INTRODUCTION
Cancer represents one of the most significant public health problems in the United States, accounting for 25% of deaths. A total of nearly 1.5 million individuals will be diagnosed with cancer in the United States in 2009 [1]. Continuing improvements in cancer therapy and health care delivery have resulted in an ever-growing population of nearly 12 million long-term cancer survivors; currently approximately 66% of adult and 80% of pediatric cancer patients survive 5 years or more.
Radiation therapy (RT), surgery, and chemotherapy are the primary treatment modalities used in cancer therapy to enhance patient survival; some 60% of all newly diagnosed cancer patients will receive RT during the course of their disease [2]. Radiation therapy represents a targeted, non-invasive and potentially organ-preserving therapy; however, radiation-induced late effects remain a significant risk. Given the increasing population of long-term survivors, the need to mitigate or treat late effects has emerged as a primary area of radiation biology research [3;4].
Numerous studies conducted over the last 20 years or so have clearly demonstrated that radiation-induced late effects arise from not simply mitotic cell death of particular target cell clonogens, but more importantly, from complex and dynamic interactions between multiple cell types within an organ [5–8]. Normal cells are active participants in the normal cellular response to injury that may initiate an active chronic process that ultimately leads to progressive damage. Indeed, radiation injury can be modulated by the application of pharmacological therapies focused on altering steps in the cascade of events leading to the clinical expression of normal tissue injury [9]. However, details of the specific pathogenic mechanisms involved in the development and progression of late, radiation-induced normal tissue morbidity, remain to be determined.
RADIATION-INDUCED BRAIN INJURY
The total dose of RT that can be administered safely to the brains of patients presenting with primary or metastatic brain tumors is limited by the risk of normal tissue morbidity. Based on the time of clinical expression, radiation-induced brain injury is described in terms of acute, early delayed, and late delayed reactions [10]. Acute injury (acute radiation encephalopathy), expressed in days to weeks after irradiation, is rare under current RT regimens. Early delayed injury occurs from 1–6 months post-irradiation and can involve transient demyelination with somnolence which is mainly seen in children but can also affect adult patients in the first 2 months after RT. While both these early injuries can result in severe reactions, they usually resolve within 1–3 months, either spontaneously or following treatment with corticosteroids and their severity is not predictive of the more severe late effects. Late delayed effects, characterized histopathologically by demyelination, vascular abnormalities and ultimate white matter necrosis [11], are observed > 6 months post-irradiation after relatively high doses (>60 Gy, fractionated [10;12]
In addition to these histopathologic endpoints, there is a growing awareness of intellectual deterioration in patients receiving brain irradiation [13] that can occur with relatively lower doses and in the absence of apparent structural lesions. Although diverse in character, this often includes hippocampal-dependent functions including learning, memory, and spatial information processing. Cognitive impairment, including dementia, induced by partial or whole-brain irradiation (WBI) is reported to occur in up to 50% of adult brain tumor patients who are long-term survivors (> 6 months postirradiation) [13–16]. The resultant impact on quality of life (QOL) has become an extremely important concern for long-term survivors, particularly for adult survivors of childhood cancer, who present with an extraordinarily high incidence of late, and often permanent, complications arising from combined RT and chemotherapy [17], including significant neurocognitive sequelae [18–20].
The need to both understand and minimize the side effects of brain irradiation is heightened by the increasing number of patients with secondary brain metastases (mets) that require RT. Some 30% of new cancer patients will develop brain mets [21;22], making this the most common neurological manifestation of cancer, and a cancer problem more common in incidence than newly diagnosed lung, breast, or prostate cancer combined. The annual incidence in the United States appears to be increasing, due in part to an aging population, better anticancer therapies for systemic disease, and the application of improved imaging techniques to detect smaller mets in asymptomatic patients [23]. Approximately 200,000 individuals will ultimately be treated with partial large field or WBI for brain mets. Over half of these patients will survive long enough to develop radiation-induced brain injury, including cognitive impairment. At present, there are no successful long-term treatments or effective preventive strategies for radiation-induced brain injury [24].
PATHOGENESIS OF RADIATION-INDUCED BRAIN INJURY
Although the precise pathogenic mechanisms involved in the development and progression of radiation-induced brain injury remain ill-defined, there is a growing acceptance of a model in which the radiation response of the brain involves all of the cell types found within the brain, including astrocytes, endothelial cells, microglia, neurons, and oligodendrocytes participating in an ongoing dynamic and interactive process [10]. An additional and important component of radiation injury to the brain is the relatively recent observation that irradiation can inhibit hippocampal neurogenesis [25;26].
