then a follow-up control application of ACh. The control ACh concentrations were 300 µM for α7 and 30 µM for muscle subunit combinations. The peak amplitude and the net charge (Papke and Porter Papke, 2002) of experimental responses were calculated relative to the preceding ACh control responses to normalize the data, compensating for the varying levels of channel expression among the oocytes. After each experimental measurement, oocytes were rechallenged with ACh at the control concentrations. Means and standard errors (SEM) were calculated from the normalized responses of at least four oocytes for each experimental concentration. The standard MTSEA treatment in the oocyte experiments was 2 mM applied for 60 s, a treatment that appears to produce a maximal effect on receptors expressed in Xenopus oocytes (Wang et al., 2010).
For concentration–response relationships, data were plotted using Kaleidagraph 3.0.2 (Abelbeck Software), and curves were generated as the best fit of the average values to the Hill equation:
where Imax denotes the maximal response for a particular agonist/subunit combination, and n represents the Hill coefficient. For the calculation of EC50 values, Imax was constrained to equal 1 and error estimates of the EC50 values are the standard errors of the parameters based on the Levenberg-Marquardt algorithm used for the generation of the fits (Press, 1988).
Outside-out patch clamp electrophysiology
Single-channel currents were recorded in the outside-out patch configuration using an Axopatch 200A amplifier (Molecular Devices) at room temperature. Cells were bathed in an external solution containing (in mM): 165 NaCl, 5 KCl, 2 CaCl2, 10 glucose, 5 HEPES, and 0.001 atropine, with pH adjusted to 7.3 with NaOH. Patch pipettes (Sutter Instrument) were pulled to a tip diameter of 1–2 µm, fire-polished to 5–10 MΩ, coated with SigmaCote (Sigma-Aldrich), and filled with an internal solution containing (in mM): 147 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES, and 2 ATP, with pH adjusted to 7.3 with CsOH. Recordings were low-pass filtered to 10 kHz with the built-in amplifier filter (four-pole Bessel) and digitized at 100 kHz with a DigiData 1440 (Molecular Devices) using Clampex 10 data acquisition software (Molecular Devices). Multiple recordings for each experimental condition were obtained from several transfection and recording dates.
Rapid drug application to outside-out patches was performed in a similar manner as described by Franke et al. (1987) and Jonas (1995). Theta glass (Sutter Instrument) was pulled, scored, and then broken by hand to create an application pipette with a diameter of 130 µM (septum thickness, ∼10 µM). The application pipette was mounted to a Burleigh piezoelectric stepper (EXFO). The signal sent by Clampex 10 (Molecular Devices) to the piezoelectric stepper was conditioned by an RC circuit (τ = 2 ms) to reduce oscillations and avoid damage to the crystal (Kabakov and Papke, 1998).
Two reservoirs (60-ml Monoject syringe bodies; Sherwood Medical Company) were connected to each channel of the theta glass application pipette with polyethylene tubing. Channel 1 of the application pipette was connected to reservoirs containing either external saline solution or 5 mM MTSEA, and channel 2 was connected to reservoirs containing either 1 mM ACh or external saline solution. Flow rates from each reservoir and channel were an equivalent 8.5 cm/s. The time required to replace solution flow in a channel was 30 s. Solution exchange times (10–90% rise times) were typically 0.4–0.7 ms and were routinely determined by movement of 50% diluted external solution over an open recording pipette. To maintain undisturbed laminar flow from the application pipette and minimize solution mixing, external saline solution was continuously perfused through the recording chamber (Warner Instruments) at a rate of 6 ml/min, and the application pipette was positioned such that streams flowing from it would directly enter the aspiration port of the chamber. In addition, the tip of the application pipette was kept free of dirt and/or cell debris by periodic cleaning in a hydrochloric acid solution. All solutions were degassed under vacuum and passed through a 0.2-µm filter to reduce the probability of particles/air bubbles obstructing solution flow and/or damaging the outside-out patch.
Once a stable outside-out patch was obtained and the application and recording pipettes were aligned, the stream of external solution exiting channel 1 was turned on, followed by the 1-mM ACh stream in channel 2. The piezoelectric stepper was then used to move the 1-mM ACh stream over the patch for the pre-MTSEA treatment ACh response. 30 s before termination of the first ACh application, the flow exiting channel 1 was replaced with the 5-mM MTSEA solution. MTSEA was then applied to the patch for 60 s, after which the 1-mM ACh stream was moved back over the patch for the post-MTSEA treatment ACh response. Any time MTSEA entered the bath chamber, whether the patch survived the entire protocol or not, the chamber was completely emptied and thoroughly rinsed, the application pipette was flushed for at least 5 min with external solution that was collected in a beaker separate from the bath chamber, and the coverslip of cells was replaced. Patches were not treated with MTSEA for the experiments in which 10 nM ACh was applied to α1β1γδL121C-mutant receptors.
All patch clamp recordings were processed, idealized, and analyzed with Clampfit 10 (Molecular Devices). Before any analysis, each recording was additionally low-pass filtered to 5 kHz with a software filter simulating an eight-pole Bessel filter, corrected for baseline drift, and any recorded artifacts or spurious noise was removed. The 5-kHz filter frequency was selected as a compromise between reliable event detection and total bandwidth. A resolution limit of 1.3× filter rise time was set at 86 µsec and imposed on all recordings (Mortensen and Smart, 2007).
Absolute Popen (NPopen) values were used as the primary measure of response to ACh for the outside-out patch clamp experiments in a manner analogous to the net charge measurements made from responses by receptors expressed in Xenopus oocytes. The NPopen value was computed for an entire response to ACh, including the nonstationary phase of activation by
where I is the recorded current relative to baseline, t is time, i is the mean single-channel amplitude, and D is the duration of the ACh application (Jackson, 1998). No attempt was made to estimate Popen for an individual channel because the total number of activatible channels in a patch could not be known with any degree of certainty, and because each patch served as its own control. Therefore, no kinetic information relating to a single channel is intended by the NPopen measurement.
Recordings containing minimal simultaneous channel openings were selected for half-amplitude idealization and analysis of single-channel burst durations. When simultaneous channel openings occurred, segments of data containing single-channel activity were selected so that nonconducting flanking regions were ≥50 ms. Apparent subconductances occurred occasionally but were ignored because they were not obvious in all traces and because they appeared to occur independently of MTSEA treatment. Data from at least four individual patches from each condition were pooled together to obtain sufficient numbers of events for analysis. Burst analysis was conducted with the intention of defining groups of one or more apparent channel openings that arise from an individual channel. Apparent channel openings separated by a closed interval less than the defined critical duration (tcrit) of 3.4 ms were called a burst of openings. The tcrit value was calculated based on the equation which misclassifies equal proportions of short and long intervals, from fit time constants of the closed duration histogram of non-MTSEA–treated α1β1γδL121C receptors (Colquhoun and Sakmann, 1985, 1995). The tcrit value determined for wild-type α1β1γδ receptors was 3.1 ms; small variations in tcrit values did not lead to significant changes in burst durations, and for consistency, the tcrit value of 3.4 ms was applied to the wild-type recordings. The value of tcrit for α1β1γδL121C patches that received 10 nM ACh was defined as 3.8 ms by the same method.
RESULTS
Identification of the α7L119C mutation as a tool to study nAChR binding sites
The α7 receptor contains a free cysteine residue at position 116. To prevent nonspecific modification and/or potential disulfide formations between the single free cysteine at position 116 and the introduced cysteine, we used a cysteine-null pseudo wild-type C116S background. Responses of pseudo wild-type α7C116S receptors to EC50 concentrations of ACh, tetramethyl ammonium, quinuclidine, and 4OH-GTS-21 are not significantly different from wild-type and are unaffected by the application of MTSEA (Papke et al., 2011). A similar pseudo wild-type background was used in other studies that introduced cysteine mutations into α7 (Barron et al., 2009).
