Abstract
It is generally assumed that one reason why white matter injury is common in preterm infants is the relatively poor vascular supply.
Aim
To examine whether blood flow to the white matter is relatively more reduced at low blood pressure than is blood flow to the brain as a whole.
Methods
Thirteen normoxic preterm infants had blood flow imaging on 16 occasions with single-photon emission computed tomography (SPECT) using 99Tc labelled hexa-methylpropylenamide oxime (HMPAO) as the tracer. Gestational age was 26–32 weeks. Transcutaneous carbon dioxide was between 4.7 and 8.5 kPa and mean arterial blood pressure between 22 and 55 mmHg.
Results
There was no statistically significant direct relation between white matter blood flow percentage and any of the variables. Using non-linear regression, however, assuming a plateau over a certain blood pressure threshold and a positive slope below this threshold, the relation to white matter flow percentage was statistically significant (p = 0.02). The threshold was 29 mmHg (95% confidence limits 26–33).
Conclusion
Our analysis supports the concept of periventricular white matter as selectively vulnerable to ischaemia during episodes of low blood pressure.
Keywords: Autoregulation, Brain, Cerebral blood flow, Haemodynamics
Brain injury in preterm infants involves predominantly white matter, and the periventricular zone is at particular risk. Parenchymatous haemorrhage and cystic leucomalacia are most often seen here. Forty years ago, the vascular anatomy was used to explain this by describing periventricular white matter as a vascular end-zone (1) at special risk for ischaemia.
It has been difficult, however, to demonstrate an association between white matter injury and ischaemia or low cerebral blood flow. Cerebral blood flow (CBF) is moderately low before major intracranial haemorrhage (2,3), and low CBF is weakly associated with neurodevelopmental deficit (4,5). One problem in these studies is that measurement of CBF was random in time. As CBF is likely to vary significantly with pCO2 and perhaps also with blood pressure, it is not likely that the minimal value was recorded.
Another problem is that if blood flow to the white matter is selectively decreased, this will not be easy to detect with a measure of global CBF as is obtained by xenon clearance or near infrared spectroscopy.
To address this issue, we used a purpose-built, mobile single-photon emission computerized tomograph to image blood flow in ill newborn infants. We have reported that flow to white matter is extraordinarily low in the human preterm neonate (6).
We have until now only reported our findings in hypotension in the form of a congress abstract (7). This was because of the limited sample size and the scatter of values. The scanner is no longer available. But to our knowledge, no better data have appeared in the literature. That is why we now publish the study in full. We used a post hoc hypothesis for analysis.
Patients and methods
Patients
Infants of <33 weeks were enrolled in the study. Exclusion criteria for this analysis were hypoxia (pulseoximeter reading below 88%) and abnormal brain ultrasound before or after the study. It was possible to perform 16 measurements of regional cerebral blood flow (rCBF) in 13 infants fulfilling these criteria. The limiting factors were the clinical condition of the infant, parental consent, and availability of primary investigator (KB), scanner, and tracer. The measurements were performed from March 1992 to March 1994.
Tracer
99mTc-Labelled HMPAO was prepared by adding 80 MBq of freshly eluted 99mTc in 10 mL of isotonic saline into one vial of HMPAO (Ceretec, Amersham International). The tracer was injected intravenously in a dose of 4 MBq/kg body weight within 30 min from preparation. HMPAO acts as a ‘chemical microsphere’; therefore, imaging can be carried out several hours after an injection of tracer that was made during an acute episode. Imaging could then be carried out when the baby had been stabilized.
SPECT equipment and procedure
SPECT measures the activity and the position of a radioactive tracer emitting one photon per decay. The activity is proportional to the number of decays with the corrections mentioned below. The position of the tracer is determined by the measurements from different angles. We used a brain dedicated fast-rotating four-head multidetector system (Tomomatic 248®, Medimatic, Denmark). The scanner was mobile, which allowed bedside studies (8).
The images were reconstructed into 1.25-mm pixels using a filtered back-projection algorithm using a Shepp-Logan filter, and an attenuation correction coefficient of 0.15/cm. The images were normalized to the mean of all slices. The tracer was fixed in the brain within 1 min, but a small part of the tracer diffused back from brain to blood. This back-diffusion is most predominant in the high-flow areas. A correction for this back diffusion of tracer from brain to blood (linearization) was performed using a correction factor of 3.4 (Ref. 2). Image analysis: The images were analysed by a semi-automated method discriminating flow to the cortical grey matter, the basal ganglia and to the white matter (Fig. 1). (Ref. 2). For this analysis the flow to the white matter was expressed as a percentage of the blood flow to the entire brain and labelled WM%.