The hippocampus is central to short-term declarative memory and spatial information processing. Active neurogenesis occurs throughout adulthood in a specialized region of the hippocampus called the dentate gyrus (DG) [27]. Neural stem/precursor cells residing in the subgranular zone (SGZ) of the DG give rise to new glia or neurons; the latter functionally integrate into the granule cell layer of the hippocampus [28]. Neurogenesis depends on the presence of a specific neurogenic microenvironment; both endothelial cells and astrocytes can promote or regulate neurogenesis [29;30]. These neural precursor cells are extremely radiosensitive [31]; rats irradiated with a single dose of 10 Gy, that fails to cause demyelination or white matter necrosis, produce only 3% of the new hippocampal neurons produced in control animals [32]. Experimental findings suggest that the detrimental effect of WBI on hippocampal neurogenesis is a key contributing factor to radiation-induced cognitive impairment. In vitro, irradiation leads to a loss of proliferative capacity of neuronal precursor cells [32]; in vivo, WBI of the mouse and rat brain leads to a significant decrease in the number of newborn mature and immature neurons in the DG [32–34]. Recent data from human patients indicate that RT for malignant brain tumors also leads to a significant reduction in the number of neurogenic cells [35]. This radiation-induced decrease in hippocampal neurogenesis is associated with hippocampal-dependent spatial learning and memory impairment [33;34], inferring a mechanistic link between these factors. Using a recently characterized rat model [36], we have applied the non-hippocampal-dependent novel object recognition task to assess recognition memory; this is significantly impaired 6 months after fractionated (f)WBI and gets worse over the next 6 months [37]. Thus, WBI leads to significant and progressive reductions in both hippocampal- and non-hippocampal-dependent cognitive function, suggesting that multiple regions of the brain are involved. In addition to decreased neurogenesis, other mechanisms may be involved, including alterations in NMDA receptor subunits [38], genetic risk factors [39], neuronal function/gene expression [40], and oxidative stress/inflammation [41;42].
The latter appears to be particularly important. There is growing appreciation for the role of acute and chronic oxidative stress/inflammation in the development and progression of radiation-induced late effects [43]. Irradiating late responding normal tissues leads to chronic increases in reactive oxygen species/reactive nitrogen oxide species (ROS/RNOS) that serve as intracellular signaling species to modulate cell phenotype, leading to chronic inflammation and organ dysfunction. The brain is particularly sensitive to oxidative stress due to its i] high oxygen consumption [44], ii] high content of oxidizable unsaturated fatty acids [44] and free iron [45], iii] limited capacity to perform anaerobic glycolysis [46], and iv] low levels of antioxidant defenses [47]. Oxidative stress and resultant changes in redox state appear critical in regulating the response of the brain to a variety of insults [48;49]. Indeed, a primary role for chronic oxidative stress/inflammation and ROS/RNOS in radiation-induced brain injury has been proposed recently [42].
Direct experimental evidence for radiation-induced oxidative/nitrosative stress has been obtained from studies using neonatal and adult rodents. Irradiating one hemisphere of postnatal day 8 rats or of postnatal day 10 mice with a single dose of 4–12 Gy of 4 MV X-rays led to time-dependent increases in nitrotyrosine in the subventricular zone and the granular cell layer of the DG 2–12 h postirradiation [50]. An oxidative stress, evidenced as a significant increase in lipid peroxidation was noted in the adult male mouse hippocampus 2 weeks after brain irradiation with a single dose of 10 Gy [51]. More recently, Rola et al [52] reported a chronic inflammatory response in the mouse SGZ 9 months following high-LET brain irradiation; expression of the CCR2 receptor, important in neuroinflammation [53;54], increased in the irradiated brains as compared to the sham-irradiated control brains. Persistent microglial activation in the rat brain has also been observed after fWBI [55]. These findings provide the rationale for applying anti-inflammatory-based interventions to prevent or ameliorate the severity of late radiation-induced brain injury.
