Abstract
Although it is believed that little recovery occurs after adult mammalian spinal cord injury, in fact significant spontaneous functional improvement commonly occurs after spinal cord injury in humans. To investigate potential mechanisms underlying spontaneous recovery, lesions of defined components of the corticospinal motor pathway were made in adult rats in the rostral cervical spinal cord or caudal medulla. Following complete lesions of the dorsal corticospinal motor pathway, which contains more than 95% of all corticospinal axons, spontaneous sprouting from the ventral corticospinal tract occurred onto medial motoneuron pools in the cervical spinal cord; this sprouting was paralleled by functional recovery. Combined lesions of both dorsal and ventral corticospinal tract components eliminated sprouting and functional recovery. In addition, functional recovery was also abolished if dorsal corticospinal tract lesions were followed 5 weeks later by ventral corticospinal tract lesions. We found extensive spontaneous structural plasticity as a mechanism correlating with functional recovery in motor systems in the adult central nervous system. Experimental enhancement of spontaneous plasticity may be useful to promote further recovery after adult central nervous system injury.
Many central nervous system (CNS) lesions lead to functional deficits that fail to improve over time. Yet, spontaneous improvement in motor, sensory, or other neurological function occurs in 41% of patients who sustain spinal cord injuries (1, 2) and in a large proportion of patients with strokes (3, 4) and head trauma (5). Although some of this improvement seems to result from rapid resolution of diaschisis (transient disruption of electrical transmission) or from functional compensation (use of uninjured systems to compensate for injured pathways) (6, 7), a significant proportion of functional recovery occurs over a more protracted time period of several weeks to months. This protracted recovery phase results in restitution of original patterns of the disrupted function, often at a less complete level compared with the preinjured state. However, mechanisms underlying long-term recovery are unknown.
The existence of spontaneous recovery over longer time periods raises the possibility that intrinsic structural rearrangements may occur after injury to the adult mammalian CNS. Previous studies have demonstrated that lesions of adult sensory and motor cortex, hippocampus, or red nucleus are followed by compensatory collateral sprouting of axons (8–16), although these compensatory changes have not been correlated with functional recovery.
The present study examined mechanisms underlying recovery after lesions of an important motor system in animals, the corticospinal projection to the spinal cord. The corticospinal system was examined because of its well-defined anatomical and functional properties (17–19), its relevance to human injury, and its frequent targeting in efforts to promote regeneration after injury (20–24). The corticospinal tract (CST) originates in primary motor cortex and projects in the spinal cord through a crossed dorsal component that contains 95% of the descending axons and an ipsilateral ventral component containing less than 5% of all CST axons (Fig. 1). Two other minor components of the CST also exist, the lateral and dorsolateral components, together constituting less than 2% of CST axons (25–27). Precise bilateral lesions were made to either the dorsal CST (dCST, including the dorsal columns) at the cervical C3 level, the ventral CST (vCST) at the cervical C2 level, the entire CST at the medullary level (MED), or both ventral and dorsal components of the CST (d/vCST) simultaneously at the C3 + C2 levels. An additional group of animals received a dorsal C3 CST lesion followed 5 weeks later by a lesion of the vCST. Effects of lesions on skilled forelimb movement, the primary functional role of the CST, were measured by using a pellet retrieval task (19). CST axons were then anterogradely labeled with a high-resolution axonal tracer, and brains and spinal cords were examined for responses of axons and their terminals to injury. We found that significant spontaneous structural modifications of CST systems occur after lesions, and these modifications correlate with functional recovery.
Figure 1.
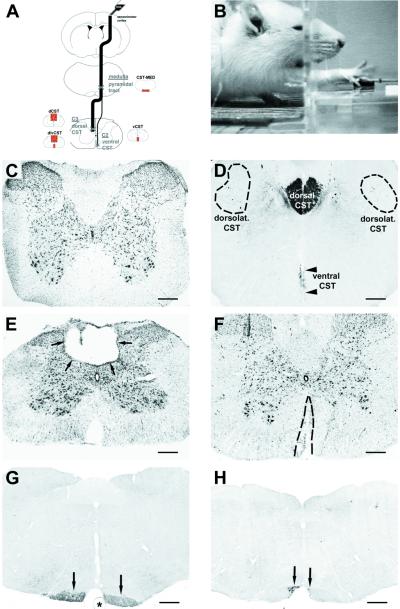
Lesion models and functional testing. (A) Lesion model. Animals received bilateral lesions of the CST in the MED just rostral to the pyramidal decussation, to the dCST at C3, to the vCST at cervical level C2, or to the d/vCST at C3 and C2. The CST was anterogradely labeled by BDA injected into sensorimotor cortex. (B) Behavioral testing: skilled forelimb task. Illustrated is an animal reaching through an aperture to retrieve a food pellet (arrow). (C–H) Morphology of lesions. Nissl stain (C) and BDA-labeled (D) coronal sections at C3 in an intact animal. Most BDA-labeled CST axons are located in the dorsal CST. The dorsolateral CST is dispersed throughout the dorsolateral columns (dashed circles). The ventral CST projects in the ventromedial portion of the cord (arrowheads). Rare BDA-labeled axons comprising the lateral CST are scattered throughout lateral aspects of the lateral columns and are not detectable at this magnification. (E) Representative Nissl-stained section through the center of a dCST lesion at C3 (arrows). The lesion affects the dorsal CST and much of the dorsal sensory columns without disrupting the surrounding spinal cord. (F) Representative Nissl-stained section through the center of a vCST lesion. The lesion is confined to the ventromedial columns bilaterally. (G) Coronal section located 200 μm rostral to a MED CST lesion showing the intact ventral BDA-labeled CST rostral to the lesion (arrows). Asterisk indicates basilar artery. (H) Coronal section from the epicenter of a bilateral medullary CST lesion. The lesion is confined to the CST bilaterally, leaving surrounding structures intact. The lateral portion of the BDA-labeled CST is completely disrupted, whereas the medial portion (arrows) is incompletely lesioned to avoid damage to the basilar artery. The wire knife could not be apposed as closely to the basilar artery on the left side as on the right side because of a restricted visual field, sparing slightly more CST axons on the left (scale bars, C–F = 283 μm; G and H = 458 μm).
