SUMMARY
BACKGROUND
Low body mass index (BMI) is a known risk factor for tuberculosis (TB) in people without human immunodeficiency virus (HIV), but there are no prospective studies linking BMI to the risk of HIV-associated TB.
DESIGN
In HIV-infected adults with CD4 counts ≥ 200 cells/µl receiving placebo in a TB booster vaccine trial in Dar es Salaam, Tanzania, we measured BMI at baseline and Year 1, and related baseline BMI and change in BMI to the risk of developing TB.
RESULTS
We documented 92 cases of TB among 979 subjects followed for a mean of 3.2 years. Compared to subjects who did not develop TB, subjects who developed TB had a lower baseline BMI (23.2 vs. 24.6 kg/m2, P = 0.006), and a greater BMI decline from baseline to Year 1 (−0.4 vs. 0.6 kg/m2, P < 0.001). In multivariate analyses, baseline BMI was associated with the risk of developing TB (hazard ratio [HR] per kg/m2 0.94, 95%CI 0.90–0.99, P = 0.028), as was the change in BMI from baseline to Year 1 (HR per kg/m2 0.79, 95%CI 0.71–0.87, P < 0.001). Subjects with a baseline BMI < 17 kg/m2 were more likely to develop TB (HR 3.72, 95%CI 1.16–12.0, P = 0.028).
CONCLUSION
Low BMI and falling BMI predict HIV-associated TB.
Keywords: tuberculosis, HIV, body mass index, malnutrition
Malnutrition, typically defined as a body mass index (BMI) of <18.5 kg/m2,1 is common among people living with the human immunodeficiency virus (HIV) in sub-Saharan Africa,2,3 and is an independent predictor of death even in the era of highly active antiretroviral therapy (HAART).4–11
Malnutrition enhances susceptibility to infection,12 and in subjects without HIV infection, low BMI is a risk factor for the development of tuberculosis (TB).13,14 Among patients with HIV, TB disease often arises during malnutrition and even exacerbates it.15–18 Furthermore, malnutrition is associated with more severe pulmonary manifestations of TB disease19 and poorer outcomes after TB therapy.20 However, there are no prospective studies showing a link between BMI and the risk of developing TB among people with HIV.
Given that TB is a leading cause of death among people with HIV,21 and malnutrition is associated with poor clinical outcomes in subjects with HIV,22,23 we studied the relation between BMI and change in BMI over time with the risk of developing TB in HIV-infected adults who received placebo in a TB vaccine trial. Our data show for the first time that low BMI and falling BMI are associated with higher risk of developing HIV-associated TB.
STUDY POPULATION AND METHODS
Study subjects
Subjects were placebo recipients of the DarDar Health Study, a 7-year Phase III randomized placebo-controlled trial in Dar es Salaam, Tanzania, of a novel TB booster vaccine.24 Subjects were enrolled if they were aged ≥18 years, had HIV infection, baseline CD4 counts of ≥200 cells/µl and a scar from childhood bacille Calmette-Guérin (BCG) immunization. We excluded pregnant women and subjects with active TB detected at baseline via clinical evaluation or cultures of sputum and blood.
Human research conduct
Subjects gave informed consent before enrollment. Human experimentation guidelines of the United States Department of Health and Human Services, as well as those of the Committee for the Protection of Human Subjects at Dartmouth College (Lebanon, NH, USA) and the Research Ethics Committee of the Muhimbili University of Health and Allied Sciences (Dar es Salaam, Tanzania), were followed in the conduct of this research. This study was registered through the National Institutes of Health (NCT00052195).
BMI assessment
We recorded height and weight at baseline, and at a second visit (‘Year 1 visit’) an average of 471 days (standard deviation 130 days) after the baseline visit. We used standard equations to calculate BMI, and categorized BMI results by quartiles.
Clinical evaluation
After enrollment, subjects were seen at 2, 4 and 6 months, and then every 3 months. Clinical evaluation for TB was initiated if subjects complained of ≥2 weeks of fever, cough or weight loss. Subjects were instructed to present for ad hoc evaluation if they experienced any of these symptoms. The evaluation for TB involved physical examination, a single view chest X-ray, three sputum collections for acid-fast bacilli smear and culture and blood culture for Mycobacterium tuberculosis. CD4 counts were performed in every subject at baseline, and annually. HIV-1 viral load data were available in a pre-planned subset of subjects.