Figure 1.
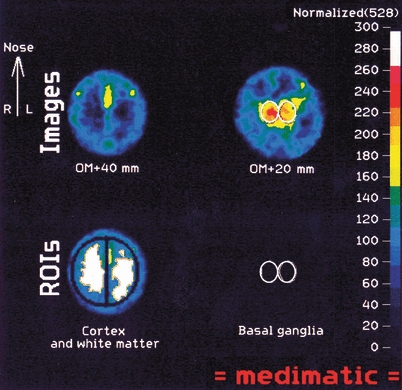
Upper row. Two imaging ‘slices’ 20 and 40 mm above the ear opening. The upper slice images the hemispheric cortex and the centrum semiovale. The lower slice images the basal ganglia/thalamus in the centre. Lower row: The regions of interest: lateral and mesial cortex, white matter, and basal ganglia/thalamus.
Informed consent was obtained from the parents of each infant. The local ethics committee of Copenhagen approved the study protocol. The maximal allowed accumulated radiation dose was 5 mSv (effective dose). In reality, average absorbed dose for one scan was 1.5 mSv, and as no infants had more than two studies carried out, maximum radiation dose was 3 mSv.
Statistical analysis
White matter flow percentage was apparently normally distributed with low skewness and normal kurtosis. Pearson correlation was used first. Then non-linear regression of white matter flow percentage (WM%) vs. mean arterial blood pressure (MABP) was made. The non-linear equation was VM% = (MABP < threshold) *(MABP−threshold) * slope+ plateau. This fits a break in the regression line: sloping at low ABP until a threshold and being horizontal at ABP above the threshold. A constraint of the non-linear regression procedure was set not allowing threshold to move outside the range 25–35. We used PASW 18.0 (SPSS inc, Chicago, IL, USA).
Results
Mean birthweight was 986 g (range 550–2680) and the mean gestational age was 27.3 weeks (range 26–32) (Table 1). Mean arterial pressure ranging from 22 to 55 mmHg. Transcutaneous pCO2 varied between 4.7 and 8.5 kPa (TINA, Radiometer, Denmark). Nine infants were healthy at 1 year of age and four died due to bronchopulmonary dysplasia without sign of brain damage.
Table 1.
Clinical and cerebral perfusion data from 16 measurements in 13 infants
| Relative perfusion (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. no. | Birthweight (g) | Gestational age (weeks) | Sex | Age at examination (days) | Postmenstrual age at examination (weeks) | Ventilation* | pCO2 (kPa) | pO2 (kPa) | Saturation (%) | MAP (mmHg) | Abnormal outcome† | White matter | Cortex | Basal ganglia |
| 1 | 2680 | 32 | Boy | 2 | 32 | V | 4.70 | 37 | 61 | 112.5 | 246.5 | |||
| 2 | 820 | 26 | Girl | 1 | 26 | V | 5.80 | 11.70 | 94 | 31 | 60.5 | 138.5 | 229 | |
| 3 | 730 | 26 | Girl | 2 | 26 | NC | 6.80 | 17.20 | 97 | 42 | 51.5 | 112 | 275 | |
| 4 | 860 | 27 | Boy | 1 | 27 | NC | 6.00 | 9.10 | 26 | 52 | 104.5 | |||
| 5 | 28 | V | 7.20 | 7.20 | 48 | 53.5 | 101 | 243.5 | ||||||
| 5 | 550 | 26 | Boy | 24 | 29 | V | 8.50 | 5.80 | 92 | 50 | Died | 70 | 104 | 232 |
| 6 | 860 | 26 | Boy | 1 | 26 | NC | 5.10 | 11.30 | 30 | Died | 59 | 100.5 | 176 | |
| 7 | 715 | 26 | Girl | 2 | 26 | V | 6.00 | 10.70 | 95 | 29 | Died | 64.5 | 104.5 | 221 |
| 21 | 29 | V | 6.10 | 8.20 | 90 | 46 | 48 | 101 | 185.5 | |||||
| 8 | 1208 | 28 | Boy | 1 | 28 | V | 5.90 | 8.70 | 92 | 22 | 44.5 | 97 | 166 | |
| 9 | 29 | NC | 6.40 | 5.50 | 95 | 55 | 60 | 99.5 | 219 | |||||
| 9 | 800 | 26 | Girl | 1 | 26 | V | 5.10 | 8.90 | 97 | 25 | 38 | 110.5 | ||
| 10 | 845 | 30 | Girl | 1 | 30 | V | 4.90 | 7.80 | 89 | 28 | 57 | 117.5 | 306.5 | |
| 11 | 845 | 30 | Girl | 10 | 31 | NC | 6.20 | 10.70 | 95 | 42 | 46.5 | 95.5 | 191 | |
| 12 | 1120 | 27 | Boy | 1 | 27 | V | 5.50 | 7.60 | 29 | Died | 55 | 122 | 205.5 | |
| 13 | 955 | 26 | Boy | 14 | 28 | NC | 6.00 | 7.50 | 92 | 48 | 71 | 107.5 | 228.5 | |
V = Mechanical ventilation; NC = Nasal CPAP.