The L119C mutation was identified as an effective tool for the investigation of nAChR binding sites because receptors containing this mutation responded normally to ACh, tetramethyl ammonium, quinuclidine, and 4OH-GTS-21 until treated with MTSEA or any of the three other cationic sulfhydryl reagents applied, after which agonist-induced responses were nearly completely abolished (Papke et al., 2011). The near 100% reduction in response to 300 µM ACh that is typical when 2 mM MTSEA is applied for 60 s to α7C116S/L119C-mutant receptors expressed in oocytes is shown in Fig. 1 B. The degree of inhibition is not significantly dependent on the ACh concentration used to evoke the responses. In the specific experiment illustrated, net charge responses to 300 µM ACh were reduced by 99.7 ± 0.1%, and responses to 3 mM ACh were reduced to a similar extent (95.9 ± 2.0%; not depicted).
Effects of α7L119C ratios in mixed α7 wild-type/mutant heteromers
We sought to test the hypothesis that α7 receptors may be activated, even if the receptors have a reduced number of activatable binding sites. We injected Xenopus oocytes with RNA coding for α7C116S (pseudo wild-type) subunits and the MTSEA-sensitive α7C116S/L119C subunits in varying ratios. The possible subunit combinations and likely distributions of those combinations as a probabilistic function of the RNA ratios, assuming equal expression and assembly of the wild-type and mutant subunits, are shown in Fig. 2 A. With the highest ratio (5:1) of α7C116S/L119C to α7C116S, less than 4% of the receptors would be predicted to have more than two MTSEA-insensitive binding sites, and 40% would be predicted to be fully MTSEA sensitive. We attempted to verify expression of the α7L119C-mutant subunits by comparison of radiolabeled α-bungarotoxin (α-btx) binding before and after MTSEA treatment. However, the α7C116S and α7L119C mutations appeared to disrupt the binding of the competitive antagonist, and such experiments were not possible to pursue. Comparison of the magnitude of non-normalized functional responses from oocytes to the same concentration of ACh (300 µM) that was injected the same day, with the same amount of RNA from the same harvest of oocytes, revealed equivalent responses among the α7C116S/L119C, 1:1, 3:1, and 5:1 groups. The functional responses of the oocytes injected with α7C116S alone were threefold greater (n ≥ 5 oocytes for each group; not depicted).
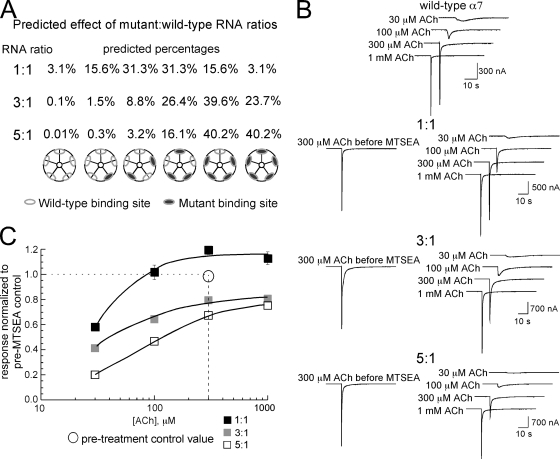
Figure 2.
Coexpression of either L119C or Y188F with wild-type α7 subunits at varying ratios. (A) The probability for the distribution of mutant subunits based on RNA ratios. Each single subunit within a pentamer is either mutant or wild-type, and if we assume X = probability of being wild-type (actually α7C116S) and Y = probability of being mutant, then 1 = X + Y, and for combinations of five subunits, (X + Y)n = 1. The expansion of this binomial is X5 + 5X4Y + 10X3Y2 + 10X2Y3 + 5XY4 + Y5 = 1. We assume that receptors are functionally equivalent regardless of the subunit positions within the pentameric structure. (B) Data traces obtained from oocytes expressing the α7C116S/L119C MTSEA-sensitive mutant and the α7C116S cysteine-null pseudo wild-type at the ratios indicated. For each panel, the traces on the left are the 300-µM ACh control responses obtained before the MTSEA treatment, and the series of traces on the right are the responses to progressively greater concentrations of ACh obtained after the MTSEA treatment. (C) Average net charge values for oocytes expressing the α7C116S/L119C MTSEA-sensitive mutant and α7C116S cysteine-null pseudo wild-type at the ratios indicated after treatment with MTSEA, normalized to the 300-µM ACh control responses before MTSEA treatment. The open circles represent the 300-µM ACh control data obtained before the MTSEA treatment (i.e., the values to which the posttreatment data are normalized). The data plotted are the means ± SEM for at least five oocytes at each of the ratios tested. See Table I for curve fit values.
Control responses to 300 µM ACh were recorded for each cell in the three injection sets before MTSEA treatment (Fig. 2 B). After treating the oocytes with MTSEA (2 mM for 60 s), oocytes were tested with a range of ACh concentrations from 30 µM to 1 mM. The averaged net charge data are shown in Fig. 2 C (see also Table I), normalized to the 300-µM ACh net charge responses obtained before the MTSEA treatment. The most obvious effect of the MTSEA treatment was on the responses to low ACh concentration in the oocytes injected with the highest fraction of MTSEA-sensitive mutants. In the control (pre-MTSEA) condition, 300 µM ACh was sufficient to produce a maximal net charge response. Responses of the oocytes injected at ratios of 1:1 and 3:1 showed no significant differences in function after MTSEA treatment (P > 0.05). Only the oocytes injected at the 5:1 ratio showed a significant decrease in the 300-µM ACh-evoked responses (P < 0.05) after MTSEA treatment; however, this average decrease of 33% was less than the percentage of receptors predicted to be fully sensitive to MTSEA (40%; Fig. 2 A). Although untreated α7 receptors (not depicted) and the treated receptors injected at 1:1 (Fig. 2 C) showed no increase in net charge from 300 µM to 1 mM ACh, the average responses of the 5:1 injected oocytes to 1 mM ACh increased to the extent that responses to 1 mM ACh were not significantly different from the pretreatment 300-µM control responses at the P < 0.05 level. One possible explanation is that α7 receptors with one or two functional binding sites may be less affected by the rapid concentration-dependent desensitization that is characteristic of α7 and, therefore, better able to respond to high concentrations of agonist.
Table I.
α7L119C ratio experiments: net charge data after MTSEA treatmenta
| mut/wt ratio | Imaxb | EC50 |
| µM | ||
| 1:1 | 1.16 ± 0.05 | 30 ± 4 |
| 3:1 | 0.83 ± 0.04 | 31 ± 4 |
| 5:1 | 0.78 ± 0.01 | 72 ± 3 |
Values are the means ± SEM of at least five oocytes.
Measured relative to ACh maximum before MTSEA treatment.