Definitions of TB
TB cases were defined by a blinded three-person expert panel according to the pre-defined and published study definitions detailed in Table 1.25
Table 1.
DarDar TB case definitions
Definite TB
|
Probable TB
|
TB = tuberculosis; cfu = colony forming units; AFB = acid-fast bacilli.
Tuberculin skin testing
At baseline all subjects had a tuberculin skin test (TST) with 0.1 ml intradermal purified protein derivative (RT-23, 2 tuberculin units [TU], Staten Serum Institute, Copenhagen, Denmark). Subjects with ≥5 mm induration at 48–72 h were considered to have a positive TST and were offered a 6-month course of isoniazid (INH), with an 88% completion rate among the placebo recipient subset.26
Statistical analysis
For univariate comparisons of BMI and clinical characteristics between subjects who did or did not develop TB during prospective follow-up, we used the Mann-Whitney U-test. For analyses of the relation of TB risk with the Year 1 BMI, or the change in BMI from baseline to Year 1, we excluded data from subjects who developed TB before the Year 1 BMI measurement. To determine if BMI was independently associated with the risk of developing HIV-associated TB, we conducted a multivariate Cox proportional hazards regression model adjusting for clinical characteristics that differed at baseline between subjects who did or did not develop TB. We confirmed that the proportional hazards assumption was not violated using log-log plots, and examined if BMI variables had a linear effect on the log hazard using penalized splines, and found no significant departure. All statistical analyses were conducted using STATA 9 (Stata Corp, College Station, TX, USA).
RESULTS
Subject characteristics
Among 979 subjects in prospective follow-up for a mean of 3.2 years, we documented 92 cases of definite or probable TB. Subjects who developed TB during prospective follow-up had lower baseline CD4 counts, were more likely to have a positive TST and were more likely to have received previous TB treatment (Table 2).
Table 2.
Baseline characteristics of subjects who did or did not develop definite or probable TB during prospective follow-up*
| n | TB (n = 92) |
No TB (n = 887) |
P value† | |
|---|---|---|---|---|
| Age, mean, years | 979 | 34.5 | 32.9 | 0.066 |
| Male, % | 979 | 29.3 | 23.7 | 0.227 |
| CD4 count, cell/µl | 977 | 362.7 | 481.6 | <0.001 |
| TST, mean, mm | 961 | 7.7 | 4.9 | 0.001 |
| TST ≥ 5 mm, % | 961 | 48.9 | 30.8 | <0.001 |
| Antiretroviral therapy at baseline, % | 979 | 0.0 | 3.7 | 0.060 |
| Previous TB treatment, % | 979 | 18.4 | 7.1 | <0.001 |
Per protocol, at study entry all subjects were HIV-infected, had a BCG scar, a CD4 count of ≥200 cells/mm3 and had no evidence of active TB (see Methods for details).
Mann-Whitney U-tests.
TB = tuberculosis; TST = tuberculin skin test; HIV = human immunodeficiency virus; BCG = bacille Calmette-Guérin.