If nothing is stated, the infant was healthy at 1 year of age.
The relative blood flow to white matter did not correlate to gestational age (p = 0.8), birth weight (p = 0.8) or transcutaneous pCO2 (p = 0.3). Therefore, correction for pCO2 was not attempted. WM% was not simply correlated to MABP either (p = 0.12).
The non-linear relation between WM% and MABP, in a shape like the conventional concept of autoregulation, was statistically significant (multiple r2 = 0.344, 13 df, p = 0.02) with the breakpoint at 29 ± 2.0 mmHg (95% confidence limits 24–34 mmHg), a slope below the breakpoint of 2.6 ± 1.3% per mmHg, and a plateau above the breakpoint of 58.2 ± 2.5% (Fig. 2).
Figure 2.
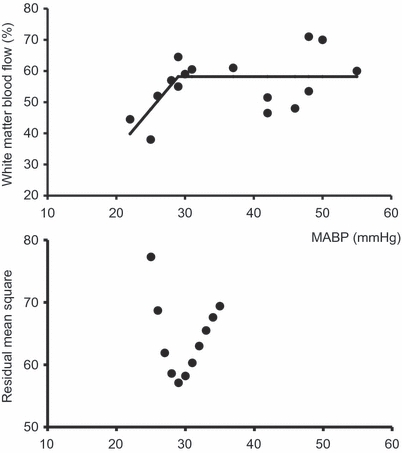
Upper panel: Flow to cerebral white matter – expressed as percentage of global cerebral blood flow as function of mean arterial blood pressure. The 16 measurements were obtained in 13 infants. A continuous function was fitted as a bi-linear regression with a preset breakpoint. Lower panel: The lowest residual mean square (best fit) was obtained with the breakpoint at 29 mmHg.
Discussion
We found support for the concept of the white matter as a vascular end zone. Blood pressure below 29 mmHg was associated with smaller share of blood flow to white matter. The implication is that white matter is selectively vulnerable to ischaemia when blood pressure is low. It also means that measures of CBF that include grey as well as white matter are less sensitive to this and therefore that studies reporting absence of a lower threshold for the autoregulatory plateau in preterm infants (9,10) should be interpreted in this light.
The method used here, HMPAO imaging, is based on the bolus distribution principle. This principle states that the concentration of tracer fixed in tissue after a bolus injection is proportional to the rate of perfusion, that is proportional to blood flow per unit of tissue volume. The uptake of HMPAO during the first pass is less than complete, and more so at higher rates of flow. This problem was compensated for by linearisation. Furthermore, images were not converted to units of flow. This would have required determination of the arterial input that is difficult for HMPAO. We circumvented this by expressing flow to the white matter in relative terms. Thus, it is was not possible to examine the threshold of autorelation in white matter directly, but only to examine whether blood flow to white matter is relatively more reduced at low blood pressure than is blood flow to the brain as a whole. That is why we interpret the result to indicate the white matter is selectively vulnerable to ischaemia during hypotension.
The statistical significance of the result, however, depends on the assumption of a non-linear relation between blood pressure and relative white matter flow of the specified shape. For the analysis we assumed the existence of a plateau and a threshold. The statistical analysis resulted in a stable estimate of the threshold that was in good agreement with expectations. Incidentally, a MABP of 30 mmHg has been used as a practical definition of hypotension in preterm infants based on early observations of risk of cerebral haemorrhage (11), and this was also the threshold for autoregulation found in extremely preterm infants treated with dopamine to support blood pressure (12).