Effects of ACh-insensitive mutant ratios in mixed α7 wild-type/mutant heteromers
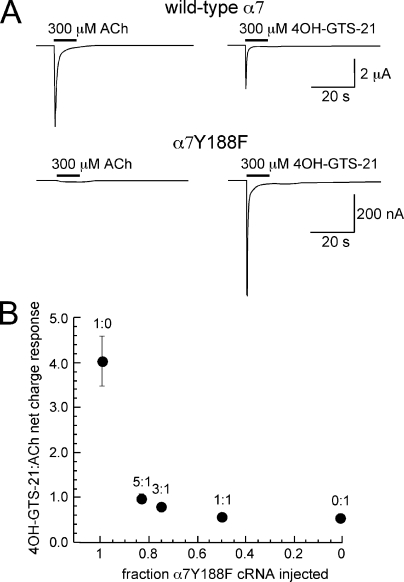
We have reported (Horenstein et al., 2007) a mutation in the primary face of the α7 ACh binding site (Y188F) that produces a 45-fold reduction in ACh potency (ACh EC50 shifted from 33 ± 4 µM for wild-type to 1,500 ± 164 µM for α7Y188F) without any significant effect on the potency of the α7-selective partial agonist 4OH-GTS-21 (4OH-GTS-21 EC50, 14 ± 1 µM for wild-type and 14 ± 2 µM for α7Y188F). As shown in Fig. 3 A, for wild-type α7, the ratio of the 300-µM 4OH-GTS-21–evoked net charge responses to 300-µM ACh-evoked responses was 0.57 ± 0.04. In contrast, for α7Y188F receptors, the ratio of the 300-µM 4OH-GTS-21–evoked responses to 300-µM ACh-evoked responses was 4.0 ± 0.6. We coexpressed ACh-sensitive wild-type α7 and ACh-insensitive α7Y188F together in Xenopus oocytes at varying ratios of RNA, similar to what was done with the L119C mutant (Fig. 2, B and C). The response evoked by 4OH-GTS-21 on α7Y188F receptors is consistent with efficient α7Y188F subunit expression and assembly in oocytes (Horenstein et al., 2007). The mutant and wild-type subunits responded alike to 300 µM 4OH-GTS-21, but wild-type subunits were required to generate responses to 300 µM ACh. However, if only single subunits are required to activate a receptor, we hypothesized that even when α7Y188F was injected at a 5:1 ratio to wild-type α7, the net charge responses to ACh should remain relatively high because 60% of the receptors would have at least one ACh-sensitive wild-type subunit. As shown in Fig. 3 B, the sensitivity of the wild-type receptor to ACh was retained well, even under the 5:1 injection condition, when it would be predicted that very few of the receptors would have more than one or two wild-type subunits. The shift in 4OH-GTS-21 to ACh response ratios was no more than would have been expected from the prediction that 40% of receptors would contain only ACh-insensitive subunits.
Figure 3.
Probing for an α7 C-loop mutation with selective and nonselective agonists. (A) Mutation of the α7 tyrosine 188 to phenylalanine reduces sensitivity to low concentrations of ACh, with little impact on sensitivity to the responses to the α7-selective agonist 4OH-GTS-21 (Horenstein et al., 2007). The upper traces are representative responses of oocytes expressing wild-type α7, for which 300 µM 4OH-GTS-21 evoked responses that are 57 ± 4% the magnitude of the responses evoked by 300 µM ACh, in net charge. In contrast, for oocytes expressing α7Y188F, 300-µM 4OH-GTS-21–evoked net charge responses that are 405 ± 55% of the magnitude of the responses evoked by 300 µM ACh. (B) Oocytes were injected with RNA for α7Y188F and wild-type α7 at (mutant/wild-type) 1:0, 5:1, 3:1, 1:1, and 0:1 ratios and then tested for their relative responses to 300 µM ACh and 300 µM 4OH-GTS-21. The values are plotted in relation to the fraction of α7Y188F RNA injected at each ratio and are the means ± SEM of at least four oocytes for every condition.
Effects of mutations homologous to α7L119C in non–α subunits of muscle-type receptors
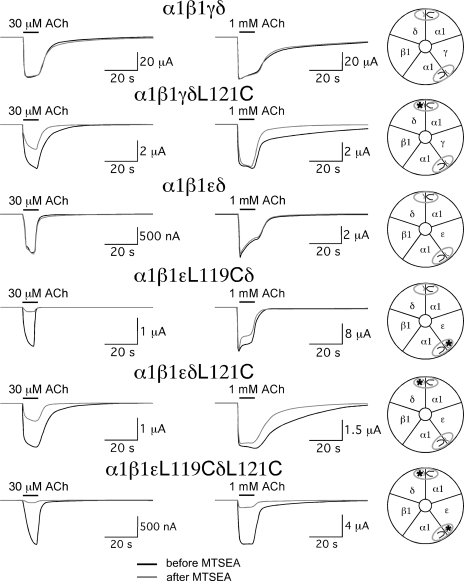
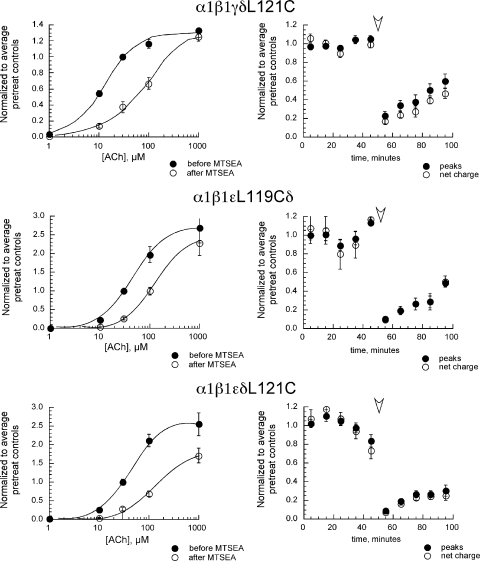
Because structural modeling of the homomeric α7 subunits places the L119 residue in the complementary face of the agonist binding site (Fig. 1 A), this site is expected to form the specialized domains corresponding to those of the non–α subunits in heteromeric nAChR. Consistent with this prediction, MTSEA treatment reduced responses to high (1-mM) and low (30-µM) ACh concentrations by >95% when the mutation homologous to α7L119C was placed in the β2 subunits of α4β2 and α3β2 receptors, whereas little MTSEA-dependent effects were observed when the homologous mutation was placed in the α subunits (Papke et al., 2011). Although the placement of the modifiable L119C residue in α7 receptors and the modifiable β2L121C in α4β2 and α3β2 receptors impacts all potential binding sites of these receptors and produces equally profound reduction in function after MTSEA treatment, the effect of the homologous mutation in the subunits of heteromeric muscle-type receptors would be expected to depend on the specific subunit(s) in which the mutation was placed because the α1 subunits are paired with different non–α subunits (δ and γ or ε), which contribute different complementary faces to the two agonist binding sites. Although MTSEA treatment produced no significant decreases in the ACh-evoked responses of wild-type α1β1εδ or α1β1γδ receptors across a range of ACh concentrations from 1 µM to 1 mM (see Fig. 4 and Table II), receptors with mutations in both ε and δ had large reductions in their responses to both low and high concentrations of ACh (Fig. 4). Specifically, peak current and net charge responses of the double mutants (α1β1εL119CδL121C) to 30 µM ACh were reduced 93 ± 1 and 96 ± 1%, respectively, and peak current and net charge responses to 1 mM ACh were reduced by 82 ± 6 and 91 ± 3%, respectively. However, if mutations were placed in only one of the two non–α subunits that contribute to agonist binding sites (α1β1γδL121C, α1β1εL119Cδ, or α1β1εδL121C), MTSEA treatment produced large decreases (P < 0.01) in the responses evoked by 30 µM ACh, with less effect on the 1-mM ACh-evoked peak current responses for either the α1β1γδL121C or α1β1εL119Cδ receptors. Although peak current responses to 1 mM ACh for the α1β1εδL121C receptors were decreased by MTSEA treatment (P < 0.01), the effect on 1-mM ACh responses (30 ± 7% decrease) was much less (P < 0.0001) than that on 30-µM ACh responses (74 ± 4% decrease). Note that although the peak amplitude of the responses evoked from α1β1γδL121C and α1β1εL119Cδ receptors by 1 mM was not decreased by the MTSEA treatment, the net charge values of the 1-mM ACh-evoked responses on all single-subunit mutants were affected because the MTSEA treatment resulted in currents with significantly (P < 0.05) faster 90–10% decay times. This would be consistent with a decreased ability of the treated oocytes to respond to the lower concentrations of ACh during the washout period.
Figure 4.