Materials and Methods
Animal Subjects.
Adult Fischer 344 rats weighing 150–165 g had free access to water throughout the experiment. For behavioral testing, animals were food deprived to no less than 80% of body weight. American Association for the Accreditation of Laboratory Animal Care guidelines for care were strictly observed.
Groups and Surgical Procedures.
To make precise lesions in specific components of the corticospinal system, rats underwent (i) a bilateral dorsal column transection to disrupt the dorsal crossed corticospinal projection at C3 (dCST; n = 9), (ii) a bilateral lesion of the ventral uncrossed corticospinal projection at C2 (vCST; n = 4), (iii) a combined bilateral dorsal column and ventral CST transection (d/vCST; n = 5), or (iv) a bilateral ventral medullary pyramidal lesion just rostral to the pyramidal decussation (MED; n = 9) (Fig. 1A). After analyzing results from the preceding groups of subjects, an additional group received dCST lesions followed 5 weeks later by vCST lesions (dCST then vCST; n = 8). Animals were anesthetized with 0.35 ml of a combination of ketamine (50 mg/kg), xylazine (2.6 mg/kg), and acepromazine (0.5 mg/kg). Lesions were made with precise microwire lesion devices as follows.
Cervical dorsal column transection.
Rats underwent laminectomy at C3, and a tungsten wire knife (Kopf Instruments, Tujunga, CA) stereotaxically positioned at the spinal dorsal midline (28) was lowered to a depth of 1.1 mm ventral to the dorsal surface, 0.6 mm to the left of midline. The tip of the knife was extruded, forming a 2.25-mm-wide wire arc that was raised 2 mm to lesion the CST bilaterally. A large proportion of the overlying dorsal funicular dorsal sensory ascending projection was also lesioned by this procedure. The wire arc was retracted back into the wire knife device and removed.
Cervical ventral CST lesion.
A 1-mm opening was made in the C2 ventral vertebral body by using an anterior approach. Using a fine microknife (Moria; Fine Science Tools, Belmont, CA), a precise 1-mm-deep, 1-mm-wide lesion of the ventral CST bilaterally was made.
Combined cervical dorsal and ventral lesion.
Procedures (i) and (ii) were performed in one session to lesion both the vCST at C2 and the dCST at C3.
Medullary pyramidal lesion.
After exposure of the clivus by using an anterior approach, the pyramidal tract was lesioned bilaterally just rostral to its decussation, by using the tungsten wire knife. The knife was stereotaxically positioned just lateral to the basilar artery on the left and was lowered to an intramedullary depth of 0.1 mm. The tip of the knife was extruded, forming a 2-mm-wide wire arc and was raised 1 mm to lesion the left CST. This procedure was repeated on the right to lesion the right CST.
Dorsal then ventral CST lesions.
Procedures (i) and (ii) were performed in two surgical sessions. The dCST lesion was first placed as described above, and the vCST lesion was performed 5 weeks later.
Behavioral Testing.
