Abstract
Aims
To evaluate the dose–response relationship of lixisenatide (AVE0010), a glucagon-like peptide-1 (GLP-1) receptor agonist, in metformin-treated patients with Type 2 diabetes.
Methods
Randomized, double-blind, placebo-controlled, parallel-group, 13 week study of 542 patients with Type 2 diabetes inadequately controlled [glycated haemoglobin (HbA1c) ≥ 7.0 and < 9.0% (≥ 53 and < 75 mmol/mol)] on metformin (≥ 1000 mg/day) treated with subcutaneous lixisenatide doses of 5, 10, 20 or 30 μg once daily or twice daily or placebo. The primary end-point was change in HbA1c from baseline to 13 weeks in the intent-to-treat population.
Results
Lixisenatide significantly improved mean HbA1c from a baseline of 7.55% (59.0 mmol/mol); respective mean reductions for 5, 10, 20 and 30 μg doses were 0.47, 0.50, 0.69 and 0.76% (5.1, 5.5, 7.5 and 8.3 mmol/mol), on once-daily and 0.65, 0.78, 0.75 and 0.87% (7.1, 8.5, 8.2 and 9.5 mmol/mol) on twice-daily administrations vs. 0.18% (2.0 mmol/mol) with placebo (all P< 0.01 vs. placebo). Target HbA1c < 7.0% (53 mmol/mol) at study end was achieved in 68% of patients receiving 20 and 30 μg once-daily lixisenatide vs. 32% receiving placebo (P< 0.0001). Dose-dependent improvements were observed for fasting, postprandial and average self-monitored seven-point blood glucose levels. Weight changes ranged from −2.0 to −3.9 kg with lixisenatide vs. −1.9 kg with placebo. The most frequent adverse event was mild-to-moderate nausea.
Conclusions
Lixisenatide significantly improved glycaemic control in mildly hyperglycaemic patients with Type 2 diabetes on metformin. Dose–response relationships were seen for once- and twice-daily regimens, with similar efficacy levels, with a 20 μg once-daily dose of lixisenatide demonstrating the best efficacy-to-tolerability ratio. This new, once-daily GLP-1 receptor agonist shows promise in the management of Type 2 diabetes to be defined further by ongoing long-term studies.
Keywords: glucagon-like peptide-1 receptor agonist, glycaemic control, lixisenatide, Type 2 diabetes
Introduction
Glycaemic control in Type 2 diabetes mellitus is generally targeted toward a glycated haemoglobin (HbA1c) level as close to normal [i.e. < 6.5 or < 7.0% (< 48 or < 53 mmol/mol)] as safely as possible [1,2]. Although a variety of pharmacological approaches are now available, current management often fails to achieve glycaemic targets [3]. Analogues of the hormone glucagon-like peptide-1 (GLP-1) have shown promise as therapeutic options in Type 2 diabetes. Endogenous GLP-1 enhances insulin secretion and inhibits postprandial glucagon secretion in a glucose-dependent fashion, slows gastric emptying, reduces food intake and promotes weight loss, with all these effects matched by GLP-1 receptor agonists [4,5]. The suppression of glucagon by GLP-1 does not occur at hypoglycaemic glucose levels, and as such exogenous GLP-1 does not impair the physiological mechanisms that counteract hypoglycaemia [6].
Pharmacological replacement with GLP-1 receptor agonists in patients with Type 2 diabetes represents an attractive strategy to improve metabolic control, particularly as we aim for near-normal glycaemia, with less risk of hypoglycaemia and no weight gain or, ideally, weight loss. In addition to improving glycaemic control, GLP-1 receptor agonists have the potential to preserve pancreatic islet B cells by enhancing proliferation and inhibiting apoptosis, based on preclinical studies in animal models and cultured pancreatic B cells [7–12], but still with no evidence in human studies.
However, exogenous native GLP-1 is not suitable as a therapeutic agent because it is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) and has a half-life of less than 2 min [4]. Thus, DPP-4-resistant GLP-1 receptor agonists with extended half-lives have been developed. Lixisenatide (AVE0010) is a new, potent, selective and synthetic 44 amino acid exendin-4-like GLP-1 receptor agonist modified C-terminally with six Lys residues and one Pro deleted. In Chinese hamster ovary (CHO) cells transfected with the human GLP-1 receptor, lixisenatide had a binding affinity approximately 4-fold greater than that of native human GLP-1 (IC50 for lixisenatide = 1.43 nmol/l vs. IC50 for GLP-1 = 5.48 nmol/l) [9,13]. Lixisenatide is being developed with the aim of improving the management of Type 2 diabetes. The primary objective of this study was to evaluate thoroughly the dose–response effect of lixisenatide using once- or twice-daily regimens (5–30 μg once or twice daily) on HbA1c changes over 13 weeks in metformin-treated patients with Type 2 diabetes.
Patients and methods
Study participants
The study population comprised male and female patients aged 30–75 years with Type 2 diabetes mellitus of at least 1 year’s duration inadequately controlled [HbA1c≥ 7.0 and < 9.0% (≥ 53 and < 75 mmol/mol)] on stable metformin monotherapy (≥ 1000 mg/day) for at least 3 months prior to screening.