The effect of MTSEA on muscle-type receptors with mutations homologous to α7L119C in muscle δ, γ, and ε subunits. Representative responses obtained before MTSEA treatments are shown as black lines, and responses obtained after MTSEA are shown as gray lines. The schematics to the right of the traces represent the subunit composition and disposition of the ACh binding sites for the different receptor subtypes. The asterisks represent the location of the mutations in the complementary face of the agonist binding site.
Table II.
MTSEA effects on muscle mutants expressed in Xenopus oocytes
| Muscle mutant | Before MTSEA | After MTSEA | ||
| Imaxa | EC50 | Imaxa | EC50 | |
| µM | µM | |||
| α1β1γδL121C (n = 5) | 1.30 ± 0.05 | 12.7 ± 1.5 | 1.41 ± 0.03 | 111 ± 8 |
| α1β1εL119Cδ (n = 3) | 2.72 ± 0.08 | 48 ± 4 | 2.40 ± 0.02 | 126 ± 4 |
| α1β1εδL121C (n = 8) | 2.56 ± 0.01 | 39.1 ± 0.4 | 1.84 ± 0.04 | 145 ± 9 |
| α1β1γδ wild-type (n = 5) | 1.15 ± 0.03 | 4.52 ± 0.39 | 1.35 ± 0.05 | 3.24 ± 0.41 |
| α1β1εδ wild-type (n = 7) | 1.28 ± 0.003 | 16.1 ± 0.10 | 1.53 ± 0.004 | 22.0 ± 0.11 |
Measured relative to average 30-µM ACh control before MTSEA treatment.
The ACh concentration–response curves for the peak current responses of the muscle-type receptors containing MTSEA-sensitive mutations are shown in Fig. 5, and the fit Imax and EC50 values are shown in Table II. In addition to obtaining responses to varying concentrations of ACh, multiple responses to ACh at the control concentration of 30 µM were obtained from each cell before and after MTSEA. These are shown in the right-hand column of plots in Fig. 5. Note that there was some recovery in the size of the 30-µM control responses after the MTSEA treatments. Given that the MTSEA modification results in a covalent bond, reversibility of the effect seems unlikely. The response reversibility could have represented the insertion of new receptors during the course of the experiment. Muscle-type receptors express better than any other nAChR subtype, and oocytes must be studied immediately on the day after injection or else currents are too large for effective voltage clamping. Another possibility is that the receptors with one unmodified binding site show less of an effect of progressive desensitization with repeated agonist applications.
Figure 5.
ACh concentration–response data for muscle-type single-subunit mutants before and after MTSEA treatment. Oocytes were stimulated alternately with control applications of 30 µM ACh and ACh at increasing concentrations: 1 µM, 10 µM, 100 µM, and 1 mM (i.e., the sequence of applications was 30, 1, 30, 10, 30, 100, 30, 1,000, and 30). Next, the oocytes were treated with 2 mM MTSEA for 60 s before being tested with the same sequence of ACh applications. All of the data were normalized to the individual oocytes’ average responses to the five 30-µM ACh applications given before the MTSEA treatment. Therefore, the 30-µM point in the pretreatment data is fixed at 1, and the SEM plotted for that point is the average SEM of the five 30-µM responses obtained from each cell. The 30-µM point in the post-MTSEA curve is based on the responses to 30 µM ACh obtained between the posttreatment 10-µM ACh and 100-µM ACh applications. The plots on the right represent the repeated 30-µM ACh responses obtained through the course of the entire experiments, normalized to the average pre-MTSEA 30-µM ACh responses from each cell. The arrowhead indicates the point at which MTSEA was applied. The values plotted are the means ± SEM of five, three, and eight oocytes for α1β1γδL121C, α1β1εL119Cδ, and α1β1εδL121C, respectively. Fit parameters are listed in Table II.
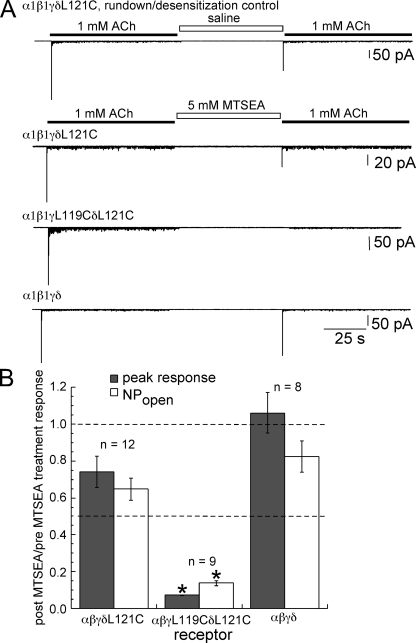
To further investigate the MTSEA-resistant activation of muscle-type receptors with one unperturbed binding site, we performed single-channel patch clamp experiments with wild-type and mutant α1β1γδ receptors transiently transfected in mammalian BOSC 23 cells. We focused on α1β1γδL121C because the fetal receptor is the subject of extensive literature as a result of its expression in BC3H1 cells (see for example, Sine and Steinbach, 1984a,b, 1987; Papke et al., 1988; Papke and Oswald, 1989). Two main types of analyses were performed on the single-channel data: (1) comparison of post-MTSEA treatment peak current and NPopen measurements with the pre-MTSEA measurements obtained from the same patch in response to 1 mM ACh; and (2) fitting of burst-duration histograms from untreated and treated receptors.
At a holding potential of −70 mV, the single-channel amplitudes before and after MTSEA treatment (5 mM for 60 s) of wild-type receptors were −2.71 ± 0.02 pA and −2.69 ± 0.01 pA, respectively, of α1β1γδL121C-mutant receptors were −2.64 ± 0.05 pA and −2.63 ± 0.03 pA, respectively, and of α1β1γL119CδL121C double-mutant receptors were −2.74 ± 0.05 pA and −2.70 ± 0.06 pA, respectively. The single-channel amplitude with 10-nM ACh concentration at −70 mV was −3.13 ± 0.02 pA. The smaller apparent single-channel amplitude observed with high concentrations of ACh would be consistent with an effect of brief episodes of channel block by agonist, limiting the detection of full amplitude events (Sine and Steinbach, 1984b). The single-channel slope conductance of α1β1γδL121C receptors was 35.5 ± 1.2 pS, and the reversal potential was −4.4 ± 2.0 mV (−80 to 80 mV; n = 3; not depicted). These values are in agreement with previously published studies on fetal muscle-type nAChR (Mishina et al., 1986; Schuetze and Role, 1987; Jaramillo and Schuetze, 1988).
Fig. 6 A shows traces from outside-out patches under the experimental protocol, which consisted of an ∼80-s initial ACh application, followed by either 5 mM MTSEA or external saline application for 60 s, and ended with a follow-up ACh application. External saline instead of 5 mM MTSEA was applied to some patches expressing α1β1γδL121C to allow channel rundown and/or desensitization that may occur independently of any MTSEA-dependent effects to be measured. Fig. 6 B summarizes the post-MTSEA treatment versus pre-MTSEA comparisons of both transient peak currents and NPopen with 1 mM ACh, normalized to the average values from eight rundown/desensitization control patches. Despite consistent experimental setup, the patch-to-patch variability of the peak current and NPopen measurements was higher than expected for unknown reasons that probably reflect the unstable and fragile nature of outside-out patches. No attempt was made to identify or eliminate outliers. There were no correlations between post-/pre-MTSEA treatment peak and NPopen measurements and transfection dates, recording dates, lower limit of N in the patch, single-channel amplitudes, or 10–90% rise times. The non-normalized average, standard error, range, and median of values from at least eight replicates of each condition, including the rundown controls, are reported in Table III. Peak and NPopen measurements of wild-type receptors were least affected by MTSEA treatment (5 mM for 60 s), with post-/pre-MTSEA treatment values of 1.1 ± 0.1 and 0.83 ± 0.09, respectively. Receptors with one MTSEA-sensitive binding site had post-/pretreatment peak current and NPopen values of 0.74 ± 0.08 and 0.65 ± 0.06, respectively. There was a much greater reduction in both peak and NPopen values when receptors contained two MTSEA-sensitive binding sites, with post-/pretreatment values of 0.072 ± 0.003 and 0.14 ± 0.02, respectively.
