Abstract
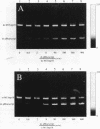
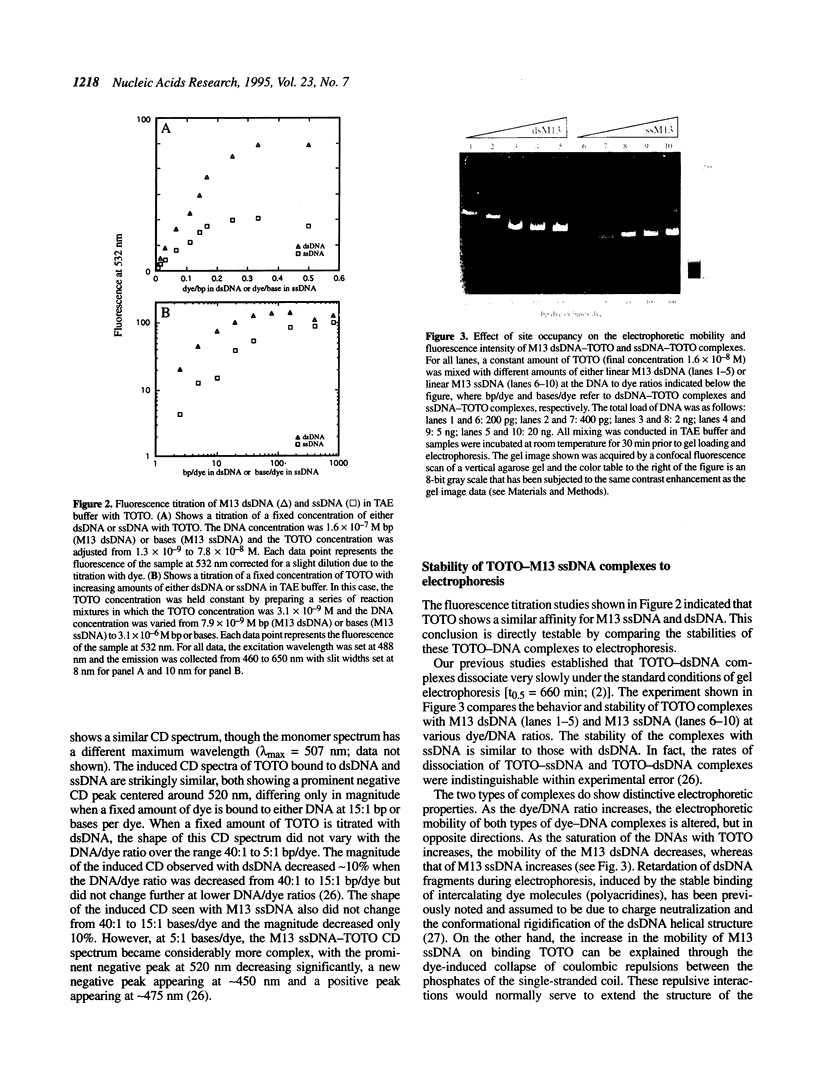
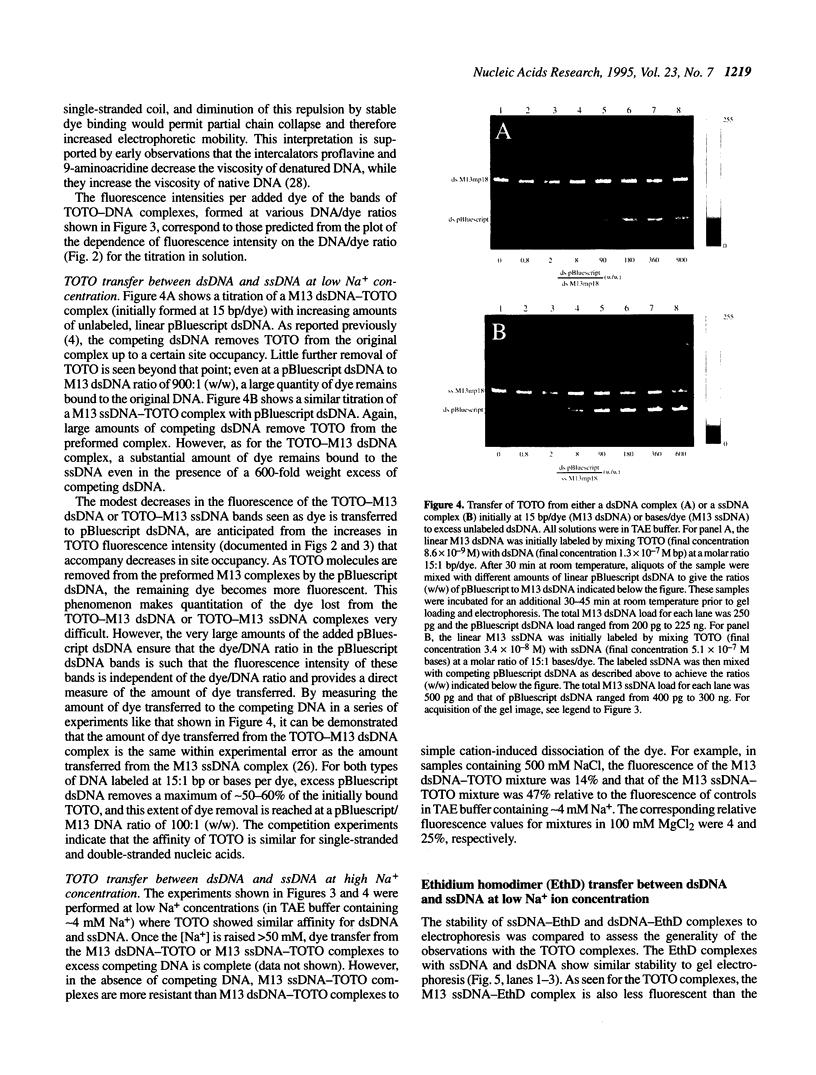
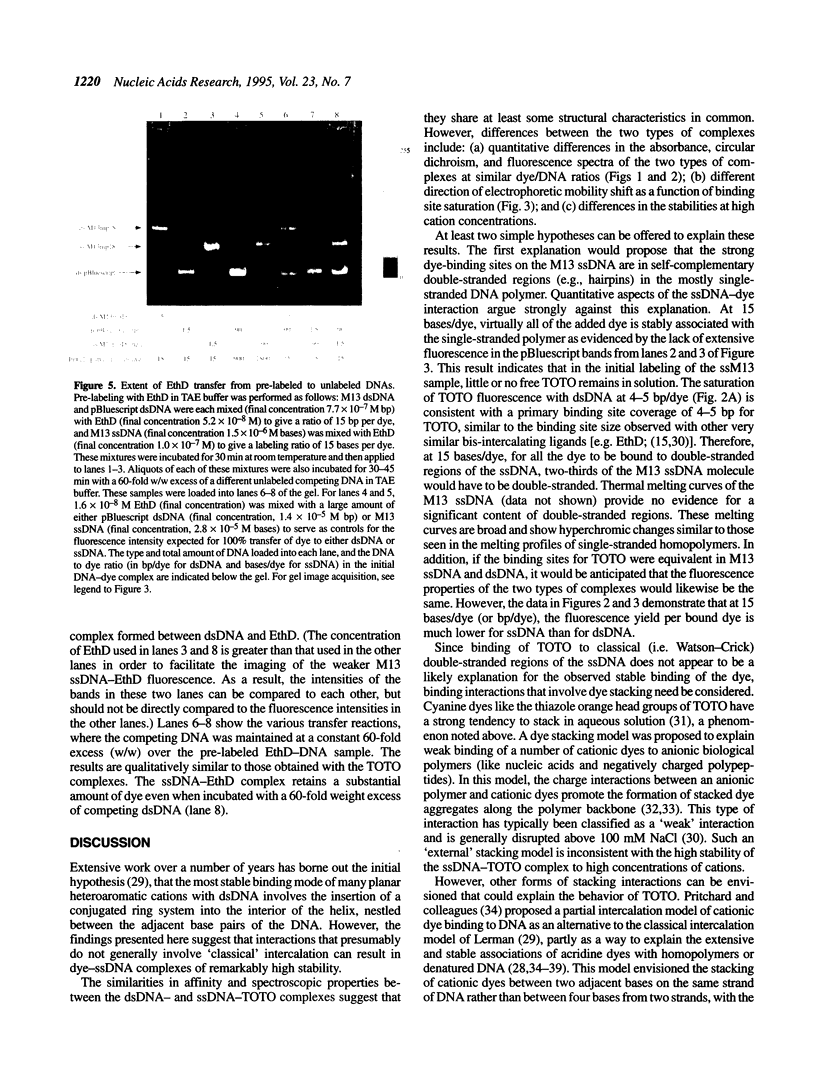
The unsymmetrical cyanine dye thiazole orange homodimer (TOTO) binds to single-stranded DNA (ssDNA, M13mp18 ssDNA) to form a fluorescent complex that is stable under the standard conditions of electrophoresis. The stability of this complex is indistinguishable from that of the corresponding complex of TOTO with double-stranded DNA (dsDNA). To examine if TOTO exhibits any binding preference for dsDNA or ssDNA, transfer of TOTO from pre-labeled complexes to excess unlabeled DNA was assayed by gel electrophoresis. Transfer of TOTO from M13 ssDNA to unlabeled dsDNA proceeds to the same extent as that from M13 dsDNA to unlabeled dsDNA. A substantial amount of the dye is retained by both the M13 ssDNA and M13 dsDNA even when the competing dsDNA is present at a 600-fold weight excess; for both dsDNA and ssDNA, the pre-labeled complex retains approximately one TOTO per 30 bp (dsDNA) or bases (ssDNA). Rapid transfer of dye from both dsDNA and ssDNA complexes is seen at Na+ concentrations > 50 mM. Interestingly, at higher Na+ or Mg2+ concentrations, the M13 ssDNA-TOTO complex appears to be more stable to intrinsic dissociation (dissociation in the absence of competing DNA) than the complex between TOTO and M13 dsDNA. Similar results were obtained with the structurally unrelated dye ethidium homodimer. The dsDNA- and ssDNA-TOTO complexes were further examined by absorption, fluorescence and circular dichroism spectroscopy. The surprising conclusion is that polycationic dyes, such as TOTO and EthD, capable of bis-intercalation, interact with dsDNA and ssDNA with very similar high affinity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auzanneau I., Barreau C., Salomé L. Imaging by fluorescence videomicroscopy of individual single stranded DNA molecules in solution. C R Acad Sci III. 1993;316(5):459–462. [PubMed] [Google Scholar]