TB incidence by BMI quartile
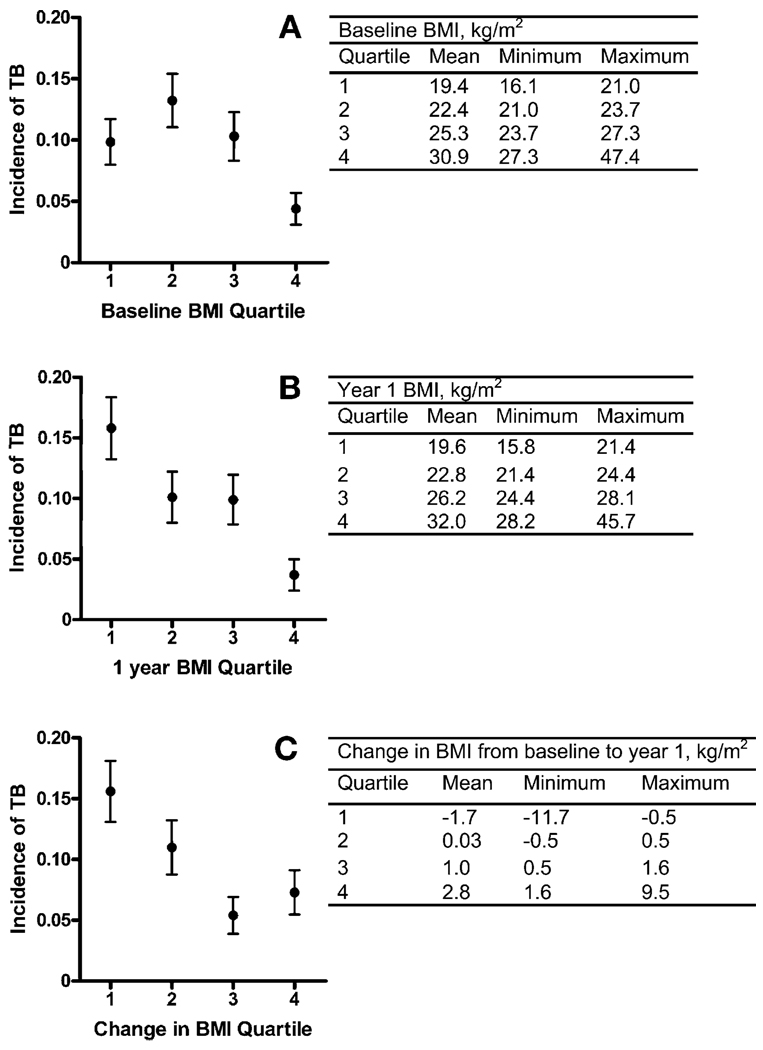
We categorized baseline BMI, Year 1 BMI, and the mean change in BMI from baseline to Year 1 by quartiles. There was a higher incidence of TB among subjects with a lower baseline BMI, a lower Year 1 BMI, and a greater BMI decrease from baseline to Year 1 (Figure 1).
Figure 1.
Relation to the incidence of developing definite or probable TB during prospective follow-up of A) baseline BMI, B) BMI at 1 year, and C) change in BMI at 1 year. Data from subjects who developed TB before the Year 1 BMI measurement were excluded from analyses depicted in B and C. BMI = body mass index; TB = tuberculosis.
BMI in subjects who did or did not develop TB
Subjects who developed TB had a lower mean baseline BMI, a lower mean Year 1 BMI, and a greater mean decrement in BMI from baseline to Year 1 than subjects who did not develop TB during prospective follow-up (Table 3). Excluding subjects who developed TB during the first year after enrollment, subjects who developed TB still had a lower mean baseline BMI compared to subjects who did not develop TB (23.3 vs. 24.6 kg/m2, P = 0.0188). Baseline weight alone was not a predictor of TB risk (data not shown).
Table 3.
BMI in subjects who did or did not develop definite or probable TB during prospective follow-up
| n | TB cases |
TB mean (range) kg/m2 |
No TB mean (range) kg/m2 |
P value† | |
|---|---|---|---|---|---|
| Baseline BMI | 979 | 92 | 23.2 (16.1–35.7) | 24.6 (16.2–47.4) | 0.0058 |
| Year 1 BMI* | 827 | 72 | 23.0 (15.8–34.0) | 25.5 (15.8–45.7) | <0.0001 |
| Year 1 change in BMI* | 827 | 72 | −0.3 (−9.6−4.4) | 0.6 (−11.7−9.5) | 0.0001 |
Data from subjects who developed TB before the Year 1 BMI measurement were excluded from these analyses.
Mann-Whitney U-test.
BMI = body mass index; TB = tuberculosis.
TB incidence by BMI thresholds
Subjects with a baseline BMI < 17 kg/m2 had a high incidence of subsequent TB disease compared to subjects with higher baseline BMI (27.3% vs. 9.2%, P = 0.0411). Of 92 TB cases, 11 occurred in subjects with a baseline BMI < 17 kg/m2. Subjects whose BMI decreased by ≥0.5 kg/m2 from baseline to Year 1 exhibited a substantially greater TB incidence compared to subjects with smaller or no BMI decrease (15.6% vs. 7.9%, P = 0.0013). Table 4 depicts the positive and negative predictive value of BMI thresholds.