Figure 6.
The effect of MTSEA treatment on peak and NPopen responses from receptors expressed in BOSC 23 cells. (A) Example traces of outside-out patches from each condition. A rapid (≤0.7-ms) drug application system was used to apply ACh and MTSEA. (B) Summary of the effect of MTSEA treatment (5 mM for 60 s) on peak current and NPopen responses to 1 mM ACh shown as the average of post-MTSEA measurements relative to the pre-MTSEA measurements of each patch. The measurements are normalized to the average peak current and NPopen responses from eight rundown/desensitization control patches. Asterisks above the values for α1β1γL119CδL121C indicate statistical significance (P < 0.01) when compared with values from either α1β1γδL121C or wild-type. See Table III for values.
Table III.
Peak current and NPopen measurements from the outside-out patch clamp experimentsa
| Statistic | α1β1γδL121C (control) (n = 8) | α1β1γδL121C (n = 12) | α1β1γL119CδL121C (n = 9) | α1β1γδ (n = 8) |
| Average post-/pre- NPopenb | 0.44 (1) | 0.28 (0.65) | 0.060 (0.14) | 0.36 (0.83) |
| SEM | 0.13 | 0.059 | 0.015 | 0.085 |
| Range | 0.078–1.13 | 0.066–0.69 | 0.0088–0.11 | 0.14–0.65 |
| Median | 0.38 | 0.25 | 0.066 | 0.36 |
| Average post-/pre- peakb | 0.41 (1) | 0.31 (0.74) | 0.030 (0.073) | 0.44 (1.06) |
| SEM | 0.081 | 0.084 | 0.0027 | 0.11 |
| Range | 0.11–0.74 | 0.051–1.10 | 0.020–0.045 | 0.14–0.78 |
| Median | 0.42 | 0.23 | 0.026 | 0.44 |
1 mM ACh applied before and after 5-mM MTSEA treatment.
Normalized NPopen and peak values are indicated in parentheses.
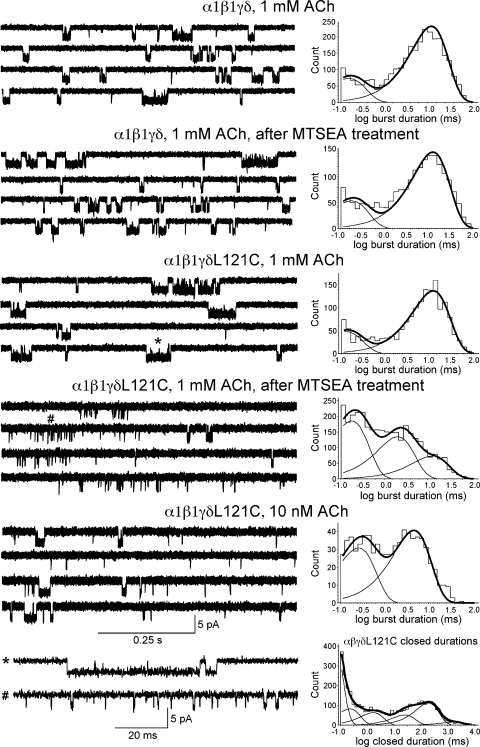
Fig. 7 presents single-channel currents obtained from wild-type and α1β1γδL121C receptors at 1 mM ACh before and after MTSEA treatment, responses from untreated α1β1γδL121C receptors at 10 nM ACh, and fit burst–duration histograms corresponding to each condition. The fit time constants are listed in Table IV. Burst durations, rather than apparent open duration histograms, are shown because open channel times are greatly affected by bandwidth limitations, such as missed brief closed intervals and ambiguities associated with idealization, whereas burst durations are much less affected by these potential confounders and are, therefore, a more reliable measure of channel opening behavior. The burst durations of wild-type α1β1γδ receptors were unaffected by MTSEA treatment, whereas the burst durations of α1β1γδL121C receptors were not different from wild-type receptors until treated with MTSEA. The number of components required to generate the best fit of the α1β1γδL121C burst-duration histogram after MTSEA increased from two to three, and most bursts consisted of brief, isolated openings, but some longer bursts remained. The proportion of total bursts of duration less than ∼2 ms increased from 0.26 ± 0.04 before MTSEA treatment to 0.81 ± 0.03 after MTSEA treatment. The longest time constant after MTSEA treatment was equivalent to the long time constant measured on nontreated patches (12.6 ± 0.2 ms vs. 12.9 ± 0.05 ms). The frequency (bursts/s) of bursts appeared to increase after MTSEA treatment by a factor of 2.78 ± 0.52 (1.88 ± 0.35 without correction for rundown). Recordings from αβγδL121 mutants were also obtained without MTSEA treatment at low ACh concentrations (10 nM), where many of the observed openings were likely to arise from singly occupied receptors (Colquhoun and Sakmann, 1981, 1985). The proportion of bursts corresponding to the brief time constant (0.26 ± 0.07 ms) was 0.43 ± 0.02, and the long time constant at 10 nM ACh was more brief than the long time constant at 1 mM ACh (4.66 ± 0.04 ms vs. 12.9 ± 0.05 ms).
Figure 7.
Single-channel traces and fit burst–duration histograms from wild-type α1β1γδ and α1β1γδL121C receptors before and after MTSEA treatment (5 mM for 60 s) as indicated. Bursts from α1β1γδL121C receptors before and after MTSEA treatment (indicated by * or #) are shown on the bottom row in higher time resolution together with the closed-duration histogram from α1β1γδL121C (before MTSEA treatment) used to define bursts. Currents were sampled at 100 kHz and ultimately low-pass filtered to 5 kHz. Each histogram represents the data pooled from at least individual four patches recorded under identical conditions, except for the 10-nM ACh concentration histogram, where data were pooled from three patches. Fit parameters are listed in Table IV.
Table IV.
Fit time constants from the burst-duration histogramsa
| Receptor; condition | τ1 ± SEM | P1 ± SEM | τ2 ± SEM | P2 ± SEM | τ3 ± SEM | P3 ± SEM |
| α1β1γδ; 1 mM ACh | 0.15 ± 0.17 | 0.24 ± 0.03 | 12.2 ± 0.04 | 0.76 ± 0.02 | — | — |
| α1β1γδ after MTSEA; 1 mM ACh | 0.18 ± 0.15 | 0.26 ± 0.03 | 13.3 ± 0.04 | 0.74 ± 0.02 | — | — |
| α1β1γδL121C; 1 mM ACh | 0.13 ± 0.2 | 0.26 ± 0.04 | 12.9 ± 0.05 | 0.74 ± 0.02 | — | — |
| α1β1γδL121C after MTSEA; 1 mM ACh | 0.18 ± 0.08 | 0.47 ± 0.02 | 2.02 ± 0.12 | 0.34 ± 0.03 | 12.6 ± 0.2 | 0.19 ± 0.03 |
| α1β1γδL121C; 10 nM ACh | 0.26 ± 0.07 | 0.43 ± 0.02 | 4.66 ± 0.04 | 0.57 ± 0.02 | — | — |
τ values indicated in milliseconds, and p-values indicate the fraction of total events estimated from histogram fit.