The main exclusion criteria were as follows: history of gastrointestinal disease with prolonged nausea and vomiting during the previous 6 months; history of chronic pancreatitis or stomach/gastric surgery; severe cardiovascular events during the previous 6 months; or hepatic or renal disease at screening [serum creatinine ≥ 114.4 μmol/l (1.5 mg/dl) for males and ≥ 106.8 μmol/l (1.4 mg/dl) for females].
The study was approved by the institutional review boards or ethics committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave written informed consent to participate in the study.
Study design
This 13 week, multinational, randomized, parallel-group, placebo-controlled study was conducted at 133 centres between March 2006 and August 2007. The study drug, added-on to stable metformin, was double-blind regarding active treatment or placebo and open-label regarding the treatment volume.
Following a 2 week screening phase, eligible patients entered into a 2 week, single-blind, placebo run-in period. Eligible patients were then randomized at visit 4 (week 0) to one of 12 treatment arms (2:2:2:2:2:2:2:2:1:1:1:1): to subcutaneous injec-tions of lixisenatide doses of 5, 10, 20 or 30 μg administered once daily within 1 h before breakfast (with volume-matched placebo before dinner); to lixisenatide doses of 5, 10, 20 or 30 μg administered twice daily (10, 20, 40 or 60 μg total daily dose, respectively) within 1 h before both breakfast and dinner; or to one of four volume-matched placebo treatments administered twice daily.
Randomization of subjects, allocation of medication and management of drug supplies were performed using an interactive voice response system.
Dose escalation was performed during the first 2–4 weeks for patients randomized to 20 and 30 μg dose levels of the study medication; the dose was initiated at 10 μg for 1 week and increased by 5 μg/week up to the target dose. The entry dosage of metformin remained unchanged throughout the study. All patients received diet and lifestyle counselling according to the American Diabetes Association guidelines [1].
Study assessments
The primary efficacy end-point was change in HbA1c from baseline to study end for the intent-to-treat population. Glycated haemoglobin was measured at a National Glycohemoglobin Standardization Program (NGSP) Level 1 certified central laboratory, measured with the high-performance liquid chro-matography method. Corresponding International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) standardized values were calculated using the relationship: IFCC value (in mmol/mol) = (NGSP value – 2.152)/0.09148 [14,15]. All HbA1c data are given as NGSP standardized values and IFCC values. The secondary efficacy measures included the percentage of patients achieving an HbA1c < 7.0 or < 6.5% (< 53 or < 48 mmol/mol), changes in body weight, fasting plasma glucose, and 2 h post-prandial plasma glucose after a standardized breakfast. Self-monitored seven-point blood glucose measurements were performed at baseline and week 13. Anti-lixisenatide antibody levels were measured.
Safety and tolerability were assessed by physical examination, adverse event reporting, blood pressure, heart rate, 12-lead electrocardiogram and standard laboratory measurements. Symptomatic hypoglycaemia was defined as symptoms consis-tent with hypoglycaemia, with an accompanying blood glucose < 3.3 mmol/l or prompt recovery with carbohydrate.
Statistical analyses
Sample sizes of 50 patients in each active treatment group and 100 patients in the placebo group were calculated to provide a statistical power of 81% to detect a 0.6% (6.6 mmol/mol) difference in HbA1c between an active treatment and placebo assuming a standard deviation of 1.2% (13.1 mmol/mol). Statistical significance was assumed at the 5% level.
Analyses of the primary efficacy variable (changes in HbA1c from baseline to end-point) were performed using analysis of covariance (ANCOVA) model, with treatment and country as fixed factors and baseline HbA1c as a covariate. Multiple testing procedure was used for the primary efficacy variable in order to control Type 1 error for the study and for multiple doses within each dose regimen (once and twice daily). The step-down trend test was used from the above ANCOVA model to assess dose–response relationship within each regimen. The continuous secondary efficacy variables (change in body weight, fasting plasma glucose, seven-point self monitored blood glucose and post-prandial plasma glucose) were analysed using the same methods used for the primary efficacy variable. Data from placebo-treated subjects were pooled for statistical analysis. Both means and least square adjusted means were calculated. The percentages of patients achieving an HbA1c < 7.0 and < 6.5% (< 53 and < 48 mmol/mol) were analysed using a Cochran–Mantel–Haenszel test stratified by country. Safety and tolerability data were analysed using descriptive statistics for all patients who received at least one dose of study medication.
Unless otherwise indicated, all efficacy data were analysed in the intent-to-treat population (all randomized subjects taking at least one dose of the study medication and having a baseline and one on-treatment value for efficacy variables); they are presented as means ± sem, unless specified otherwise.
Results
Demographic and baseline characteristics
A total of 542 patients were randomized from 1466 patients screened. The reasons for screening failure (n = 924) were as follows: ineligible inclusion criteria (n = 850), patient’s wish (n = 34) and other (n = 40). Approximately 90% of patients completed 13 weeks of active treatment, and the percentages ranged from 83% in the 30 μg lixisenatide once daily group to 96% in the 5 μg lixisenatide once daily and twice daily groups, compared with 95% in the placebo group (Table 1). Nearly all patients were at their randomized dose level by study end, ranging from 85 and 89% in the 30 μg once daily and twice daily groups to 100% in the 5 μg once daily and twice daily and 10 μg twice daily groups.