For functional analysis, rats were trained daily on a modified skilled forelimb reaching task as previously described (19, 29). Rats were free to reach with either forelimb into a Plexiglas chamber through a 1.4 × 5.0-cm aperture, across a 2-cm gap, to reach a food pellet (Fig. 1B). Animals were trained for 2 weeks preoperatively, 30 trials per day, then tested for an additional 3 weeks postoperatively, 30 trials per day, beginning on the second postoperative week. Animals that underwent dorsal-then-ventral CST lesions received an additional 2 weeks of behavioral testing after the second lesion. The number of successful pellet retrievals was recorded. Analysis of specific features of forelimb use while retrieving pellets was also performed to gain insight into mechanisms underlying motor recovery; measurements were based on descriptions of Whishaw et al. (19). Briefly, pellet retrieval requires coordinated activation of both proximal and distal forelimb muscles, including advancing the forepaw to the pellet (proximal muscles), grasping the pellet (distal muscles), then retracting the limb into the testing box (proximal muscles) while the paw maintains a grip on the pellet (distal muscles). In executing this task, activation of proximal forelimb muscles is generated primarily by neurons of the spinal cord medial motor neuron column, and distal forelimb muscles are innervated primarily by neurons of the lateral motor neuron column (30). Four features of pellet retrieval were quantified on the last postoperative day of functional testing in each animal: failed advances, the number of times that the forelimb failed to advance fully to reach the pellet (a failure of proximal muscle function); missed aims, the number of reaching attempts that aimed for but missed the pellet (a failure of aiming the limb properly, a proximal muscle function); digit spread, the spreading of digits upon reaching for the food reward (reflecting integrity of distal muscle function); and pellet drops, the number of times that a pellet was lifted but then dropped (a reflection of either proximal or distal muscle function). Digit spread was measured in all trials; failed advances, missed aims, and pellet drops were measured on trials in which the pellet was not successfully retrieved. Each trial was videotaped, and frame-by-frame analysis based on sampling of 30 frames/s was conducted. An observer blinded to group identity rated the behavioral performance of each animal.
Anterograde CST Labeling and Quantification.
Three-hundred nanoliters of a 10% solution of biotinylated-dextran amine (BDA; 10,000 MW; Molecular Probes) were injected into each of 18 sites per hemisphere of the rat forelimb sensorimotor cortex (31). Two weeks later, animals were perfused and 35-μm coronal sections were cut from 3-mm-thick tissue blocks at medullary, C2, and C3 cervical lesion sites. An additional block from C4 was processed to quantify CST axons caudal to the lesion. BDA-labeled CST axons were visualized by using avidin–biotin peroxidase incubation followed by diaminobenzidine, H2O2, and nickel chloride. Ventral horn motoneurons were identified in the same section by immunolabeling for choline acetyltransferase (goat anti-ChAT; Chemicon) (28, 32). BDA-labeled CST axons were quantified in the dCST, vCST, dorsolateral CST, and lateral CST in two coronal sections spaced 200 μm apart at the C4 level by using stereological methods. The total number of BDA-labeled dorsal CST axons was quantified stereologically by using a high numerical aperture 60× oil-immersion objective with a 5% sampling frame on images captured with NIH IMAGE software. The total number of BDA-labeled coronal CST profiles contacting ChAT-labeled motoneurons in the medial or lateral motoneuron columns of Rexed's lamina IX was also quantified in ten coronal sections per subject located 40 μm apart by using a 60× oil-immersion objective, sequentially moving through each section at 0.1-μm increments by using a motorized z axis stage (Ludl, Inc., Hawthorne, NY). Thus, in the sampled sections, all putative BDA contacts with motor neurons were quantified.
Statistics.
Group differences were compared by ANOVA and post hoc comparisons by Fisher's least square difference. Data are presented as mean ± SEM.
Results
CST Lesions Are Accurately Targeted and Reduce or Eliminate Projecting Axons.
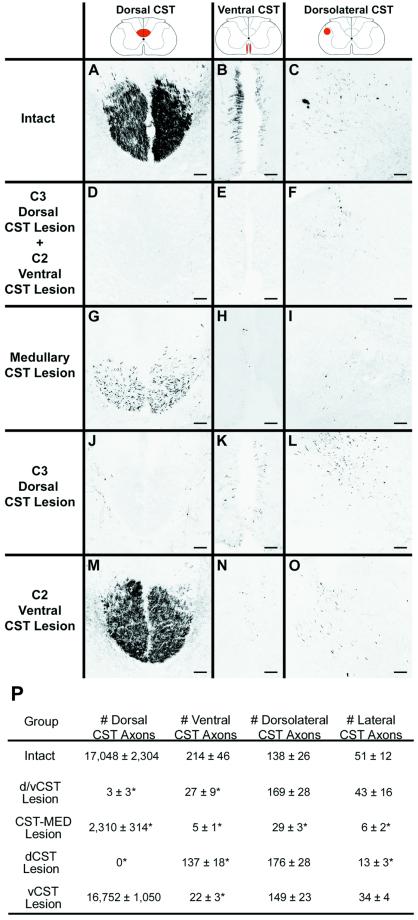
Specific labeling of the CST with BDA was used to assess lesion completeness and morphological response of lesioned axons. Dorsal bilateral CST lesions at C3 completely interrupted this pathway (Fig. 2 J–L and P), reducing the number of BDA-labeled dorsal CST axons from 17,000 in intact animals to zero in lesioned subjects. Similarly, ventral bilateral CST lesions at C2 reduced the number of ventral axons by 90% (Fig. 2 M–P). Combined lesions of both the dorsal and ventral pathway eliminated nearly all CST axons, leaving only a few lateral and dorsolateral axons (Fig. 2 D–F and P). Finally, bilateral medullary lesions eliminated ≈85% of all CST axons; these lesions were incomplete because of the need to avoid damaging the basilar artery that straddles the medial component of the CST (Fig. 2 G–I and P).