DISCUSSION
Other studies have investigated the relationship between available binding sites and agonist-evoked responses in muscle-type receptors (Sine and Taylor, 1980; Jha and Auerbach, 2010) and in an α7/5-HT3 chimeric receptor (Rayes et al., 2009). Sine and Taylor (1980) estimated the fractional blockade of binding sites with α-cobratoxin and then measured ion flux in toxin-bound vesicles from BC3H1 cells (fetal receptor). The conclusion was that only receptors with two free agonist binding sites could be activated. However, Groebe et al. (1995) reported that the application of 500 nM α-conotoxin M1 to BC3H1 cells almost completely inhibits agonist-evoked responses, even though the 500-nM concentration of α-conotoxin M1 theoretically results in <3% occupation of the low-affinity binding site (α-γ) by the toxin on the BC3H1 nAChR (Groebe et al., 1995). In addition, Hansen et al. (2005) solved crystal structures for three states in the agonist binding site of the ACh binding protein, one of which was not part of the minimal model for the nAChR (closed, open, and desensitized states). This state was induced by the binding of α-cobratoxin, but not by the binding of small competitive antagonists like methyllycaconitine. In this state, the C-loop extended in the opposite direction from which it presumably moves during the activation process (Hansen et al., 2005). Therefore, it is possible that in the original Sine and Taylor (1980) experiments, the large conformational change produced by the binding of just one α-cobratoxin molecule was sufficient to render the entire channel unable to gate. Jha and Auerbach (2010) used mutations at position αW149 in adult receptors to produce binding sites with reduced sensitivity to ACh, with the conclusions being that a single agonist binding site can activate the receptor, but with much less efficiency than two binding sites (equilibrium gating constants: E1ACh ≅ 4.3 × 10−3 vs. E2ACh ≅ 28). However, although expression of αW149 mutant subunits resulted in receptors with two mutant binding sites and greatly reduced responses to ACh, the coexpression of αW149-mutant subunits with wild-type α subunits to produce receptors with single functional binding sites resulted in mixed populations of receptors consisting of all wild-type subunits, single αW149-mutant subunits, and two αW149-mutant subunits. Rayes et al. (2009) used an α7/5-HT3 receptor chimera with subunits containing α7Y190T and/or α7W55T mutations to reduce ACh sensitivity. In addition, the mutant subunits contained reporter mutations in the 5-HT3 sequence that altered unitary channel conductance so that receptor subunit combinations could be identified. The conclusions of this study were that α7/5-HT3 chimeric receptors activated partially when receptors contained fewer than three wild-type subunits or when the three wild-type subunits were located at adjacent subunit interfaces. In addition, the authors concluded that receptors with fewer wild-type binding sites experienced less desensitization after strong stimulation by ACh. Unfortunately, the α7/5-HT3 chimera is a “man-made” receptor with a much higher open probability than native α7 nAChR, and so it is unclear to what extent the conclusions of this study may be applied to α7. Although our approach is not without its own limitations, it provides the significant strength over the loss-of-function mutation approach that we can record responses from the same receptor population before and after binding site modification. Notwithstanding the Sine and Taylor (1980) experiments, the general consensus from early single-channel recordings of muscle-type receptors (see below) and experiments using loss-of-function mutations suggests that a single-agonist binding site is sufficient to open the nAChR channel, but that openings from a single binding site have a low Popen relative to that of openings arising from multiple binding sites. Our results are not inconsistent with this observation, given that responses to ACh were reduced at low concentrations after binding site modification.
Early single-channel studies of muscle-type nAChR noted that the component of brief openings at low agonist concentrations behaved as if it arose from mono-liganded receptors. The ratio of total events corresponding to brief, isolated openings (fast time constant) versus longer-lived openings (slow time constant) that were attributed to di-liganded receptors was relatively high at low agonist concentrations, but decreased as agonist concentrations increased (Colquhoun and Sakmann, 1981, 1985; Takeda and Trautmann, 1984; Labarca et al., 1985; Jaramillo and Schuetze, 1988; Papke and Oswald, 1989). Interestingly, however, a component of short-lived openings persisted even at high agonist concentrations, accounting for ∼10% of all apparent channel openings (Colquhoun and Sakmann, 1985; Jaramillo and Schuetze, 1988). Before performing the patch clamp experiments, we hypothesized that only brief, isolated openings would be observed after MTSEA treatment of δL121C receptors, with the frequency of such events increasing as agonist concentration increased. The majority (81%) of bursts were indeed brief (≤2 ms); however, some longer bursts (∼12.6 ms) were also observed. This observation, together with previous observations that brief, isolated single-channel openings persist at high agonist concentrations (Colquhoun and Sakmann, 1985), suggests the interesting possibility that both short- and longer-lived channel activations may arise from either mono- or di-liganded receptors, with a single-liganded binding site opening with greatest probability to the shorter-lived opening, and the di-liganded receptor opening to the longer-lived open state with greater probability. In general, our data support the interpretation that conversion to the desensitized state occurs as in relation to the total time spent in the open state. Individual openings from singly liganded receptors may with higher likelihood be brief; nonetheless, if entry into desensitized states depends strictly on time in the open state, such receptors would generate the same net amount of current before desensitizing as di-liganded receptors. This hypothesis, that entry of receptors into the desensitized state depends strictly on time in the open state, is consistent with classical Markov models used to describe ion channel behavior (Katz and Thesleff, 1957; Sakmann et al., 1985; Sine and Steinbach, 1986).
Ideally, MTSEA modification occurred to completion at all “L119C” sites, producing binding sites with no affinity for ACh, and with no effect on channel gating itself. The observation that some currents were recorded after MTSEA was applied to the α1β1γL121CδL119C double mutants in both two-electrode voltage clamp and patch clamp experiments is problematic. Does the MTSEA modification only partially reduce the binding site sensitivity to ACh, or were some receptors left unmodified? Unfortunately, neither question is straightforward to answer. In a study evaluating residues that contribute to α-btx binding in muscle-type receptors, Sine (1997) demonstrated that [2-(trimethylammonium)ethyl] methanethiosulfonate (MTSET) modification of receptors containing δL121C, γL119C, or εL119C residues resulted in a 50% decrease in α-btx binding, as would be expected if the receptors contained only one modified binding site and modification completely prevented α-btx binding. Furthermore, analysis of the binding site–selective antagonists dimethyl-d-tubocurarine and α-conotoxin M1 confirmed that the effect was a result of specific modification at the selected binding site. Electrostatic repulsion, rather than effects on channel conformation, was hypothesized to be responsible for the disruption of α-btx binding after MTSET modification (Sine, 1997). In addition, a recently constructed homology model of MTSET-modified α7L119C suggests that the modification places a hard positive charge in close proximity to where ACh is expected to bind. Because MTSET differs from MTSEA only in the substitution of methyl groups for hydrogens on the quaternary nitrogen of the sulfhydryl reagent, the model for MTSEA-modified receptors is expected to be equivalent (Papke et al., 2011). From these observations, and given that ACh contains a positively charged ammonium group, it seems most likely that the residual current by the double mutants was a result of a small fraction of incompletely modified receptors. Could the modification have spared some or all of the binding site affinity for ACh, primarily affecting the ability of the binding site to initiate the gating cascade once ACh bound? Protocols using α7C116S/L119C mutants, MTSEA, and the positive allosteric modulator N-(5-chloro-2,4-dimethoxy phenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596), which converts some desensitized states into conducting states, suggest that MTSEA modification may stabilize mutant α7 receptors in PNU-120596–sensitive desensitized states because the application of PNU-120596 alone after MTSEA modification produces activation. Importantly, MTSEA-modified receptors remain insensitive to ACh, even after they have been primed by the powerful allosteric modulator PNU-120596 (Wang et al., 2010). In addition, the conversion of MTSEA-modified α7L119C-mutant receptors into the PNU-sensitive desensitized state only occurs when all five subunits contain the L119C mutation (unpublished data), suggesting that even though other binding sites may be in a desensitized state as a result of MTSEA modification, the receptor retains functionality with one or two unperturbed binding sites. However, although the receptor modeling and data favor the position that ACh is excluded from the ligand binding site, the modification by MTSEA could conceivably mimic a permanently bound weak partial agonist, potentially increasing the ability of the unmodified binding site(s) to activate more readily upon binding of the full agonist ACh.