Table 4.
PPV and NPV of BMI thresholds in predicting the development of HIV-associated tuberculosis
| All | TST <5 mm | TST ≥5 mm | |
|---|---|---|---|
| Baseline BMI <17 kg/m2 | |||
| n | 979 | 649 | 312 |
| PPV | 0.27 | 0.40 | 0.17 |
| NPV | 0.91 | 0.93 | 0.86 |
| BMI decrease ≥0.5 kg/m2 | |||
| n | 837 | 548 | 272 |
| PPV | 0.16 | 0.11 | 0.24 |
| NPV | 0.93 | 0.93 | 0.91 |
| TST ≥5 mm* | |||
| n | 961 | — | — |
| PPV | 0.14 | — | — |
| NPV | 0.93 | — | — |
Data available for 961 subjects.
PPV = positive predictive value; NPV = negative predictive value; HIV = human immunodeficiency virus; BMI = body mass index; TST = tuberculin skin test.
Relation of BMI data to CD4 count, HIV-1 viral load and receipt of antiretroviral therapy
There was a significant positive correlation between baseline CD4 count and baseline BMI (n = 977, Spearman rho [ρ] 0.1657, P < 0.0001). Among subjects with available CD4 count data at Year 1, the Year 1 CD4 count was lower among subjects who developed TB (n = 244, 296 vs. 461, P < 0.0001), and there was a positive correlation between the Year 1 CD4 count and the Year 1 BMI (n = 241, Spearman ρ 0.1684, P = 0.0088). However, there was no correlation between the change in CD4 count from baseline to Year 1 with the change in BMI from baseline to Year 1 (n = 241, Spearman ρ −0.0945, P = 0.1437). Among subjects with available baseline HIV-1 viral load data, there was a negative correlation between HIV-1 viral load and baseline BMI (n = 341, Spearman ρ −0.2514, P < 0.0001). Subjects who were on HAART by Year 1 did not have a significantly different Year 1 BMI compared to subjects not on HAART (25.8 vs. 25.2 kg/m2, P = 0.3260).
Relation of BMI data to TST status
BMI was lower among subjects with a positive baseline TST compared to subjects with a negative baseline TST (23.9 vs. 24.7 kg/m2, P = 0.0051), and at Year 1 (24.7 vs. 25.5 kg/m2, P = 0.0156). There was, however, no difference in the change in BMI from baseline to Year 1 between subjects with positive and negative baseline TST (0.49 vs. 0.53, P = 0.3994).
Multivariate analyses
We related baseline BMI, Year 1 BMI and the change in BMI from baseline to Year 1 to the risk of subsequently developing TB using a multivariate Cox proportional hazards regression model adjusting for CD4 count, TST status and previous TB treatment history. As shown in Table 5, we found that baseline BMI, Year 1 BMI and change in BMI from baseline to Year 1 were all significantly and independently associated with the risk of developing TB during prospective follow-up. These results were unchanged by adjustment for sex or receipt of HAART.
Table 5.