Figure 2.
Extent of CST lesions. CST axons were anterogradely labeled by bilateral BDA injections into sensorimotor cortex. Coronal sections were examined at C4, caudal to all lesions. (A, D, G, J, and M) Dorsal CST. (B, E, H, K, and N) Ventral CST. (C, F, I, L, and O) Dorsolateral CST. In intact animals (A–C), the tightly bundled dorsal CST is evident, together with the less dense ventral CST aligned along the median fissure. The dorsolateral tract is loosely organized, and the lateral CST is diffusely interspersed within lateral regions of the white matter (not shown). In animals with combined dorsal and ventral CST lesions (d/vCST; D–F), virtually all dorsal and ventral CST axons are eliminated, and numbers of dorsolateral CST axons are unchanged (see also P). Subjects with MED CST lesions (G–I) show a loss of 86% of dorsal CST axons, 94% of ventral CST axons, 79% of dorsolateral CST axons, and 88% of lateral CST axons. Animals with dCST lesions (J–L) exhibit a loss of all dorsal CST axons. In addition, although dorsal CST lesions were restricted to the midline dorsal white matter of the cord, numbers of ventral CST axons were reduced by 36%, and numbers of lateral CST axons were reduced by 76%, possibly because of collateralization of CST axons at the medullary level (49). Animals with ventral CST lesions (vCST; M–O) showed a 90% reduction in ventral CST axons but no losses in any other portions of the CST projection. (P) Quantification of axon numbers in each component of the CST after specific lesions (* ANOVA, P < 0.001; scale bars, A–O = 73 μm).
Spontaneous Functional Recovery Occurs After Complete Dorsal Corticospinal Tract Lesions.
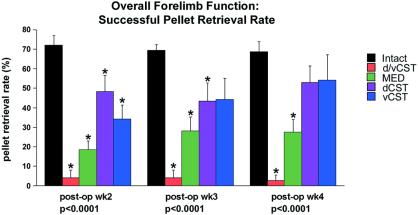
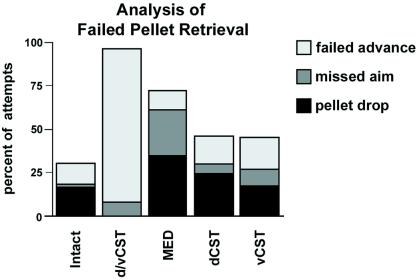
During preoperative training, all groups performed the pellet retrieval task equally well; group differences were not evident (ANOVA, P = 0.45; data not shown). Postoperatively, lesions of specific CST components resulted in significant disturbances of skilled forelimb function (ANOVA, P < 0.0001; Fig. 3), but spontaneous recovery occurred depending on the location of the lesion. Whereas intact animals retrieved food pellets in 70.2% of trials, animals with simultaneous lesions of the dorsal and ventral components of the CST bilaterally (d/vCST) exhibited a severe and persistent reduction in performance, retrieving pellets in only 3.5% of trials (Fig. 3). Animals with bilateral medullary lesions (MED) also exhibited significant and persistent deficits, retrieving pellets in 24.7% of all trials. Deficits were presumably less severe after MED lesions than in d/vCST lesions because of sparing of 15% of CST axons in MED subjects, and possibly because of some damage to dorsal column sensory axons in d/vCST animals. Animals with simultaneous dorsal and ventral CST lesions (d/vCST and MED groups) exhibited increased errors attributable to both proximal and distal forelimb motor functions (failed advances, missed aims, and pellet drops; Fig. 4). Animals with d/vCST lesions in particular showed severe reductions in ability to advance forelimbs (Fig. 4) and absence of digit spread. Significant differences in digit spread were not detected in other experimental groups relative to intact subjects (P = 0.23; data not shown).
Figure 3.
Behavior of pellet retrieval. Data are presented for each group as percentage of successful pellet retrievals on the last day of testing 2, 3, and 4 weeks postoperatively. Preoperatively, groups did not differ significantly from one another (P = 0.45; data not shown). Significant postoperative group differences were present; asterisks indicate significant post hoc differences compared with intact animals (post hoc Fisher's). Animals with lesions of ventral plus dorsal components of the CST exhibited long-lasting, stable deficits in skilled forelimb use. Animals with isolated lesions of either the dorsal CST or ventral CST showed significant deficits compared with intact animals 2 weeks postlesion, which improved by 4 weeks postlesion. Thus, recovery occurred over several weeks after isolated lesions of the dorsal or ventral CST.
Figure 4.