Because the proportion of the total synaptic current from mono-liganded muscle-type receptors under normal physiological conditions is likely negligible, our data are arguably most interesting in their possible application to neuronal nAChRs in the brain, where evidence for nicotinic synaptic transmission is slim and agonist concentrations are expected to be low. In fact, some have wondered whether nAChRs in the brain even see ACh at all (Sivilotti and Colquhoun, 1995). Our data show that Xenopus oocytes injected with a high percentage of α7L119C-mutant RNA, and therefore likely to have a reduced number of available agonist binding sites after MTSEA treatments, can give responses to high agonist concentrations after MTSEA treatment that are comparable to the responses of receptors with all binding sites intact obtained at lower concentrations of agonist. Likewise, combinations of wild-type and α7Y188F receptors likely to have very few ACh-sensitive wild-type subunits nonetheless respond well to ACh.
If submaximal occupancy by agonist is sufficient to activate heteromeric and homomeric receptors, what is the role of the additional binding sites? Especially in the case of α7, the functional consequence of having multiple ACh binding sites may be sensitivity to low levels of agonist rather than to require high levels of occupancy for activation. High levels of agonist occupancy only appear to promote desensitization, or at least to provide sufficient activation during the process of achieving high levels of agonist site occupancy for the receptors to become desensitized. When challenged with a high concentration of agonist, α7 receptors open with highest probability during the leading edge of the solution exchange, when only a fraction of the agonist binding sites would be occupied (Papke and Thinschmidt, 1998; Papke et al., 2000; Papke and Porter Papke, 2002; Uteshev et al., 2002, 2003), after which the receptors are predominantly closed or desensitized. This is true whether recordings are made on a slow time scale in oocytes (Papke and Thinschmidt, 1998) or on a rapid time scale with dissociated neurons (Uteshev et al., 2002), and this is so pronounced that with our normal experimental protocol, the time-integrated (i.e., net charge) response to 3 mM ACh shows no significant increase over that evoked by 100 µM ACh. The rapid desensitization of α7 may be a protective mechanism against cytotoxicity induced by excess entry of calcium. We also hypothesize that although the desensitized state is nonconducting, channel desensitization itself may provide another dimension of neuronal nAChR-mediated transduction of cellular signals (Suzuki et al., 2006; de Jonge and Ulloa, 2007) of particular importance for α7.
The nature of the desensitized state appears to be rather different for heteromeric and homomeric receptors. For heteromeric receptors, desensitization is associated with an approximately thousand-fold increase in the apparent affinity of the agonist binding site for ACh, whereas in α7 receptors there does not appear to be any more than a 10-fold increase in agonist affinity with desensitization (Uteshev et al., 2002). The conversion of heteromeric receptors to a high-affinity desensitized state means they will be likely to retain agonist at the binding sites or rebind agonist even as agonist concentration decreases. This may have the effect of stabilizing the desensitized state and slowing recovery. In contrast, agonist will readily dissociate from homomeric α7 receptors, so that in the presence of low concentrations of agonist, the receptors may easily cycle between activation and desensitization, and generate a significant time-integrated calcium signal over a prolonged period of time. This modality of prolonged stimulation by low levels of agonist has been shown to be what is required to achieve cyto-protective effects via α7 receptors (Li et al., 1999) and is probably important for other roles played by α7 receptors in the brain (Uteshev et al., 2002) and in non-neuronal tissues (Wang et al., 2003). Therefore, because it is likely that in vivo α7 receptors are tonically exposed to low level stimulation (via choline and diffuse release of ACh), receptor activation, based on partial occupancy of the multiple binding sites, may be an important functional modality mediating the cyto-protective and perhaps also the cognitive effects documented for α7-selective agonists.
Acknowledgments
We thank Lisa Jacobs, Jenny Hughes, Chad Brodbeck, Ke Ren, and Sara Braley for technical assistance. We thank Drs. Edwin Meyer, Robert Oswald, Joe Henry Steinbach, Stephen Baker, Jan Torleif Pedersen, and Jan Egebjerg for helpful comments, and Dr. Steven Sine for providing the wild-type muscle receptor cDNAs subcloned in pRBG4.
This work was supported by National Institutes of Health grants RO1-GM057481 and T32-AG000196.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- 4OH-GTS-21
- 3-(4-hydroxy, 2-methoxybenzylidene) anabaseine
- α-btx
- α-bungarotoxin
- ACh
- acetylcholine
- MTSEA
- 2-aminoethyl methanethiosulfonate
- MTSET
- [2-(trimethylammonium)ethyl] methanethiosulfonate
- nAChR
- nicotinic ACh receptor
- PNU-120596
- N-(5-chloro-2,4-dimethoxy phenyl)-N′-(5-methyl-3-isoxazolyl)-urea
References
- Amin J., Weiss D.S. 1996. Insights into the activation mechanism of rho1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc. Biol. Sci. 263:273–282 10.1098/rspb.1996.0042 [DOI] [PubMed] [Google Scholar]
- Barron S.C., McLaughlin J.T., See J.A., Richards V.L., Rosenberg R.L. 2009. An allosteric modulator of alpha7 nicotinic receptors, N-(5-Chloro-2,4-dimethoxyphenyl)-N’-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol. Pharmacol. 76:253–263 10.1124/mol.109.056226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M., Groot-Kormelink P.J., Colquhoun D., Sivilotti L.G. 2004. The activation mechanism of alpha1 homomeric glycine receptors. J. Neurosci. 24:895–906 10.1523/JNEUROSCI.4420-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K., van Dijk W.J., Klaassen R.V., Schuurmans M., van Der Oost J., Smit A.B., Sixma T.K. 2001. Crystal structure of an Ach-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 411:269–276 [DOI] [PubMed] [Google Scholar]
- Celie P.H., van Rossum-Fikkert S.E., van Dijk W.J., Brejc K., Smit A.B., Sixma T.K. 2004. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 41:907–914 10.1016/S0896-6273(04)00115-1 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. 1981. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 294:464–466 10.1038/294464a0 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. 1985. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J. Physiol. 369:501–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. 1995. Fitting and statistical analysis of single-channel records. Single-Channel Recording. Sakmann B., Neher E., Plenum Press, New York: 483–585 [Google Scholar]
- Dani J.A., Bertrand D. 2007. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47:699–729 10.1146/annurev.pharmtox.47.120505.105214 [DOI] [PubMed] [Google Scholar]
- de Jonge W.J., Ulloa L. 2007. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 151:915–929 10.1038/sj.bjp.0707264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Gisiger V., Steriade M. 1997. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 53:603–625 10.1016/S0301-0082(97)00050-6 [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. 1987. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci. Lett. 77:199–204 10.1016/0304-3940(87)90586-6 [DOI] [PubMed] [Google Scholar]
- Groebe D.R., Dumm J.M., Levitan E.S., Abramson S.N. 1995. alpha-Conotoxins selectively inhibit one of the two acetylcholine binding sites of nicotinic receptors. Mol. Pharmacol. 48:105–111 [PubMed] [Google Scholar]
- Guex N., Peitsch M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 18:2714–2723 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- Hansen S.B., Sulzenbacher G., Huxford T., Marchot P., Taylor P., Bourne Y. 2005. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24:3635–3646 10.1038/sj.emboj.7600828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein N.A., McCormack T.J., Stokes C., Ren K., Papke R.L. 2007. Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. J. Biol. Chem. 282:5899–5909 10.1074/jbc.M609202200 [DOI] [PubMed] [Google Scholar]