Risk of developing definite or probable TB during prospective follow-up according to baseline BMI, BMI at 1 year and change in BMI from baseline to Year 1 in a multivariate Cox regression model adjusting for baseline CD4 count, previous TB treatment history and TST status*
| Variable | Hazard ratio† |
95%CI | P value |
|---|---|---|---|
| Risk according to baseline BMI | |||
| Age per 10 years | 1.07 | 0.80–1.43 | 0.630 |
| CD4 count, per 100 cells/µl | 0.70 | 0.60–0.81 | <0.001 |
| Previous TB | 2.52 | 1.47–4.32 | 0.001 |
| Positive TST | 2.01 | 1.32–3.07 | 0.001 |
| Baseline BMI, unadjusted, kg/m2 | 0.92 | 0.88–0.97 | 0.001 |
| Baseline BMI, adjusted, kg/m2 | 0.94 | 0.90–0.99 | 0.028 |
| Risk according to BMI at Year 1 | |||
| Age per 10 years | 1.17 | 0.87–1.59 | 0.306 |
| CD4 count, per 100 cells/µl | 0.73 | 0.62–0.85 | <0.001 |
| Previous TB | 2.0 | 1.09–3.66 | 0.025 |
| Positive TST | 1.68 | 1.07–2.63 | 0.024 |
| Year 1 BMI, unadjusted, kg/m2 | 0.88 | 0.83–0.94 | <0.001 |
| Year 1 BMI, adjusted, kg/m2 | 0.90 | 0.84–0.95 | <0.001 |
| Risk according to change in BMI from baseline to Year 1 | |||
| Age, per 10 years | 1.02 | 0.75–1.38 | 0.924 |
| CD4 count, per 100 cells/µl | 0.71 | 0.60–0.82 | <0.001 |
| Previous TB | 1.97 | 1.07–3.63 | 0.029 |
| Positive TST | 1.81 | 1.16–2.83 | 0.009 |
| Change in BMI, undajusted, kg/m2 | 0.79 | 0.71–0.87 | <0.001 |
| Change in BMI, adjusted, kg/m2 | 0.79 | 0.71–0.87 | <0.001 |
Data from subjects who developed TB before the Year 1 BMI measurement were excluded from these analyses.
All hazard ratios are adjusted unless otherwise indicated.
TB = tuberculosis; BMI = body mass index; TST = tuberculin skin test; CI = confidence interval.
If we restricted our analyses to subjects with a negative baseline TST, there was a trend toward an association between the risk of developing TB with baseline BMI (hazard ratio [HR] 0.93, 95% confidence interval [CI] 0.87–1.01, P = 0.069) and a decrement in BMI from baseline to Year 1 of ≥0.5 kg/m2 (HR 1.67, 95%CI 0.89–3.12, P = 0.108), and significant associations of the risk of TB with Year 1 BMI (HR 0.92, 95%CI 0.85–0.99, P = 0.020) and baseline BMI < 17 kg/m2 (HR 6.09, 95%CI 1.46–25.43, P = 0.013).
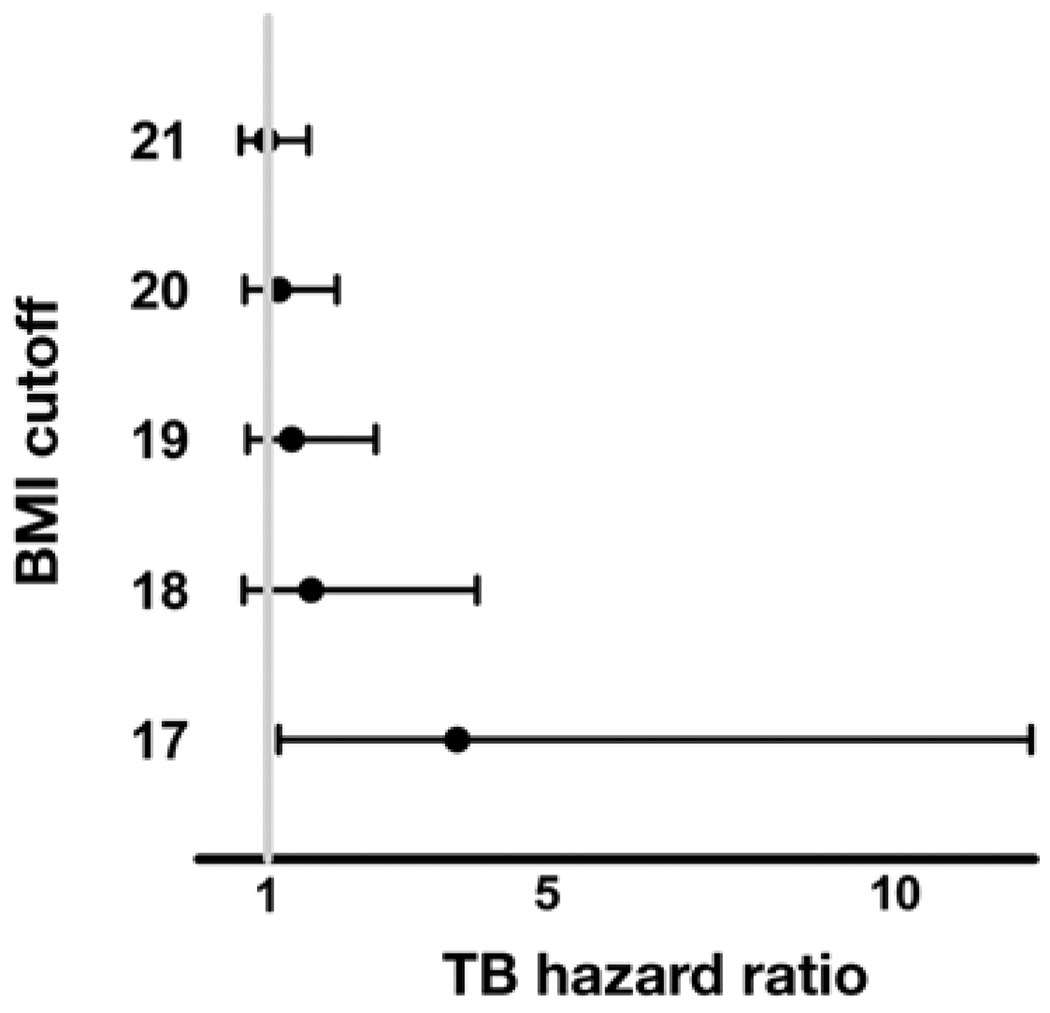
Subjects with a baseline BMI < 17 kg/m2 had a greater risk of developing TB during prospective follow-up (HR 3.72, 95%CI 1.16–12.0, P = 0.028, Figure 2). Similarly, subjects whose BMI fell by >0.5 kg/m2 from baseline to Year 1 had a greater risk of developing TB (HR 2.03, 95%CI 1.29–3.20, P = 0.002).