Table 1.
Patient disposition, demographics and baseline characteristics (safety population)
| Lixisenatide | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | 5 μg QD | 10 μg QD | 20 μg QD | 30 μg QD | 5 μg BID | 10 μg BID | 20 μg BID | 30 μg BID | |
| Patient disposition, n (%) | |||||||||
| Randomized | 109 | 55 | 52 | 55 | 54 | 53 | 56 | 54 | 54 |
| Completed | 103 (94.5) | 53 (96.4) | 47 (90.4) | 46 (83.6) | 45 (83.3) | 51 (96.2) | 51 (91.1) | 46 (85.2) | 47 (87.0) |
| Discontinued | 6 (5.5) | 2 (3.6) | 5 (9.6) | 9 (16.4) | 9 (16.7) | 2 (3.8) | 5 (8.9) | 8 (14.8) | 7 (13.0) |
| Reasons for discontinuation, n (%) | |||||||||
| Adverse event | 2 (1.8) | 1 (1.8) | 2 (3.8) | 3 (5.5) | 6 (11.1) | 0 | 2 (3.6) | 8 (14.8) | 5 (9.3) |
| Lack of efficacy | 0 | 1 (1.8) | 0 | 0 | 1 (1.9) | 0 | 0 | 0 | 0 |
| Other | 4 (3.7) | 0 | 3 (5.8) | 6 (10.9) | 2 (3.7) | 2 (3.8) | 3 (5.4) | 0 | 2 (3.7) |
| Demographics and baseline characteristics | |||||||||
| Mean age (years ± sd) | 56.3 ± 9.2 | 56.8 ± 7.8 | 55.4 ± 9.2 | 55.4 ± 9.9 | 56.5 ± 8.7 | 57.1 ± 8.2 | 56.0 ± 7.9 | 56.7 ± 8.3 | 55.3 ± 9.1 |
| Male, n (%) | 61 (56.0) | 26 (47.3) | 31 (59.6) | 28 (50.9) | 27 (50.0) | 25 (47.2) | 29 (51.8) | 20 (37.0) | 23 (42.6) |
| Race, n (%) | |||||||||
| Caucasian | 84 (77.1) | 38 (69.1) | 36 (69.2) | 45 (81.8) | 43 (79.6) | 46 (86.8) | 45 (80.4) | 42 (77.8) | 35 (64.8) |
| Black | 12 (11.0) | 5 (9.1) | 6 (11.5) | 1 (1.8) | 6 (11.1) | 2 (3.8) | 2 (3.6) | 2 (3.7) | 9 (16.7) |
| Other | 13 (11.9) | 12 (21.8) | 10 (19.3) | 9 (16.4) | 5 (9.3) | 5 (9.4) | 9 (16.0) | 10 (18.5) | 10 (18.5) |
| Mean duration of diabetes diagnosis (years ± sd) | 7.1 ± 5.4 | 7.2 ± 4.9 | 6.2 ± 4.1 | 6.4 ± 6.8 | 6.0 ± 4.8 | 6.2 ± 6.0 | 6.4 ± 5.0 | 6.6 ± 5.1 | 7.0 ± 5.4 |
| Mean HbA1c (% ± sd; NGSP) | 7.53 ± 0.6 | 7.58 ± 0.7 | 7.52 ± 0.6 | 7.58 ± 0.7 | 7.52 ± 0.7 | 7.60 ± 0.6 | 7.54 ± 0.6 | 7.61 ± 0.7 | 7.46 ± 0.5 |
| Mean HbA1c (mmol/mol ± sd; IFCC)* | 58.8 ± 5 | 59.3 ± 6 | 58.7 ± 5 | 59.3 ± 6 | 58.7 ± 6 | 59.6 ± 5 | 58.9 ± 5 | 59.7 ± 6 | 58.0 ± 4 |
| Mean weight (kg ± sd) | 87.7 ± 14 | 84.6 ± 16 | 90.5 ± 17 | 89.4 ± 17 | 87.6 ± 15 | 86.5 ± 14 | 89.8 ± 17 | 88.5 ± 17 | 87.5 ± 14 |
| Mean BMI (kg/m2 ± sd) | 31.7 ± 4.2 | 30.7 ± 4.6 | 31.9 ± 4.0 | 32.0 ± 4.3 | 31.6 ± 3.6 | 31.6 ± 4.2 | 32.8 ± 4.4 | 32.7 ± 4.4 | 32.3 ± 4.5 |
| Mean fasting plasma glucose (mmol/l ± sd) | 8.8 ± 2.1 | 8.4 ± 2.1 | 8.7 ± 2.0 | 8.4 ± 1.8 | 8.9 ± 2.1 | 8.9 ± 1.8 | 9.2 ± 2.4 | 9.0 ± 2.0 | 8.8 ± 2.3 |
| Mean 2 h post prandial plasma glucose (mmol/l ± sd) | 11.8 ± 3.2 | 12.8 ± 2.4 | 12.8 ± 3.3 | 11.5 ± 2.5 | 13.1 ± 3.3 | 12.5 ± 2.6 | 12.0 ± 3.3 | 12.3 ± 2.7 | 12.2 ± 3.0 |
Abbreviations: IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; NGSP, National Glycohemoglobin Standardization Program; Postprandial plasma glucose measured in a subgroup of patients undergoing standardized breakfast in selected sites. The standardized 500 kcal breakfast, which consisted of orange juice (180 ml), toasted bread (60 g), jam or preserves (20 g), butter or margarine (10 g), whole milk (120 ml) and coffee or tea with non-nutritive sweetener (if desired), was consumed within a 15 min period. The safety population consisted of all randomized patients who took at least one dose of the study medication during the double-blind treatment phase.