Behavior. Detailed analysis of forelimb movements revealed the nature of errors in pellet retrieval 4 weeks postoperatively. Animals with long-lasting deficits in pellet retrieval (d/vCST, MED) exhibited particular dysfunction of proximal forelimb use. Animals with d/vCST lesions showed a 9-fold increase in failed advances and a 6.4-fold increase in missed aims compared with intact animals. In contrast, animals with dCST lesions had only a small increase in failed advances (1.4-fold), missed aims (2-fold), and pellet drops (1.9-fold) and failed to exhibit long-term deficiencies in pellet retrieval rate (see Fig. 3).
Animals with vCST lesions had an initial deficit on the first postoperative week of testing, despite the fact that the lesion affected fewer than 5% of all CST axons (Fig. 3). However, this deficit rapidly resolved by the second week of testing compared with intact animals. Most pellet retrieval errors in vCST-lesioned animals were attributable to proximal forelimb deficits, with increased numbers of failed advances and missed aims (Fig. 4).
Interestingly, animals with lesions of the dorsal component of the CST, which contains 95% of all corticospinal axons, also exhibited only transient impairment in skilled forelimb use. Significant impairments in limb use were present 2 and 3 weeks postoperatively in dCST animals; however, these deficits resolved by the fourth postoperative week relative to intact animals, and food pellets were successfully retrieved in 58.5% of trials, an amount that did not differ from intact animals on post hoc Fisher's. Analysis of pellet retrieval pattern on the fourth postoperative week in dCST-lesioned animals revealed a modest increase in the number of errors attributable to either proximal or distal forelimb motor function (failed advances, missed aims, and pellet drops; Fig. 4). Thus, lesions of greater than 95% of the CST only transiently impaired function on the skilled forelimb task, and functional deficits resolved by the fourth postoperative week.
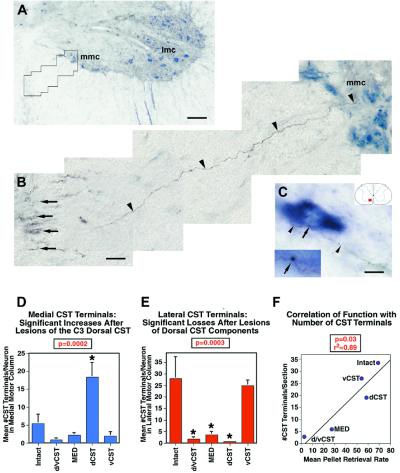
Corticospinal Axon Terminals Sprout onto Neurons of the Medial Motor Neuron Pool After Lesions.
The number of CST axon terminals on motor neurons in the medial and lateral motor columns in lamina IX of spinal gray matter was quantified in all groups by using detailed analysis of putative BDA-labeled axon contacts with ChAT-labeled motoneurons at C4 in ten sections per subject (Fig. 5). Direct projections from ventral CST axons to neurons of the medial motor neuron pool were revealed for the first time (Fig. 5 A–C). Notably, total numbers of CST contacts with the medial motor neuron pool significantly increased following lesions to the dCST, reaching levels 331% greater than those of intact animals 6 weeks postoperatively (P < 0.001; Fig. 5D). Animals with lesions that included the ventral component of the CST did not exhibit this increase (nor did they show functional recovery). In contrast, CST contacts with the lateral motor neuron pool were significantly reduced in all groups with lesions that affected the dorsal CST, including dCST-, MED-, and d/vCST-lesioned animals (Fig. 5E). Thus, substantial lesion-induced structural changes in CST contacts with spinal cord targets occurred. Lesions to the dorsal system resulted in compensatory increases from ventral inputs, if such inputs were not also lesioned.
Figure 5.
Spontaneous compensatory CST sprouting after injury. Contacts of BDA-labeled CST axons (brown-black) with ChAT-immunolabeled motoneurons (blue) were quantified in the medial motoneuron column (mmc) and lateral motoneuron columns (lmc) at C4. (A) Ventral horn and ventral white matter are shown, with ChAT-labeled motoneurons in the medial and lateral motor columns. Animal with dorsal CST lesion. (B) Higher magnification of the boxed area in A. A BDA-labeled axon (arrowheads) branches off the ventral CST (arrows) and projects to the medial motoneuron column, Rexed's lamina IX. (C) A BDA-labeled CST axon (arrowheads) projects from the ventral white matter into the medial motoneuron column and contacts a ChAT-labeled motoneuron, exhibiting a bouton-like swelling at the site of this contact (arrow). This point of putative contact is shown at higher magnification in the Inset. The total number of bouton-like contacts was quantified through the full thickness of ten coronal sections at C4 in every animal. (D and E) Total number of BDA-labeled CST axons contacting ChAT-labeled motoneurons in lamina IX at C4. (D) BDA-labeled CST axon contacts in the medial motoneuron column of lamina IX are significantly increased only in dCST-lesioned animals compared with intact animals (ANOVA, P < 0.01). (E) All lesions disrupting the dorsal CST (d/vCST, MED, dCST) yield a significant reduction of BDA-labeled CST terminations in the lateral motoneuron column of lamina IX (ANOVA, P < 0.001). (F) The total number of CST terminations on medial + lateral motor neurons correlates significantly with functional performance on the pellet retrieval task (r2 = 0.89; P = 0.03; scale bars: A = 91 μm, B = 13.4 μm, C = 11.1).