Figure 2.
Risk of developing definite or probable TB during prospective follow-up according to baseline BMI cut-off. Data from subjects who developed TB before the Year 1 BMI measurement were excluded from these analyses. BMI = body mass index; TB = tuberculosis.
DISCUSSION
In this prospective study in a large cohort of high-risk HIV-infected adults living in a high TB prevalence setting, we found that low BMI and falling BMI are associated with an elevated risk of subsequently developing HIV-associated TB. These data provide the first prospective support for the hypothesis that malnutrition contributes to the development of HIV-associated TB.
Malnutrition aggravates the risk of infection,12 and, in subjects without HIV infection, lower BMI is associated with greater TB risk in low-prevalence areas and in older populations.13,14 However, while cross-sectional studies in people with HIV have demonstrated that subjects with TB disease have lower BMI,16,17,27 and that low BMI is related to a greater bacillary burden during HIV-associated TB,28 these studies left in question whether low BMI was the cause or the result of TB disease. Our prospective study in subjects in whom active TB was excluded at baseline suggests that low BMI contributes to the development of TB, perhaps by exacerbating HIV-associated deficits in cell-mediated immunity.29,30 Clearly, nutritional interventions aimed at preventing TB and other complications of malnutrition merit additional study.31,32
Our study identifies important predictors of TB risk. Specifically, BMI < 17 kg/m2 and a decrease in BMI of ≥0.5 kg/m2 were associated with increased risk of TB in univariate and multivariate models. Although all HIV-infected adults should be screened for TB disease, our data suggest that the risk of TB is even higher among HIV-infected adults with low or falling BMI. While subjects with low or falling BMI constitute the minority of subjects at risk for developing HIV-associated TB, detection of falling BMI may be a particularly useful predictor of TB risk among subjects with a negative TST, in part because malnutrition can lead to TST anergy33 and thus thwart the identification of individuals who would benefit from INH preventive therapy. Intensified TB screening or even consideration of preventive therapy independently of TST status should be assessed among HIV-infected adults with low or falling BMI.