Demographic and baseline characteristics were well matched, and there were no clinically relevant differences between groups (Table 1).
Efficacy
There were significantly greater improvements in the primary efficacy end-point of HbA1c change from a mean overall baseline of 7.55% (59.0 mmol/mol) in all the lixisenatide groups (P < 0.01 vs. placebo), with reductions ranging from 0.47 to 0.87% (from 5.1 to 9.5 mmol/mol) among the different dosing regimens (mean reductions for 5, 10, 20 and 30 μg doses of 0.47, 0.50, 0.69 and 0.76% [5.1, 5.5, 7.5 and 8.3 mmol/mol] on once-daily administration, respectively, and 0.65, 0.78, 0.75 and 0.87% (7.1, 8.5, 8.2 and 9.5 mmol/mol) on twice-daily administration, respectively), compared with a decrease of 0.18% (2.0 mmol/mol) for placebo (Fig. 1). A dose–response relationship with HbA1c level was seen for both the once daily and twice daily regimens of lixisenatide, with improvements in HbA1c observed as early as week 5 (Fig. 1).
FIGURE 1.

Changes in glycated haemoglobin (HbA1c) levels following 13 weeks' treatment with lixisenatide once daily or twice daily, according to dosage and regimen. Top panel shows change in mean (±sem) HbA1c over time. Bottom panel shows least square (LS) mean change in HbA1c from baseline to 13 weeks.
Significantly more patients in the lixisenatide groups achieved an HbA1c < 7.0% (53 mmol/mol; ranging from 47 to 69% with once daily dosing and from 51 to 77% with twice daily dosing), compared with 32% of those in the placebo group (P <0.05) at week 13 (Fig. 2). Further improvement in glycaemic control to HbA1c < 6.5% (48 mmol/mol) at study end was observed in significantly more patients in the lixisenatide groups than in the placebo group (7.5%); one-third of patients receiving 20 or 30 μg once daily and 5, 10 and 20 μg twice daily achieved this goal (P <0.0001 for all of these groups vs. placebo and P = 0.0315 vs. placebo for 5 μg once daily; Fig. 2).
FIGURE 2.
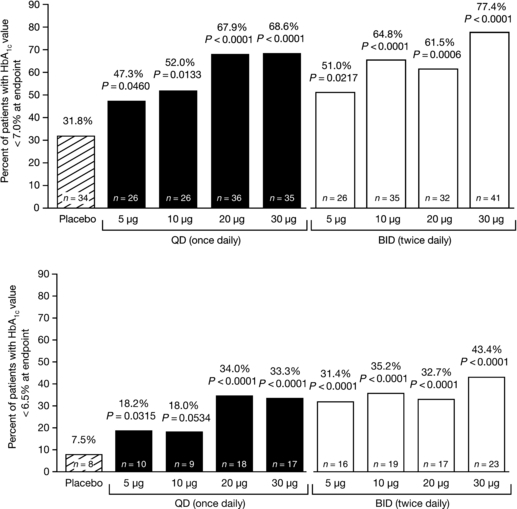
Percentage of patients with glycated haemoglobin (HbA1c) level of < 7.0% (53 mmol/mol; top panel) and < 6.5% (48 mmol/mol; bottom panel) following 13 weeks’ treatment with lixisenatide once daily or twice daily, according to dosage and regimen.
As noted in Table 2, there were dose-dependent reductions from baseline in fasting plasma glucose and also in daily averaged seven-point self monitored blood glucose, 2 h post-prandial plasma glucose concentrations, and in body weight with lixisenatide.
Table 2.