The Number of Corticospinal Axon Contacts with Motor Neurons Correlates with Functional Performance.
Regression analysis demonstrated that the total number of medial + lateral CST axon contacts with motor neuron pools at the C4 level of the cord correlated significantly with ability to perform skilled forelimb tasks (r2 = 0.89; P = 0.03; Fig. 5F). Of note, animals with lesions of the dCST attain a quantity of CST axon contacts with motor neuron pools that is intermediate in number between animals with more extensive CST lesions (d/vCST or MED) and less extensive (vCST) lesions, as a result of compensatory increases in the number of contacts with medial motor neuron pools. Thus, compensatory increases in CST contacts with motor neurons correlate with recovery of skilled motor function and occur spontaneously after CNS injury. These compensatory changes, and functional recovery, are not observed if the vCST is also lesioned when the dCST is lesioned.
Functional Recovery After dCST Lesions Is Abolished by a Subsequent Lesion of the vCST.
The preceding observations implicate spontaneous sprouting of ventral CST axons as a mechanism of functional recovery after dCST lesions. To confirm the presence of functionally significant ventral sprouting, eight additional animals underwent dCST lesions followed by vCST lesions 4 weeks later to determine if recovery was abolished. Two weeks after the first (dCST) lesion, animals exhibited significant functional deficits in the forelimb reaching task (32.7 ± 9.3% reduction in accuracy of pellet retrieval compared with intact animals, P < 0.01) that recovered to a nonsignificant difference of 18.3 ± 7.4% compared with intact animals (P value nonsignificant). The second (vCST) lesion was then made, resulting in a complete loss of functional recovery 2 weeks later; rats exhibited a severe 70.1 ± 6.9% reduction in pellet retrieval (P < 0.0001) 2 weeks after placement of the vCST lesion and a 71.8 ± 7.3% reduction in pellet retrieval (P < 0.0001) 3 weeks after the vCST lesion compared with intact animals. Thus, recovery required ventral pathways.
Discussion
This study reveals that significant spontaneous structural compensation by axons of the corticospinal motor tract occurs after adult CNS injury, and this compensation correlates with functional recovery. The number of putative CST axon contacts on neurons of the medial motor column increased to 331% of levels of the uninjured system following loss of the majority dorsal component of the corticospinal system. Parallel with these changes, rodents recover on a skilled motor task that is sensitive to corticospinal function. Such sprouting may explain protracted improvement in function in many humans after lesions to the CNS (1–5).
The increase of putative CST contacts with medial motor neurons likely represents compensatory sprouting from uninjured ventral CST axons; evidence for this is 3-fold. First, BDA-labeled CST axons extended from the ventral CST directly to medial motor neurons, the first observation of such direct projections. Second, sprouting occurred only if lesions spared the ventral CST; sprouting was eliminated in animals with lesions to both the ventral and dorsal components of the CST and was not present in animals with vCST lesions alone. Third, functional recovery was abolished if the vCST was lesioned after the dCST lesion, or if the dorsal and ventral CST were simultaneously lesioned. Thus, sprouting originated from the vCST. Elimination of sprouting after combined dorsal and ventral CST lesions indicated that axons did not sprout from the minor but spared components of the lateral and dorsolateral CST.
Because lesions were accurately placed and functional recovery only occurred when the vCST was spared, it is likely that recovery resulted from compensatory responses of the corticospinal tract rather than other systems. In particular, the highly accurate vCST lesions would affect few if any axons of other systems that influence motor function, including rubrospinal, reticulospinal, propriospinal, raphespinal, or vestibulospinal pathways (6, 7, 33–35). Furthermore, the corticospinal system is directly implicated in the recovery because medullary lesions, which affected all components of the CST and did not injure other motor systems, were not followed by functional recovery. It is possible that functional recovery in animals with dCST lesions may have resulted purely from compensatory behavioral strategies mediated through the vCST and that the observed sprouting of ventral CST axons was not necessary for behavioral compensation. However, analysis of the pattern of qualitative limb movements (missed aims, failed advances) in recovered animals revealed no fundamental differences from intact animals, suggesting that a purely behavioral compensation was a less likely mechanism of recovery, although more extensive analysis of fine features of qualitative limb use (7) would be required to draw firm conclusions. It is also possible that structural rearrangements of axons, dendrites, or synapses in fact underlie some cases of behavioral “compensation” (36, 37).