FUNCTIONAL COMPONENTS OF THE BRAIN RAS AS POTENTIAL MEDIATORS OF RADIATION-INDUCED INJURY
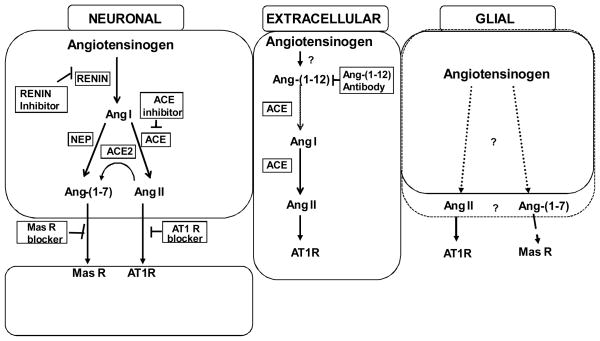
As reviewed recently [56], there is still controversy about the presence and localization of components of the brain RAS. Three possibilities for which there is support are shown in Figure 1. It is well known that angiotensinogen (Aogen) is an extracellular component of the cerebrospinal/interstitial fluid and constitutes one of the more abundant proteins in cerebrospinal fluid [57]; production of the precursor protein is primarily glial [56]. Overexpression of antisense to Aogen behind a glial-fibrillary acidic protein (GFAP) promoter results in loss of 90% of the brain Aogen [58]. However, as illustrated in the first panel of Figure 1, Aogen is also found in neurons [59], most often in brain centers involved in cardiovascular regulation such as the subfornical organ, paraventricular nucleus, nucleus of the solitary tract and rostroventrolateral medulla. In addition, Aogen immunoreactivity is present at sites other than those associated with blood pressure (BP), fluid and electrolyte homeostasis providing evidence that the brain RAS may serve in other capacities and is not limited to cardiovascular regulatory functions. Questions remain, however, regarding local synthesis of renin in brain, especially given that prorenin or active renin can be sequestered from the circulation and other enzymes can exhibit similar proteolytic profiles. There is evidence within the brain of renin mRNA [60] and cells in the pituitary, choroid plexus, medulla oblongata, and hypothalamus are positive for renin immunoreactivity. The renin present in cells of the choroid plexus would be positioned for release and have the ability to act on the Aogen in the extracellular milieu. Renin immunoreactivity is localized with neurons, but in the medulla oblongata and subfornical organ, it has been demonstrated in glial elements as well. However, renin mRNA as an indicator of synthesis of the protein, is predominantly but not exclusively in neurons [61;62].
Fig 1.
Summary of various pathways and sources hypothesized to exist for the generation of Ang peptides. From left to right - An intracellular, renin-dependent pathway is well documented in paraventricular nucleus, nucleus of the solitary tract and rostroventrolateral medulla, that may mediate formation of both angiotensin (Ang) II and Ang-(1–7) involved in stress responses and regulation of arterial pressure. Ang-(1–12) is thought to be extracellular, contributing to hypertension and impairment of Baroreflex function in (mRen2)27 rats, but may not play a role in normal animals. Angiotensinogen of glial origin is the predominant source of the precursor protein in brain (~90%), but whether angiotensin peptide processing represents an intracellular or extracellular event is not known. ACE, angiotensin converting enzyme; ACE2, angiotensin converting enzyme 2; NEP, neprilysin; AT1, Ang II type 1 receptor; Mas, Ang-(1–7) receptor.
All enzymes required for subsequent processing of Ang I into active peptides including Ang converting enzyme (ACE) for Ang II, ACE2 and neprilysin for Ang-(1–7) and aminopetidases for Ang III and IV are present on the basis of immunocytochemistry or molecular approaches throughout the central nervous system (CNS). The predominant localization of these secondary enzymes on plasma membranes points to extracellular formation of the final bioactive peptide products, however evidence for intracellular staining for the major active peptides Ang II and Ang-(1–7) does not rule out a completely intracellular processing pathway [63]. From previous studies whether the processing of Ang I and other active peptides takes place within the same glial or neuronal cell, extracellularly by membrane-bound enzymes located on glia or neurons, or a combination of both, is not established as there is evidence for all of these possibilities.