- Jackson M.B. 1984. Spontaneous openings of the acetylcholine receptor channel. Proc. Natl. Acad. Sci. USA. 81:3901–3904 10.1073/pnas.81.12.3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.B. 1986. Kinetics of unliganded acetylcholine receptor channel gating. Biophys. J. 49:663–672 10.1016/S0006-3495(86)83693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.B. 1998. Single-channel recording. Curr. Protoc. Neurosci. 6.8:1–32 [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S.M. 1988. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J. Physiol. 396:267–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A., Auerbach A. 2010. Acetylcholine receptor channels activated by a single agonist molecule. Biophys. J. 98:1840–1846 10.1016/j.bpj.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P. 1995. Fast application of agonists to isolated membrane patches. Single-Channel Recording. Sakmann B., Neher E., Plenum Press, New York: 231–243 [Google Scholar]
- Jope R.S., Gu X. 1991. Seizures increase acetylcholine and choline concentrations in rat brain regions. Neurochem. Res. 16:1219–1226 10.1007/BF00966699 [DOI] [PubMed] [Google Scholar]
- Kabakov A.Y., Papke R.L. 1998. Ultra fast solution applications for prolonged gap-free recordings: controlling a Burleigh piezo-electric positioner with Clampex7. Axobits. January:6–9 [Google Scholar]
- Karlin A., Cowburn D.A. 1973. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc. Natl. Acad. Sci. USA. 70:3636–3640 10.1073/pnas.70.12.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Thesleff S. 1957. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 138:63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Montal M.S., Lindstrom J.M., Montal M. 1985. The occurrence of long openings in the purified cholinergic receptor channel increases with acetylcholine concentration. J. Neurosci. 5:3409–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Papke R.L., He Y.-J., Millard W.J., Meyer E.M. 1999. Characterization of the neuroprotective and toxic effects of α7 nicotinic receptor activation in PC12 cells. Brain Res. 830:218–225 10.1016/S0006-8993(99)01372-4 [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. 1986. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 321:406–411 10.1038/321406a0 [DOI] [PubMed] [Google Scholar]
- Mortensen M., Smart T.G. 2007. Single-channel recording of ligand-gated ion channels. Nat. Protoc. 2:2826–2841 10.1038/nprot.2007.403 [DOI] [PubMed] [Google Scholar]
- Mott D.D., Erreger K., Banke T.G., Traynelis S.F. 2001. Open probability of homomeric murine 5-HT3A serotonin receptors depends on subunit occupancy. J. Physiol. 535:427–443 10.1111/j.1469-7793.2001.00427.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numa S., Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Kikyotani S. 1983. Molecular structure of the nicotinic acetylcholine receptor. Cold Spring Harb. Symp. Quant. Biol. 48:57–69 [DOI] [PubMed] [Google Scholar]
- Palma E., Bertrand S., Binzoni T., Bertrand D. 1996. Neuronal nicotinic alpha 7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J. Physiol. 491:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R.L., Oswald R.E. 1989. Mechanisms of noncompetitive inhibition of acetylcholine-induced single-channel currents. J. Gen. Physiol. 93:785–811 10.1085/jgp.93.5.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R.L., Porter Papke J.K. 2002. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 137:49–61 10.1038/sj.bjp.0704833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R.L., Thinschmidt J.S. 1998. The correction of alpha7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci. Lett. 256:163–166 10.1016/S0304-3940(98)00786-1 [DOI] [PubMed] [Google Scholar]
- Papke R.L., Millhauser G., Lieberman Z., Oswald R.E. 1988. Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys. J. 53:1–10 10.1016/S0006-3495(88)83059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R.L., Bencherif M., Lippiello P. 1996. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α 7 subtype. Neurosci. Lett. 213:201–204 [DOI] [PubMed] [Google Scholar]
- Papke R.L., Meyer E., Nutter T., Uteshev V.V. 2000. alpha7 receptor-selective agonists and modes of alpha7 receptor activation. Eur. J. Pharmacol. 393:179–195 10.1016/S0014-2999(00)00009-1 [DOI] [PubMed] [Google Scholar]
- Papke R.L., Stokes C., Williams D.K., Wang J., Horenstein N.A. 2011. Cysteine accessibility analysis of the human alpha7 nicotinic acetylcholine receptor ligand-binding domain identifies L119 as a gatekeeper. Neuropharmacology. 60:159–171 10.1016/j.neuropharm.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press W.H. 1988. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press, Cambridge, UK: 768 pp [Google Scholar]
- Rayes D., De Rosa M.J., Sine S.M., Bouzat C. 2009. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J. Neurosci. 29:6022–6032 10.1523/JNEUROSCI.0627-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Methfessel C., Mishina M., Takahashi T., Takai T., Kurasaki M., Fukuda K., Numa S. 1985. Role of acetylcholine receptor subunits in gating of the channel. Nature. 318:538–543 10.1038/318538a0 [DOI] [PubMed] [Google Scholar]
- Schuetze S.M., Role L.W. 1987. Developmental regulation of nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 10:403–457 10.1146/annurev.ne.10.030187.002155 [DOI] [PubMed] [Google Scholar]
- Scremin O.U., Jenden D.J. 1991. Time-dependent changes in cerebral choline and acetylcholine induced by transient global ischemia in rats. Stroke. 22:643–647 [DOI] [PubMed] [Google Scholar]
- Sine S.M. 1997. Identification of equivalent residues in the gamma, delta, and epsilon subunits of the nicotinic receptor that contribute to alpha-bungarotoxin binding. J. Biol. Chem. 272:23521–23527 10.1074/jbc.272.38.23521 [DOI] [PubMed] [Google Scholar]
- Sine S.M. 2002. The nicotinic receptor ligand binding domain. J. Neurobiol. 53:431–446 10.1002/neu.10139 [DOI] [PubMed] [Google Scholar]
- Sine S.M., Steinbach J.H. 1984a. Activation of a nicotinic acetylcholine receptor. Biophys. J. 45:175–185 10.1016/S0006-3495(84)84146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S.M., Steinbach J.H. 1984b. Agonists block currents through acetylcholine receptor channels. Biophys. J. 46:277–283 10.1016/S0006-3495(84)84022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S.M., Steinbach J.H. 1986. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J. Physiol. 373:129–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S.M., Steinbach J.H. 1987. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J. Physiol. 385:325–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S.M., Taylor P. 1980. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J. Biol. Chem. 255:10144–10156 [PubMed] [Google Scholar]
- Sivilotti L., Colquhoun D. 1995. Acetylcholine receptors: too many channels, too few functions. Science. 269:1681–1682 10.1126/science.7569892 [DOI] [PubMed] [Google Scholar]
- Stokes C., Papke J.K.P., Horenstein N.A., Kem W.R., McCormack T.J., Papke R.L. 2004. The structural basis for GTS-21 selectivity between human and rat nicotinic alpha7 receptors. Mol. Pharmacol. 66:14–24 10.1124/mol.66.1.14 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hide I., Matsubara A., Hama C., Harada K., Miyano K., Andrä M., Matsubayashi H., Sakai N., Kohsaka S., et al. 2006. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 83:1461–1470 10.1002/jnr.20850 [DOI] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. 1984. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J. Physiol. 349:353–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev V.V., Meyer E.M., Papke R.L. 2002. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 948:33–46 10.1016/S0006-8993(02)02946-3 [DOI] [PubMed] [Google Scholar]
- Uteshev V.V., Meyer E.M., Papke R.L. 2003. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. J. Neurophysiol. 89:1797–1806 10.1152/jn.00943.2002 [DOI] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., et al. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 421:384–388 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang J., Horenstein N.A., Stokes C., Papke R.L. 2010. Tethered agonist analogs as site-specific probes for domains of the human α7 nicotinic acetylcholine receptor that differentially regulate activation and desensitization. Mol. Pharmacol. 78:1012–1025 10.1124/mol.110.066662 [DOI] [PMC free article] [PubMed] [Google Scholar]