The least square adjusted mean ± sem changes in fasting plasma glucose, daily averaged self-monitored seven-point blood glucose and 2 h postprandial plasma glucose following 13 weeks of treatment with lixisenatide once daily or twice daily according to dosage and regimen in the ITT population
| Lixisenatide | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 108) | 5 μg QD(n = 55) | 10 μg QD(n = 51) | 20 μg QD(n = 53) | 30 μg QD(n = 52) | 5 μg BID(n = 51) | 10 μg BID(n = 54) | 20 μg BID(n = 52) | 30 μg BID(n = 53) | |
| Fasting plasma glucose (mmol/l) | −0.21 ± 0.19 | −0.62 ± 0.24 | −0.54 ± 0.25 | −0.80 ± 0.25 | −1.02 ± 0.25* | −0.19 ± 0.24 | −0.98 ± 0.24* | −1.13 ± 0.25* | −1.42 ± 0.25* |
| Average self-monitored seven-point blood glucose (mmol/l) | −0.53 ± 0.18 | −1.23 ± 0.24* | −1.27 ± 0.24* | −1.74 ± 0.24* | −1.77 ± 0.25* | −0.88 ± 0.24 | −1.60 ± 0.24* | −1.83 ± 0.24* | −2.08 ± 0.24* |
| 2 h postprandial glucose (mmol/l)a | −0.41 ± 0.46 | −2.12 ± 0.67† | −3.57 ± 0.62* | −3.65 ± 0.68* | −4.33 ± 0.71* | −2.01 ± 0.61† | −3.51 ± 0.62* | −4.12 ± 0.68* | −4.61 ± 0.68* |
| Body weight (kg) | −1.94 ± 0.32 | −2.00 ± 0.40 | −2.39 ± 0.42 | −3.01 ± 0.41* | −3.47 ± 0.41* | −2.10 ± 0.41 | −2.21 ± 0.41 | −2.61 ± 0.41 | −3.89 ± 0.41* |
The ITT population consisted of all randomized patients who took at least one dose of the study medication and had a baseline and at least one post-baseline on-treatment value for efficacy variables.
P < 0.01
P < 0.05 in the step-down linear trend test.
In a subgroup of patients undergoing standardized breakfast in selected sites (approximately half of total sites). The standardized 500 kcal breakfast, which consisted of orange juice (180 ml), toasted bread (60 g), jam or preserves (20 g), butter or margarine (10 g), whole milk (120 ml) and coffee or tea with non-nutritive sweetener (if desired), was consumed within a 15 min period.
Data are mean ± SEM.
Safety and tolerability
The most frequent adverse events were gastrointestinal, primarily dose-dependent nausea (Table 3). The onset of gastrointestinal adverse reactions was observed during the first 5 weeks of the study in the majority of cases, and these were usually mild-to-moderate in intensity. No cases of pancreatitis were experienced. There was no evidence of a dose relationship with symptomatic hypoglycaemic episodes [ranging from 1 to 3 events (0.9–5.7%) per group), which were mostly mild in intensity. No patients experienced severe hypoglycaemia.
Table 3.
Number (%) of patients with treatment-emergent adverse events occurring in ≥ 10% in any one group and symptomatic hypoglycaemia in the safety population
| Lixisenatide | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of adverse event | Placebo (n = 109) | 5 μg QD (n = 55) | 10 μg QD (n = 52) | 20 μg QD (n = 55) | 30 μg QD (n = 54) | 5 μg BID(n = 53) | 10 μg BID (n = 56) | 20 μg BID (n = 54) | 30 μg BID (n = 54) |
| Any treatment-emergent adverse events | 65 (59.6) | 31 (56.4) | 26 (50.0) | 37 (67.3) | 42 (77.8) | 30 (56.6) | 32 (57.1) | 38 (70.4) | 40 (74.1) |
| Any serious treatment-emergent adverse events | 3 (2.8) | 0 | 1 (1.9) | 1 (1.8) | 3 (5.6) | 0 | 1 (1.8) | 2 (3.7) | 0 |
| Nausea | 5 (4.6) | 4 (7.3) | 6 (11.5) | 14 (25.5) | 19 (35.2) | 4 (7.5) | 8 (14.3) | 12 (22.2) | 18 (33.3) |
| Vomiting | 1 (0.9) | 2 (3.6) | 3 (5.8) | 3 (5.5) | 10 (18.5) | 3 (5.7) | 4 (7.1) | 5 (9.3) | 2 (3.7) |
| Diarrhoea | 8 (7.3) | 3 (5.5) | 4 (7.7) | 5 (9.1) | 4 (7.4) | 3 (5.7) | 4 (7.1) | 6 (11.1) | 14 (25.9) |
| Headache | 11 (10.1) | 7 (12.7) | 3 (5.8) | 7 (12.7) | 7 (13.0) | 7 (13.2) | 5 (8.9) | 6 (11.1) | 4 (7.4) |
| Dizziness | 7 (6.4) | 1 (1.8) | 4 (7.7) | 4 (7.3) | 6 (11.1) | 3 (5.7) | 5 (8.9) | 2 (3.7) | 5 (9.3) |
| Symptomatic hypoglycaemia | 1 (0.9) | 1 (1.8) | 2 (3.8) | 1 (1.8) | 1 (1.9) | 3 (5.7) | 1 (1.8) | 3 (5.6) | 1 (1.9) |
Data are n (%). Treatment-emergent adverse events were defined as adverse events that developed or worsened during the on-treatment period (the time from the first dose of study medication up to 3 days after the last dose). The safety population was composed of all randomized patients who took at least one dose of the study medication during the double-blind treatment phase.