Although the possibility that TST status confounds the relation between BMI and TB risk is raised by the fact that subjects with a positive baseline TST had a higher incidence of developing TB and a lower baseline and Year 1 BMI, our data suggest that, in fact, BMI is independently associated with TB risk. First, adjusting for TST status in our multivariate model did not abrogate the association between BMI and the risk of developing TB. Second, BMI < 17 kg/m2 and Year 1 BMI remained significantly associated with the risk of TB even among subjects with a negative TST. Furthermore, we believe the loss of significant relationships between baseline BMI and change in BMI from baseline to Year 1 with TB risk reflects a loss of statistical power in this smaller cohort.
Strengths of this study include its size and prospective nature, as well as the rigorous and pre-defined case definitions used for TB diagnoses. However, we did not measure other indices of malnutrition in this study, and thus cannot exclude the possibility that low BMI was associated with other non-nutritional factors that could themselves influence TB risk. However, in sub-Saharan Africa, where malnutrition is common especially among people living with HIV,5 we believe it is a reasonable presumption to suppose that low BMI among our subject population is predominantly driven by malnutrition. Low BMI is also associated with HIV disease progression34 and with low CD4 count in our study, but the fact that BMI remained associated with the risk of developing TB even when adjusting for the baseline CD4 count suggests that the impact of malnutrition on TB risk is not entirely explained by the relation of BMI to HIV disease progression. We assessed HIV-1 viral load in only a minority of subjects at baseline, and thus could not adjust for this factor in our multivariate analyses. As low BMI has been associated with higher viral load, as confirmed in our subset analysis, it is conceivable that this relationship contributes somewhat to the observed relationship.19 Importantly, however, we believe BMI remains an important contributor to TB risk stratification among HIV-infected adults in sub-Saharan Africa, not solely because in such settings measuring the BMI is usually much more feasible than measuring the HIV-1 viral load.
We have reported the phenomenon of subclinical TB among people with HIV in Tanzania.35 It is conceivable that active TB emerges slowly in patients with HIV and that low baseline BMI resulted in part from incipient TB, and thus that low BMI represents a symptom of rather than a predisposition to TB disease. Two findings suggest, however, that incipient TB is not the sole or even the major driver of the strong correlation between BMI and TB risk. First, Year 1 BMI remained an independent predictor of TB risk although subjects who developed TB in the year after enrollment were excluded from those analyses. Second, most subjects with a BMI < 17 kg/m2, as is consistent with severe malnutrition,1 did not develop TB. However, even if subclinical TB does contribute to low BMI in a subset of HIV-infected adults in sub-Saharan Africa, the detection of low and falling BMI is an inexpensive and non-invasive approach to raising suspicion for that diagnosis.
CONCLUSIONS
Among HIV-infected adults with CD4 counts of ≥200 cells/µl living in a high TB prevalence area, low BMI and falling BMI were independently and significantly associated with greater risk of developing TB. These results suggest that malnutrition contributes to HIV-associated TB, and that subjects with BMI < 17 kg/m2 or a 1-year BMI decrease of ≥0.5 kg/m2 have a heightened risk of developing HIV-associated TB.
Acknowledgements
Funding: National Institutes of Health (NIH), Division of Acquired Immunodeficiency Syndrome, AI 45407 (CFR) and Fogarty International Center, D43-TW006807 (IM, CFR); NIH, National Center for Research Resources, Centers of Biomedical Research Excellence 5P20RR016437-08 (TL).