Animals with isolated vCST lesions also exhibited short-term functional deficits, a surprising finding because this tract comprises less than 5% of all axons in the corticospinal projection. This finding, together with the observation that increases in ventral CST axon contacts correlate with functional recovery in dCST-lesioned animals, suggests that the ventral CST may exert a more important contribution to skilled forelimb function than previously appreciated. Indeed, the magnitude of early functional deficits in animals with isolated dorsal or ventral CST lesions was nearly equivalent, suggesting that both of these systems exert important effects on pellet retrieval in rodents. The existence of direct ventral CST projections to medial motor neurons of the cord suggests that transient disruption of these inputs may cause functional deficits related to proximal forelimb dysfunction. In fact, detailed analysis of errors made on forelimb reaching in animals with vCST lesions disclosed such proximal errors, including missed aims and failed advances (Fig. 4). The mechanism of recovery in vCST-lesioned subjects over the 4-week period of this study may have also involved sprouting of dCST projections, but this effect would be difficult to resolve at the morphological level because 95% of the CST axons were unaffected by vCST lesions.
Return of motor function in lesioned compared with intact animals over the 4-week postinjury period correlated significantly with total numbers of corticospinal terminal contacts with spinal cord motor neurons (Fig. 5F). Thus, a critical number of direct supraspinal inputs to local motor circuits is necessary to sustain function, and recovery may depend on reestablishing this critical number. Two means of generating such recovery exist: sprouting of nearby axons or regeneration of lesioned axons. The former mechanism is likely to account for the spontaneous motor recovery observed here; the latter mechanism is the subject of numerous experiments (22, 23, 38–45).
Studies of neonatal spinal cord injury have also reported spontaneous partial functional and morphological plasticity (46, 47). However, plasticity and functional recovery was not known to emerge from lesioned corticospinal systems of the adult. Rather, recent studies focus on augmenting CNS regeneration in the belief that little morphological plasticity occurs after adult CNS motor lesions. The present findings may help to place regeneration studies in an appropriate context: inherent plasticity does exist in injured spinal motor systems and can lead to recovery of motor function in some circumstances. Thus, efforts to enhance spontaneous plasticity may be a means of promoting further recovery. Recent studies have demonstrated that axonal sprouting can be enhanced by certain compounds (24, 48). Application of growth factors to local sites of injury, in regions where some plasticity is already occurring, may be another strategy for amplifying axon growth, sprouting, and recovery in the CNS.
Acknowledgments
This work was supported by National Institutes of Health Grant NS37083, Veterans Affairs, Canadian Spinal Research Organization, the Swiss Foundation for International Research into Paraplegia on behalf of the Sandoz Family Foundation, and the Hollfelder Foundation.
Abbreviations
- CST
corticospinal tract
- dCST
lesion of the dorsal component of the corticospinal tract
- vCST
lesion of the ventral component of the corticospinal tract
- d/vCST
lesion of the dorsal and ventral components of the corticospinal tract
- MED
lesion of the entire corticospinal tract at the medullary level
- BDA
biotinylated dextran amine
- ChAT
choline acetyltransferase
- CNS
central nervous system
References
- 1.Bracken M B, Shepard M J, Collins W F, Jr, Holford T R, Baskin D S, Eisenberg H M, Flamm E, Leo-Summers L, Maroon J C, Marshall L F, et al. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 2.Frankel H K. In: Outcomes in Neurological and Surgical Disorders. Swash M, editor. Cambridge: Cambridge Univ. Press; 1998. pp. 181–194. [Google Scholar]
- 3.Ferrucci L, Bandinelli S, Guralnik J M, Lamponi M, Bertini C, Falchini M, Baroni A. Stroke. 1993;24:200–205. doi: 10.1161/01.str.24.2.200. [DOI] [PubMed] [Google Scholar]
- 4.Naeser M A, Palumbo C L, Prete M N, Fitzpatrick P M, Mimura M, Samaraweera R, Albert M L. Brain Lang. 1998;62:1–28. doi: 10.1006/brln.1997.1866. [DOI] [PubMed] [Google Scholar]
- 5.Sbordone R J, Liter J C, Pettler-Jennings P. Brain Inj. 1995;9:285–299. doi: 10.3109/02699059509008199. [DOI] [PubMed] [Google Scholar]
- 6.Alstermark B, Lundberg A, Pettersson L G, Tantisira B, Walkowska M. Neurosci Res. 1987;5:68–73. doi: 10.1016/0168-0102(87)90024-1. [DOI] [PubMed] [Google Scholar]