The middle panel in Figure 1 shows Ang-(1–12), the most recent Ang peptide identified in brain tissue [64]. This C-terminal extended sequence is detected in heart, kidney, and brain in equal or higher amounts relative to traditional Ang peptides [64]. Ang-(1–12) has a vasoconstrictive action in the isolated perfused rat aorta and increases mean arterial pressure following intravenous administration; effects that were abolished by ACE inhibition or Ang II type 1 receptor (AT1R) blockade. Preliminary studies [65] indicate that Ang-(1–12) is metabolized in a non-renin dependent manner in heart, plasma and kidney to either Ang II or Ang-(1–7), depending upon the processing enzymes present, ACE or neprilysin. An antibody to Ang-(1–12) given intracerebroventricularly lowered BP in hypertensive but not normotensive rats [66;67]. Therefore, Ang-(1–12) is thought to arise from processing of Aogen extracellularly, since it is not expected that an antibody would gain access to intracellular peptide. In (mRen2)27 rats chronic immunoneutralization of Ang-(1–12) with an antibody specific to the unique C-terminal end of the peptide, reduced BP over a 2 wk period, and the combination of Ang-(1–12) antibody and a renin inhibitor had a greater effect than the antibody alone [66;67]. Thus, we suggest that an Ang-(1–12)/ACE pathway exists in brain in parallel with other known pathways that involve renin, and that to fully block the formation of the Ang peptides in brain tissue, both pathways must be targeted.
As discussed above Aogen is expressed widely in brain tissue, as is ACE for generation of Ang II, and ACE2 and neprilysin for generation of Ang-(1–7) from Ang II or Ang I, respectively [68;69]. However, each Ang peptide could come from Ang-(1–12) as an intermediate as well. If the further conversion of Ang-(1–12) to Ang I is ACE dependent, then blockade by ACE inhibitors (ACEIs) in brain may reduce both Ang II and Ang-(1–7), as would a renin inhibitor. Indeed, knock-down of glial Aogen in transgenic animals is associated with less age-related cardiovascular morbidity and an increased lifespan [70]. ACE inhibitors as well as AT1R antagonists (AT1RAs) given systemically appear to have beneficial long-term effects [71;72] and may shift the balance from Ang II to Ang-(1–7) actions through a variety of mechanisms [73] as further discussed below. Thus, in the absence of total knock-down of both limbs of the RAS, elevation of Ang-(1–7) or reduction of Ang II may also provide substantial neuroprotection.
The AT1 and AT2 receptors for Ang II and mas receptor for Ang-(1–7) are found in many brain areas. In fact, the widespread distribution of the receptors in areas not consistent with either localization of the precursor processing components or cardiovascular actions has been a vexing challenge in understanding the configuration of the system. Although it is well known that molecular or pharmacologic RAS inhibitors lower BP [74], knowledge of the impact of disruption of this system on non-cardiovascular endpoints is severely lacking as are data on the effects of long-term interruption of the system in the cognitive decline with aging. However, the concept has evolved that the Ang peptides play a role in cellular growth and metabolism, injury and repair processes as well as vasculo-neural communications [75–78]. Thus, the fact that AT1, AT2 and mas receptors are localized along with other RAS components on neuronal, glial, vascular and epithelial elements (Figure 2) may represent far more than typical transmitter like functions in brain. The distribution is certainly compatible with effects of the peptides on a variety of modulatory functions reflecting the presence of the receptors on all of the elements of the brain mentioned in the sections above as responding to or involved in the late effects of radiation-induced injury. Therefore, studies investigating the involvement of locally derived brain, or systemically produced, renin, Aogen and Ang peptides in the late effects of brain irradiation are eagerly awaited.
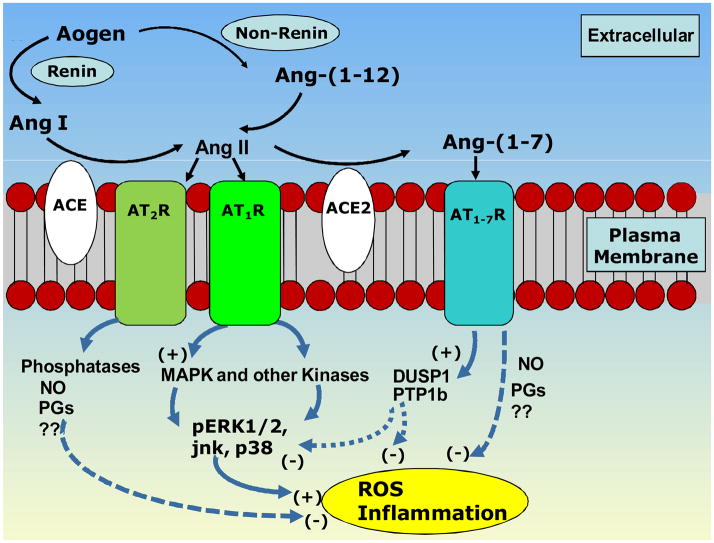
Fig 2.