References
- 1.World Health Organization. Report of a WHO Expert Consultation. Geneva, Switzerland: WHO; 1995. Physical status: the use and interpretation of anthropometry. Report no. 854. [PubMed] [Google Scholar]
- 2.de Onis M, Blossner M, Borghi E, Frongillo EA, Morris R. Estimates of global prevalence of childhood underweight in 1990 and 2015. JAMA. 2004;291:2600–2606. doi: 10.1001/jama.291.21.2600. [DOI] [PubMed] [Google Scholar]
- 3.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103:541–548. doi: 10.1016/j.trstmh.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Melchior JC, Niyongabo T, Henzel D, Durack-Bown I, Henri SC, Boulier A. Malnutrition and wasting, immunodepression, and chronic inflammation as independent predictors of survival in HIV-infected patients. Nutrition. 1999;15:865–869. [PubMed] [Google Scholar]
- 5.Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:239–246. doi: 10.1097/00042560-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006;7:323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 7.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler DA, Gibert CL, Launer CA, et al. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- 10.van der Sande MA, Schim van der Loeff MF, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr. 2004;37:1288–1294. doi: 10.1097/01.qai.0000122708.59121.03. [DOI] [PubMed] [Google Scholar]
- 11.Yotebieng M, Van Rie A, Moultrie H, Meyers T. Six-month gain in weight, height, and CD4 predict subsequent antiretroviral treatment responses in HIV-infected South African children. AIDS. 2010;24:139–146. doi: 10.1097/QAD.0b013e328332d5ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra RK. Nutritional deficiency and susceptibility to infection. Bull World Health Organ. 1979;57:167–177. [PMC free article] [PubMed] [Google Scholar]
- 13.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis. 1986;69:355–362. [PubMed] [Google Scholar]
- 14.Leung CC, Lam TH, Chan WM, et al. Lower risk of tuberculosis in obesity. Arch Intern Med. 2007;167:1297–1304. doi: 10.1001/archinte.167.12.1297. [DOI] [PubMed] [Google Scholar]
- 15.Paton NI, Castello-Branco LR, Jennings G, et al. Impact of tuberculosis on the body composition of HIV-infected men in Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:265–271. doi: 10.1097/00042560-199903010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S, Padmapriyadarsini C, Sukumar B, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis. 2008;46:946–949. doi: 10.1086/528860. [DOI] [PubMed] [Google Scholar]
- 17.Niyongabo T, Henzel D, Idi M, et al. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition. 1999;15:289–293. doi: 10.1016/s0899-9007(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 18.Lawson L, Yassin MA, Thacher TD, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis. 2008;40:30–35. doi: 10.1080/00365540701509899. [DOI] [PubMed] [Google Scholar]
- 19.Van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004;8:211–217. [PubMed] [Google Scholar]
- 20.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96:291–294. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 21.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 22.Moh R, Danel C, Messou E, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 23.Johannessen A, Naman E, Ngowi BJ, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Reyn CF, Mtei L, Arbeit RD, et al. Prevention of tuberculosis in bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24:675–685. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahey T, Matee M, Mtei L, Bakari M, Pallangyo K, von Reyn CF. Lymphocyte proliferation to mycobacterial antigens is detectable across a spectrum of HIV-associated tuberculosis. BMC Infect Dis. 2009;9:21. doi: 10.1186/1471-2334-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis. 2008;12:1037–1041. [PubMed] [Google Scholar]
- 27.Scalcini M, Occenac R, Manfreda J, Long R. Pulmonary tuberculosis, human immunodeficiency virus type-1 and malnutrition. Bull Int Union Tuberc Lung Dis. 1991;66(1):37–41. [PubMed] [Google Scholar]
- 28.Villamor E, Saathoff E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 co-infection, socio-economic status, and severity of tuberculosis. Eur J Clin Nutr. 2006;60:163–171. doi: 10.1038/sj.ejcn.1602281. [DOI] [PubMed] [Google Scholar]
- 29.McMurray DN. Cellular immune changes in undernourished children. Prog Clin Biol Res. 1981;67:305–318. [PubMed] [Google Scholar]
- 30.Chandra RNP. Nutrition, immunity and infection: mechanisms of interactions. New York, NY, USA: Plenum Press; 1977. [Google Scholar]
- 31.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 32.Koethe JR, Chi BH, Megazzini KM, Heimburger DC, Stringer JS. Macronutrient supplementation for malnourished HIV-infected adults: a review of the evidence in resource-adequate and resource-constrained settings. Clin Infect Dis. 2009;49:787–798. doi: 10.1086/605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelly TF, Santillan CF, Gilman RH, et al. Tuberculosis skin testing, anergy and protein malnutrition in Peru. Int J Tuberc Lung Dis. 2005;9:977–984. [PMC free article] [PubMed] [Google Scholar]
- 34.Shah S, Whalen C, Kotler DP, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001;131:2843–2847. doi: 10.1093/jn/131.11.2843. [DOI] [PubMed] [Google Scholar]
- 35.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]