- Benson S. C., Mathies R. A., Glazer A. N. Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res. 1993 Dec 11;21(24):5720–5726. doi: 10.1093/nar/21.24.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. Extrinsic Cotton effects of proflavine bound to polynucleotides. Biopolymers. 1967 Apr-May;5(4):383–397. doi: 10.1002/bip.1967.360050407. [DOI] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourlent M., Hélène C. A quantitative analysis of proflavine binding to polyadenylic acid, polyuridylic acid, and transfer RNA. Eur J Biochem. 1971 Nov 11;23(1):86–95. doi: 10.1111/j.1432-1033.1971.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Drummond D. S., Pritchard N. J., Simpson-Gildemeister V. F., Peacocke A. R. Interaction of aminoacridines with deoxyribonucleic acid: viscosity of the complexes. Biopolymers. 1966 Oct-Nov;4(9):971–987. doi: 10.1002/bip.1966.360040903. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Giesman-Cookmeyer D., Ding B., Lommel S. A., Lucas W. J. Cell-to-Cell Trafficking of Macromolecules through Plasmodesmata Potentiated by the Red Clover Necrotic Mosaic Virus Movement Protein. Plant Cell. 1993 Dec;5(12):1783–1794. doi: 10.1105/tpc.5.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Capelle N., Roques B. P., Le Pecq J. B. DNA Bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Oberlin R., Roques B. P., Le Pecq J. B. DNA bifunctional intercalators. I. Synthesis and conformational properties of an ethidium homodimer and of an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5071–5078. doi: 10.1021/bi00617a001. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Peck K., Mathies R. A. A stable double-stranded DNA-ethidium homodimer complex: application to picogram fluorescence detection of DNA in agarose gels. Proc Natl Acad Sci U S A. 1990 May;87(10):3851–3855. doi: 10.1073/pnas.87.10.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Rye H. S. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature. 1992 Oct 29;359(6398):859–861. doi: 10.1038/359859a0. [DOI] [PubMed] [Google Scholar]
- Goodwin P. M., Johnson M. E., Martin J. C., Ambrose W. P., Marrone B. L., Jett J. H., Keller R. A. Rapid sizing of individual fluorescently stained DNA fragments by flow cytometry. Nucleic Acids Res. 1993 Feb 25;21(4):803–806. doi: 10.1093/nar/21.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirons G. T., Fawcett J. J., Crissman H. A. TOTO and YOYO: new very bright fluorochromes for DNA content analyses by flow cytometry. Cytometry. 1994 Feb 1;15(2):129–140. doi: 10.1002/cyto.990150206. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Denaturation of nucleic acids induced by intercalating agents. Biochemical and biophysical properties of acridine orange-DNA complexes. J Biomol Struct Dyn. 1984 Jun;1(6):1485–1499. doi: 10.1080/07391102.1984.10507532. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Increased accessibility of bases in DNA upon binding of acridine orange. Nucleic Acids Res. 1983 Nov 11;11(21):7555–7568. doi: 10.1093/nar/11.21.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Melamed M. R. Interactions of acridine orange with nucleic acids. Properties of complexes of acridine orange with single stranded ribonucleic acid. Biochem Pharmacol. 1983 Dec 15;32(24):3679–3694. doi: 10.1016/0006-2952(83)90136-3. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Markovits J., Roques B. P., Le Pecq J. B. Ethidium dimer: a new reagent for the fluorimetric determination of nucleic acids. Anal Biochem. 1979 Apr 15;94(2):259–264. doi: 10.1016/0003-2697(79)90357-9. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Zhen W. P., Henriksen U., Buchardt O. Sequence-influenced interactions of oligoacridines with DNA detected by retarded gel electrophoretic migrations. Biochemistry. 1988 Jan 12;27(1):67–73. doi: 10.1021/bi00401a012. [DOI] [PubMed] [Google Scholar]
- Noueiry A. O., Lucas W. J., Gilbertson R. L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell. 1994 Mar 11;76(5):925–932. doi: 10.1016/0092-8674(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Quake S. R., Smith D. E., Chu S. Relaxation of a single DNA molecule observed by optical microscopy. Science. 1994 May 6;264(5160):822–826. doi: 10.1126/science.8171336. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Smith D. E., Chu S. Direct observation of tube-like motion of a single polymer chain. Science. 1994 May 6;264(5160):819–822. doi: 10.1126/science.8171335. [DOI] [PubMed] [Google Scholar]
- Pritchard N. J., Blake A., Peacocke A. R. Modified intercalation model for the interaction of amino acridines and DNA. Nature. 1966 Dec 17;212(5068):1360–1361. doi: 10.1038/2121360a0. [DOI] [PubMed] [Google Scholar]
- Quesada M. A., Rye H. S., Gingrich J. C., Glazer A. N., Mathies R. A. High-sensitivity DNA detection with a laser-excited confocal fluorescence gel scanner. Biotechniques. 1991 May;10(5):616–625. [PubMed] [Google Scholar]