Proposed signaling pathways involved in the opposing actions of Ang II versus Ang-(1–7). Generation of Ang II by converting enzyme (ACE) from Ang I dervided from either renin-dependent or independent [Ang-(1–12)] pathways. Ang II acts on AT1 receptors to activate kinase pathways associated with increases in reactive oxygen species (ROS) and inflammation. ACE2 can cleave Ang II to form Ang-(1–7). Either mas activation via Ang-(1–7) or AT2 activation via Ang II have been linked to protective prostanoids (PGs) or nitric oxide (NO) in addition to phosphatases know to counterbalance kinase activity.
In terms of actions of Ang II on cellular mechanisms leading to inflammation or oxidative stress thought to participate in the late effects of radiation therapy, the localization of the receptors is of primary interest. In studies of cardiovascular areas of the brain such as the nucleus of the solitary tract, the AT1R is present on glial, neuronal, vascular endothelial and smooth muscle cells, as well as on the epithelial components of brain tissue such as the choroid plexus. Brain regions with evidence of the AT1R or AT2R receptor include cortex, basal ganglia, thalamic and limbic regions. Importantly for memory and learning, functional receptors are reported in the hippocampus [79]. Less is known about the mas receptor, but it is reportedly localized in many of these same brain regions [80]. Evidence that Ang II receptors appear in areas of inflammation and response to injury further support a role for the peptide on invading cells related to immune or defense reactions [81–84]
Some of the potential signaling pathways for Ang II and Ang-(1–7) are shown in Figure 2. These cellular pathways are common to many other transmitters and hormones in the brain and periphery. For example, Ang II activates PI3 kinase and MAP kinase pathways to induce rapid signaling responses in many cells and tissues including the brain. The PI3 kinase pathway is involved in maintenance of Ang II-induced hypertension as PI3 kinase blockade with wortmannin in the nucleus tractus solirarii [85] of (mRen2)27 rats or rostro-ventrolateral medulla of spontaneously hypertensive rats [86] decreases arterial pressure and increases reflex control of heart rate. The MAP kinase pathway is implicated in the support of resting, as opposed to elevated, arterial pressure and in response to Ang II there are increases in phosphorylated ERK-1/2 in cells from both normotensive and hypertensive rats [87]. The activity of MAP kinases and PI3 kinases is tightly regulated by coordinated regulation of phosphatases. DUSP-1 acts to negatively regulate the MAP kinase pathway while PTP1b acts to negatively regulate the PI3K pathway [88–91]. Inhibition of PTP1b, the phosphatase acting on Akt, has the opposite effect to PI3 kinase blockade, in that it reduces reflex control of heart rate in Sprague-Dawley rats [92]. Our preliminary data suggest that when PI3K signaling pathways are activated in (mRen2)27 rats leading to impaired reflex function, there is very little PTP1b tone to offset the impairment [92]. This would be consistent with low endogenous levels of Ang-(1–7) in the medulla of these hypertensive animals and, potentially, down-regulation of the PTP1b. While this hypothesis is being explored in present studies, Figure 2 illustrates how Ang-(1–7) is proposed to regulate Ang II-stimulated signaling pathways by up-regulating DUSP-1 and PTP1b expression [93]. Whether expression of DUSP-1 and PTP1b are regulated in brain areas involved in memory and learning, or anxiety and depression, in response to long-term alterations in brain or circulating Ang peptides is not known, but the differential effects of PTP1b blockade in the nucleus of the solitary tract in animals over-expressing or under-expressing Ang-(1–7) as mentioned above is consistent with this overall concept. Even in the absence of an upregulation of the kinases in normal animals, the phosphatases could provide a buffering tone, such that phosphatase inhibition would cause a functional shift towards Ang II. Whether similar shifts in the balance of these kinases and phosphatases occur in response to radiation, and whether blockade of the RAS can alter this response, is a subject of current investigation. In addition to the role of the kinase-phosphatase pathways in the actions of Ang peptides, as reviewed in more detail below, there is ample evidence for counterbalancing NADPH oxidase and diaphorase pathways. Ang II is proposed to exert a pressor response through activation of NADPH oxidase in the subfornical organ and perhaps the paraventricular nucleus. In contrast, there is close association of Ang-(1–7) immunoreactivity with NADPH diaphorase in the brain, and a functional relationship is suggested given that nitric oxide (NO) release by Ang-(1–7) occurs in ischemia [94–96]. Certainly the localization of Ang receptors throughout the brain parenchyma, activated blood and glial cells, vasculature and epithelium provides an anatomical substrate for the myriad of actions associated with RT as further discussed in the following sections of this review.