There were zero to three (5.6%) serious adverse events in the lixisenatide groups and three (2.8%) in the placebo group (Table 3). These events included one patient in the lixisenatide 30 μg once daily group who discontinued treatment owing to a few seconds of loss of consciousness and one in the lixisenatide 10 μg once daily group who discontinued secondary to an allergic reaction (a 30 min episode of pruritus over the entire body within 10 min of injecting study drug after 3 weeks of study treatment, and a second episode 10 min after the next injection, given 3 days later, with swollen lips/tongue and difficulty in breathing that resolved within minutes of receiving an oral antihistamine). Two non-serious cases of urticaria were reported with lixisenatide and three with placebo. There was evidence for a relationship between the lixisenatide dose and frequency of adverse events (mainly due to gastrointestinal adverse events), but not with the number of serious adverse events (Table 3).
The frequencies of patient discontinuations from the study due to treatment-emergent adverse events ranged from 1.8 to 11.1% in the once daily lixisenatide groups and from 0 to 14.8% in the twice daily lixisenatide groups, while 1.8% of patients taking placebo discontinued.
No clinically significant changes were detected by laboratory safety assessments and on 12-lead electrocardiogram. Mean systolic and diastolic blood pressure levels were 130/80 mmHg, respectively, at baseline. A clear trend of systolic and diastolic blood pressure reductions from baseline to end-point occurred with each lixisenatide dose (ranging from −2 to −9 mmHg for the systolic, and −2 to −4 mmHg for the diastolic blood pressures), and also with placebo (−3 and −2 mmHg for systolic and diastolic blood pressures, respectively). The apparent reductions in blood pressure were observed as early as week 1 in most of the groups and, therefore, appeared to be independent of reductions in HbA1c and body weight. There were no relevant changes in heart rate from baseline to end-point in any of the groups.
The percentages of anti-lixisenatide antibody-positive subjects at end-point ranged from 43.1% in the 10 μg once daily group to 71.2% in the 20 μg twice daily group. No relevant differences were observed in terms of safety and efficacy between the patient populations with antibody-positive and negative status at study-end for all dose regimens.
Discussion
In this study, the new GLP-1 receptor agonist lixisenatide significantly improved glycaemic control from a mildly elevated mean baseline HbA1c [∼7.55% (∼59.0 mmol/mol)] in patients with Type 2 diabetes mellitus inadequately controlled with metformin.
A total of eight regimens, comparing four doses each administered once or twice daily, were compared with placebo in order to characterize fully the dose–effect profile of lixisenatide when added to previous metformin monotherapy. At week 13, statistically significant reductions in the primary end-point—the HbA1c level—were observed for each dose of lixisenatide. The efficacy of lixisenatide was dose related across the once daily dose range with regard to improvements in the primary and secondary end-points of fasting plasma glucose, daily averaged seven-point self monitored blood glucose and 2 h post-prandial plasma glucose. Notably, the once daily and twice daily lixisenatide regimens achieved similar levels of efficacy, and doubling the daily dose (in the twice daily regimens) did not provide relevant additional improvements in glycaemic control over the once daily regimens. The efficacy of lixisenatide appeared to reach a plateau at a dose of 20 μg once daily, with further increases offering limited benefit relative to the increase in drug exposure. This is in accordance with a pharmacodynamic study that found that both 20 μg once daily and 20 μg twice daily of lixisenatide significantly improved HbA1c to a similar extent vs. placebo, despite the short (4 week) treatment period [13].
There have been a few previous dose-ranging studies of exenatide or liraglutide [16–19]. A Phase II dose-ranging study of exenatide (2.5–10.0 μg) was performed over 4 weeks [18]. Initial dose-ranging studies of liraglutide monotherapy evaluated doses of 0.045–0.75 mg once daily over 12 weeks [16,17], with the highest doses giving HbA1c reductions similar to that observed with lixisenatide 20 μg once daily in the present study, but from a higher baseline HbA1c [16]. A subsequent study appeared to establish a dose–effect plateau at 1.25 and 1.90 mg once daily in patients with poorer glycaemic control at baseline [HbA1c 8.1–8.5% (65–69 mmol/mol)] [19].
Importantly, over two-thirds of patients on lixisenatide 20 μg once daily and 30 μg once daily reached the target of HbA1c < 7.0% (53 mmol/mol), compared with 32% of those taking placebo. This suggests that lixisenatide could be a useful option for helping the large fraction of patients in clinical practice who do not achieve recommended HbA1c goals [20], as well as for overcoming the limited therapeutic response provided by some existing therapies when the baseline is mildly elevated.
Of note, improvements in glycaemic control with lixisenatide were coupled with reductions in body weight. This is an important finding in light of the high prevalence of obesity and overweight in this population and the relationship of weight with insulin resistance and cardiovascular disease [21], as well as the tendency of current intensive therapy to cause weight gain [22].