- Rodríguez-Núez A., Camiña F., Lojo S., Rodríguez-Segade S., Castro-Gago M. Concentrations of nucleotides, nucleosides, purine bases and urate in cerebrospinal fluid of children with meningitis. Acta Paediatr. 1993 Oct;82(10):849–852. doi: 10.1111/j.1651-2227.1993.tb17625.x. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Dabora J. M., Quesada M. A., Mathies R. A., Glazer A. N. Fluorometric assay using dimeric dyes for double- and single-stranded DNA and RNA with picogram sensitivity. Anal Biochem. 1993 Jan;208(1):144–150. doi: 10.1006/abio.1993.1020. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Drees B. L., Nelson H. C., Glazer A. N. Stable fluorescent dye-DNA complexes in high sensitivity detection of protein-DNA interactions. Application to heat shock transcription factor. J Biol Chem. 1993 Nov 25;268(33):25229–25238. [PubMed] [Google Scholar]
- Rye H. S., Quesada M. A., Peck K., Mathies R. A., Glazer A. N. High-sensitivity two-color detection of double-stranded DNA with a confocal fluorescence gel scanner using ethidium homodimer and thiazole orange. Nucleic Acids Res. 1991 Jan 25;19(2):327–333. doi: 10.1093/nar/19.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Picogram detection of stable dye-DNA intercalation complexes with two-color laser-excited confocal fluorescence gel scanner. Methods Enzymol. 1993;217:414–431. doi: 10.1016/0076-6879(93)17080-o. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Wemmer D. E., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications. Nucleic Acids Res. 1992 Jun 11;20(11):2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K., Morris S. C., Girard J. E., Kline M. C., Reeder D. J. Enhanced detection of PCR products through use of TOTO and YOYO intercalating dyes with laser induced fluorescence--capillary electrophoresis. Appl Theor Electrophor. 1993;3(5):235–239. [PubMed] [Google Scholar]
- Zhu H., Clark S. M., Benson S. C., Rye H. S., Glazer A. N., Mathies R. A. High-sensitivity capillary electrophoresis of double-stranded DNA fragments using monomeric and dimeric fluorescent intercalating dyes. Anal Chem. 1994 Jul 1;66(13):1941–1948. doi: 10.1021/ac00085a004. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Schwarz G. Complex formation of acridine orange with single-stranded polyriboadenylic acid and 5'-AMP: cooperative binding and intercalation between bases. Biophys Struct Mech. 1979 Mar 21;5(1):75–90. doi: 10.1007/BF00535774. [DOI] [PubMed] [Google Scholar]