- 7.McKenna J E, Whishaw I Q. J Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raisman G. Brain Res. 1969;14:25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- 9.Cotman C W, Matthews D A, Taylor D, Lynch G. Proc Natl Acad Sci USA. 1973;70:3473–3477. doi: 10.1073/pnas.70.12.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukahara N, Hultborn H, Murakami F, Fujito Y. J Neurophysiol. 1975;38:1359–1372. doi: 10.1152/jn.1975.38.6.1359. [DOI] [PubMed] [Google Scholar]
- 11.Steward O. Science. 1976;194:426–428. doi: 10.1126/science.982024. [DOI] [PubMed] [Google Scholar]
- 12.Lynch G, Gall C, Cotman C. Exp Neurol. 1977;54:179–183. doi: 10.1016/0014-4886(77)90244-8. [DOI] [PubMed] [Google Scholar]
- 13.Blight A R. Neuroscience. 1983;10:521–543. doi: 10.1016/0306-4522(83)90150-1. [DOI] [PubMed] [Google Scholar]
- 14.Merzenich M M, Nelson R J, Stryker M P, Cynader M S, Schoppmann A, Zook J M. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz L I, Rodriguez W R, Neve R L. Brain Res Mol Brain Res. 1990;8:17–23. doi: 10.1016/0169-328x(90)90004-w. [DOI] [PubMed] [Google Scholar]
- 16.Jones T A, Kleim J A, Greenough W T. Brain Res. 1996;733:142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence D G, Kuypers H G. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Oxford Univ. Press; 1993. [Google Scholar]
- 19.Whishaw I Q, Pellis S M, Gorny B, Kolb B, Tetzlaff W. Behav Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- 20.Schnell L, Schneider R, Kolbeck R, Barde Y A, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Cao Y, Olson L. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 22.Grill R, Murai K, Blesch A, Gage F H, Tuszynski M H. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Field P M, Raisman G. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 24.Zgraggen W J, Metz G A S, Kartje G L, Thallmair M, Schwab M E. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahlsing H L, Feringa E R. Exp Neurol. 1980;70:282–287. doi: 10.1016/0014-4886(80)90027-8. [DOI] [PubMed] [Google Scholar]
- 26.Joosten E A, Schuitman R L, Vermelis M E, Dederen P J. J Comp Neurol. 1992;326:133–146. doi: 10.1002/cne.903260112. [DOI] [PubMed] [Google Scholar]
- 27.Brösamle C, Schwab M E. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Weidner N, Grill R J, Tuszynski M H. Exp Neurol. 1999;160:40–50. doi: 10.1006/exnr.1999.7200. [DOI] [PubMed] [Google Scholar]
- 29.Schrimsher G W, Reier P J. Exp Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- 30.Holstege G. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San. Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 32.Blurton-Jones M M, Roberts J, Tuszynski M H. J Comp Neurol. 1999;405:529–542. [PubMed] [Google Scholar]
- 33.Alstermark B, Lundberg A, Sasaki S. Exp Brain Res. 1984;56:308–322. doi: 10.1007/BF00236286. [DOI] [PubMed] [Google Scholar]
- 34.Whishaw I Q, Tomie J A, Ladowsky R L. Behav Brain Res. 1990;40:131–144. doi: 10.1016/0166-4328(90)90005-y. [DOI] [PubMed] [Google Scholar]
- 35.Pettersson L G, Lundberg A, Alstermark B, Isa T, Tantisira B. Neurosci Res. 1997;29:241–256. doi: 10.1016/s0168-0102(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 36.Kleim J A, Swain R A, Armstrong K A, Napper R M, Jones T A, Greenough W T. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- 37.Klintsova A Y, Greenough W T. Curr Opin Neurobiol. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- 38.Blesch A, Uy H S, Grill R J, Cheng J G, Patterson P H, Tuszynski M H. J Neurosci. 1999;19:3556–3566. doi: 10.1523/JNEUROSCI.19-09-03556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Kim D, Himes B T, Chow S Y, Schallert T, Murray M, Tessler A, Fischer I. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X M, Guenard V, Kleitman N, Bunge M B. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 41.Snyder E Y, Park K I, Flax J D, Liu S, Rosario C M, Yandava B D, Aurora S. Adv Neurol. 1997;72:121–132. [PubMed] [Google Scholar]
- 42.Diener P S, Bregman B S. J Neurosci. 1998;18:779–793. doi: 10.1523/JNEUROSCI.18-02-00779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weidner N, Blesch A, Grill R J, Tuszynski M H. J Comp Neurol. 1999;413:495–506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 44.Ramon-Cueto A, Cordero M I, Santos-Benito F F, Avila J. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 45.Schwab M E, Bartholdi D. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 46.Bregman B S, Goldberger M E. Brain Res. 1983;285:119–135. doi: 10.1016/0165-3806(83)90046-9. [DOI] [PubMed] [Google Scholar]
- 47.Bregman B S, Goldberger M E. Science. 1982;217:553–555. doi: 10.1126/science.7089581. [DOI] [PubMed] [Google Scholar]
- 48.Benowitz L I, Goldberg D E, Madsen J R, Soni D, Irwin N. Proc Natl Acad Sci USA. 1999;96:13486–13490. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terashima T. Neurosci Res. 1995;22:139–161. doi: 10.1016/0168-0102(95)00895-9. [DOI] [PubMed] [Google Scholar]