Overall, lixisenatide was well tolerated. Consistent with other GLP-1 receptor agonists [16,17,23–25], gastrointestinal adverse events were the most common with lixisenatide, and nausea was the most frequent of these. Nevertheless, it appears that fewer patients experienced nausea with the 20 μg once daily dose than figures previously reported in clinical studies of twice-daily exenatide [23–25], but only head-to-head studies can substantiate this suggestion. The onset of nausea occurred predominantly during the first half of the study and was mild to moderate in intensity. Only one patient discontinued the study due to nausea (and none for vomiting) in the 20 μg once daily lixisenatide group. The risks of hypoglycaemia and serious adverse events were low and similar across the lixisenatide dose range. Based on these data, a dose of 20 μg once daily appears to balance maximal efficacy with good tolerability. However, the present study has the limitation of a relatively short treatment period (13 weeks), and the full long-term effect of lixisenatide on glycaemic control and body weight remains to be determined.
In conclusion, in this thorough dose-ranging study of four doses and two regimens, lixisenatide significantly improved glycaemic control in mildly hyperglycaemic patients previously on metformin monotherapy, with associated weight loss and without causing significant hypoglycaemia. Clear dose–response relationships and similar levels of efficacy were seen for the once daily and twice daily regimens, with a 20 μg once daily dose showing the best efficacy-to-tolerability ratio. This new, once-daily GLP-1 receptor agonist shows promising efficacy, safety and tolerability in the management of Type 2 diabetes, but further investigations in long-term studies are needed.
Acknowledgments
We would like to thank all of the investigators (see below), co-ordinators and patients who took part in this study, Quest Diagnostics (Middlesex, UK) and Van Nuys (CA, USA) for analysing the blood samples for efficacy and safety parameters, and MDS Pharma Services (Fehraltorf, Switzerland) for analysing plasma samples for anti-lixisenatide antibody deter-mination. sanofi-aventis sponsored this study. Editorial support was provided by Susan Crawford.
These data were presented as a poster at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 6–10 June 2008 and the 44th European Association for the Study of Diabetes, Rome, 7–11 September 2008.
Glossary
Abbreviations
- DPP-4
dipeptidyl-peptidase-4
- GLP-1
glucagon-like peptide-1
- IFCC
International Federation of Clinical Chemistry and Laboratory Medicine
- NGSP
National Glycohemoglobin Standardization Program
- QD
once daily
Competing interests
Dr Ratner has received research support from Amylin, Boehringer Ingelheim, GlaxoSmithKline, Merck, Novo Nordisk, Pfizer, Roche, sanofi-aventis and Takeda Pharmaceuticals, and has acted as a consultant for Amylin, Eli Lilly, Novo Nordisk, Roche, sanofi-aventis and Takeda. Dr Rosenstock has served on advisory boards and received honorarium or consulting fees from Pfizer, Roche, sanofi-aventis, Novo Nordisk, Eli Lilly, MannKind, GlaxoSmithKline, Forest, Takeda, Daiichi Sankyo, Boehringer Ingelheim, Johnson & Johnson, Emisphere, Novartis and Amylin. He has also received research grants from Merck, Pfizer, sanofi-aventis, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Forest, Takeda, Novartis, AstraZeneca, Amylin, Johnson & Johnson, Daiichi Sankyo, Boehringer Ingelheim and MannKind. Dr Boka is an employee of sanofi-aventis.
The study was funded by sanofi-aventis, the manufacturer of lixisenatide. The investigators and representatives from sanofi-aventis were responsible for the study design, protocol, statistical analysis plans, analysis and reporting of the results. Final responsibility for the decision to submit the manuscript for publication was made jointly by all authors.
Investigators for the DRI6012 Study Group
Brazil Antonio Chacra, Marco Antonio Dias, Mauro Dinato, Freddy Eliaschewitz, Vivian Ellinger, Adriana Forti, Amelio Godoy, Marilia Gomes, Alfredo Halpern, Miguel Hissa, Antonio Carlos Lerario, Geisa Macedo, Euler Manenti, Jose Egidio Oliveira, Hermelinda Pedrosa, Nelson Rassi, Giuseppe Repetto, Joao Salles, Jose Augusto Sgarbi, Ruy Lyra da Silva Filho, Henrique Suplicy, Balduino Tschiedel, Rosa Vargas and Maria Zanella.
Canada Ronnie Aronson, James Cha, Ronald Goldenberg, Neil Hudson, Mustafa Kamouna, Lisa Klassen, Jacobus Kooy, Randy MacKinnon, Karl Misik, Michael O’Mahony, Alexander Skamene, Yaw Twum-Barima, Anthony Wade and Vincent Woo.
Poland Marek Konieczny, Jerzy Kuleta, Grzegorz Laskawiec, Piotr Mader, Adam Pieniazek, Marek Szolkiewicz, Ireneusz Szymczyk and Danuta Zytkiewicz-Jaruga.
Romania Alexandru Barnea, Mihaela Busegeanu, Carmen Crisan, Radu Lichiardopol, Nicoleta Mindrescu, Magdalena Morosanu, Lavinia Pop, Milivoi Stamoran and Iosif Szilagyi.
Russian Federation Olga Barbarash, Nina Chernova, Galina Chumakova, Tatjana Raskina and Elena Zonova.