Our study has a number of weaknesses. First, because of the highly technically demanding method, the group of infants was small and was not sampled systematically. Second, in 1992–1994, arterial hypotension was treated more actively than now. This meant that we have only a few studies during significant hypotension. Third, whereas the relation between blood pressure and WM% was statistically significant, two-thirds of the variance of WM% was still unexplained. Finally, two imaging studies were achieved in only three of the 13 infants, precluding any meaningful paired statistical analysis.
The result should not be used for clinical decision-making. At least it should be realised that the decrease in relative flow to white matter at pressures below 30 mmHg was gradual, being only moderate at the pressures recorded. It should also be realised that normal CBF includes a comfortable reserve (13). Measures of cerebral oxygenation with near-infrared spectroscopy in preterm infants suggest that it is also the case for term and preterm infants in general (14,15). In situations where blood flow to cerebral white matter is already marginal, however, such as in cases of low pCO2, low blood glucose, or low blood haemoglobin concentration, a low blood pressure may be detrimental.
In conclusion, our data support the concept of periventricular white matter in preterm infants as a vascular end-zone, particularly vulnerable to ischaemia.
References
- 1.Wigglesworth JS, Pape KE. An integrated model for haemorrhagic and ischaemic lesions in the newborn brain. Early Hum Dev. 1978;2:179–99. doi: 10.1016/0378-3782(78)90010-5. [DOI] [PubMed] [Google Scholar]
- 2.Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J Pediatr. 1989;115:638–45. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- 3.Meek JH, Tyszczuk L, Elwell CE, Wyatt JS. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child. 1999;81:F15–8. doi: 10.1136/fn.81.1.f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou HC, Skov H, Pedersen H. Low cerebral blood flow: a risk factor in the neonate. J Pediatr. 1979;95:606–9. doi: 10.1016/s0022-3476(79)80779-9. [DOI] [PubMed] [Google Scholar]
- 5.Pryds O. Low neonatal cerebral oxygen delivery is associated with brain injury in preterm infants. Acta Paediatr. 1994;83:1233–6. doi: 10.1111/j.1651-2227.1994.tb13002.x. [DOI] [PubMed] [Google Scholar]
- 6.Børch K, Greisen G. Blood flow distribution in the normal human preterm brain. Pediatr Res. 1998;43:28–33. doi: 10.1203/00006450-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Børch K, Greisen G. Regional cerebral blod flow during hypotension and hypoxaemia in preterm infants (abstract) Pediatr Res. 1997;42:389–9. [Google Scholar]
- 8.Børch K, Greisen G. 99mTc-HMPAO as a tracer of cerebral blood flow in newborn infants. J Cereb Blood Flow Metab. 1997;17:448–54. doi: 10.1097/00004647-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Greisen G, Trojaborg W. Cerebral blood flow, PaCO2 changes, and visual evoked potentials in mechanically ventilated, preterm infants. Acta Paediatr Scand. 1987;76:394–400. doi: 10.1111/j.1651-2227.1987.tb10488.x. [DOI] [PubMed] [Google Scholar]
- 10.Tyszczuk L, Meek J, Elwell CE, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998;102:337–41. doi: 10.1542/peds.102.2.337. [DOI] [PubMed] [Google Scholar]
- 11.Miall-Allen VM, de Vries LS, Whitelaw AGL. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child. 1987;62:1068–9. doi: 10.1136/adc.62.10.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munro MJ, Wong F, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114:1591–6. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 13.Astrup J. Energy-requiring cell functions in the ischaemic brain. J Neurosurg. 1982;56:482–97. doi: 10.3171/jns.1982.56.4.0482. [DOI] [PubMed] [Google Scholar]
- 14.Buchvald FF, Keshe K, Greisen G. Measurement of cerebral oxyhaemoglobin saturation and jugular blood flow in term healthy newborn infants by near-infrared spectroscopy and jugular venous occlusion. Biol Neonate. 1999;75:97–103. doi: 10.1159/000014084. [DOI] [PubMed] [Google Scholar]
- 15.Wardle SP, Yoxall CW, Weindling AM. Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab. 2000;20:272–9. doi: 10.1097/00004647-200002000-00008. [DOI] [PubMed] [Google Scholar]