Ukraine Volodymyr Botsyurko, Vitaliy Katerenchuk, Boris Mankovsky, Tetyana Pertseva, Alexander Serhiyenko and Maryna Vlasenko.
USA John Abernethy, Andrew J. Ahmann, Richard F. Arakaki, Suresh Baliga, Anthony J. Bartkowiak Jr, Bantwal Richard Bernstein, Loray Blair-Britt, Michael Bolognese, Robert S. Busch, Robert Buynak, LeRoy J. Byrd, David W. Cardona, Christopher M. Chappel, Richard B. Christensen, Lisa Cohen, Terry Nizo Copeland, Clinton Corder, Yvette Crabtree, Arcot Dwarakanathan, Daniel S. Donovan, H. Jackson Downey, Robert M. Evans, Robert Eyzaguirre, Ronald B. Goldberg, Barry Jay Goldstein, G. M. Gollapudi, Gregory M. Gottschlich, Michael Guice, Yehuda Handelsman, Israel Hartman, John Arthur Hoekstra, Priscilla Hollander, Robert L. Jackson, David Jasper, Marvin Kalafer, William A. Kaye, Lawrence R. Koehler, Dan Lender, Peter Lodewick, Charles F. Lovell, Kathryn Jean Lucas, Timothy J. Lyons, Marco Marcelli, Wendell Miers, Sam S. Miller, Paul S. Norwood, Fernando Ovalle, Phillip J. Peters, Fatima Phillips, Brian R. Riveland, Syed W. Rizvi, Julio Rosenstock, Douglas R. Schumacher, Sherwyn L. Schwartz, Robert B. Schwartz, Cranford L. Scott, Anuja Jasvant Shah, Loknath Shandilya, Mansur Shomali, Mohammad I. Siddiqqi, Jeremy Soule, Robert Strzinek, Danny Sugimoto, James Underberg, Thomas A. Wade, Bret A. Wittmer, Berto M. Zamora and Howard C. Zisser.
References
- 1.American Diabetes Association. Standards for diabetes care—2008. Diabetes Care. 2008;31:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Global Guideline for Type 2 Diabetes, 2005. Available at http://www.idf.org/home/index.cfm?node=1457 Last accessed 29 January 2009.
- 3.Alvarez Guisasola F, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab. 2008;10(Suppl 1):8–15. doi: 10.1111/j.1463-1326.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–2940. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 5.Holst JJ, Deacon CF, Vilsbøll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–168. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Schmiegel WH, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 7.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX–1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide–1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 9.Thorkildsen C, Neve S, Larsen BD, Meier E, Petersen JS. Glucagon-like peptide 1 receptor agonist ZP10A increases insulin mRNA expression and prevents diabetic progression in db/db mice. J Pharmacol Exp Ther. 2003;307:490–496. doi: 10.1124/jpet.103.051987. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 11.Tews D, Werner U, Eckel J. Enhanced protection against cytokine- and fatty acid-induced apoptosis in pancreatic beta cells by combined treatment with glucagon-like peptide–1 receptor agonists and insulin analogues. Horm Metab Res. 2008;40:172–180. doi: 10.1055/s-2008-1042426. [DOI] [PubMed] [Google Scholar]
- 12.Werner U, Vandewalle B, Kerr Conte J, Pattou F, Pruniaux M-P, Herling AW. The GLP–1 receptor agonist AVE0010 improves GSIS, prevents lipotoxicity-induced insulin depletion and preserves beta-cell function in human pancreatic islets [abstract] Diabetes. 2008;57(Suppl 1):A3–A4. [Google Scholar]
- 13.Distiller LA, Ruus P, on behalf of the ACT6011 Study Group Pharmacokinetics and pharmacodynamics of a new GLP–1 agonist AVE0010 in type 2 diabetes patients [abstract] Diabetes. 2008;57(Suppl 1):A154–A155. [Google Scholar]
- 14.Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 15.Sacks DB, ADA/EASD/IDF Working Group of the HbA1c Assay Global harmonization of hemoglobin A1c. Clin Chem. 2005;51:681–683. doi: 10.1373/clinchem.2004.047431. [DOI] [PubMed] [Google Scholar]
- 16.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, NN2211-1310 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12–week, double-blind, randomized, controlled trial. Diabetes Care. 2007;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 17.Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O, Liraglutide Dose-Response Study Group Effects of liraglutide (NN2211), a long-acting GLP–1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med. 2005;22:1016–1023. doi: 10.1111/j.1464-5491.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- 18.Poon T, Nelson P, Shen L, Mihm M, Taylor K, Fineman M, et al. Exenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a dose-ranging study. Diabetes Technol Ther. 2005;7:467–477. doi: 10.1089/dia.2005.7.467. [DOI] [PubMed] [Google Scholar]
- 19.Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, et al. Liraglutide, a long-acting human glucagon-like peptide–1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 20.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol. 2008;18:222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson PM. Is weight loss beneficial for reduction of morbidity and mortality? What is the controversy about? Diabetes Care. 2008;31:S278–S283. doi: 10.2337/dc08-s268. [DOI] [PubMed] [Google Scholar]
- 22.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin–4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 24.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin–4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 25.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin–4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]