RAS BLOCKERS AND TREATMENT OF RADIATION-INDUCED BRAIN INJURY
Extensive studies in the kidney and to a lesser extent the lung have provided clear evidence that ACEIs and AT1RAs can modulate radiation-induced late effects [9]. However, although these empirical observations demonstrate that RAS blockade works, the specific mechanisms involved remain unclear. Nonetheless, given the presence of a functioning brain RAS and its importance in normal cognitive processing and potential treatment of dysfunctional memory disease states [97], the use of RAS blockers in the treatment of radiation-induced brain injury appears logical [98]. Indeed, a key role for the brain RAS in the development of dyscirculatory encephalopathy (DE) in Chernobyl cleanup workers has been proposed recently [99].
Using a recently established rat model of radiation-induced injury to the optic nerve, Kim et al [100] were the first to demonstrate ACEI-mediated neuroprotection against late radiation-induced brain injury. The ACEI ramipril is an ester-containing prodrug that upon ingestion is rapidly taken up by the liver and converted to its active form, ramiliprat. Of importance, and unlike many other ACEIs, the drug can cross the blood brain barrier [101]. Both optic nerves and chiasm of young adult male Fischer 344 (F344) rats were irradiated stereotactically with 30 Gy using a single collimated beam. Starting 2 weeks after irradiation, groups of rats were assigned to receive either normal drinking water or drinking water containing ramipril (1.5 mg/kg). Six months after brain irradiation, rats were assessed for functional optic nerve damage using visually evoked potential (VEP), followed by histological analyses after euthanization. Rats receiving radiation alone exhibited a 3-fold lengthening in the mean peak latency in the VEP. In contrast, 75% of rats receiving radiation followed by ramipril had VEPs that resembled those of control unirradiated rats. Moreover, histological analyses of the tissue removed from rats receiving radiation followed by ramipril revealed essentially normal optic nerves, while there was significant demyelination in both optic nerves of the irradiated rats. Thus, these findings indicate that ramipril can mitigate radiation-induced optic neuropathy.
Additional studies focused on determining the optimum dose and time of administration of ramipril [102]. Varying the dose administered from 0.5, 1.0 or 1.5 mg/kg for 6 months starting from 2 weeks after irradiation revealed a reduction in the effectiveness of ramipril to decrease the incidence of severe necrosis; a dose of 0.5 mg/kg failed to mitigate this morbidity. Moreover, delaying the start of ramipril administration until 4 weeks after irradiation failed to prevent the radiation-induced increase in VEP. These results suggest that effective mitigation of radiation-induced brain injury with ramipril necessitates early administration of the drug. Ryu et al [102] also noted that administering the AT1RA, losartan, 20 mg/kg, again starting 2 weeks after irradiation, did not mitigate the radiation-induced optic neuropathy. It was suggested that this might reflect the low drug dose and subsequent low availability. However, they noted that losartan similarly failed to protect mouse skin against radiation-induced injury.
Our more recent findings do support a role for AT1RAs in protecting against radiation-induced cognitive impairment. As noted above, fWBI (40 Gy in 4 weeks, 2 fractions of 5 Gy/week) of the young adult male F344 × Brown Norway rat leads to a chronic, progressive reduction in cognitive function [37;103]. Administration of the AT1RA, L-158,809 (20 mg/L drinking water), starting 3 days before, during, and for 28 or 54 weeks postirradiation, prevented the radiation-induced cognitive impairment observed 26 and 52 weeks postirradiation [104]. Chronic administration of L-158,809 was associated with significant increases in systemic levels of Ang I, Ang II, and Ang-(1–7), consistent with previous observations of effective AT1R blockade [72] and indicative of loss of the RAS feedback mechanism [105]. Thus, long-term AT1R blockade with L-158,809 appears to prevent radiation-induced cognitive impairment. Continued RAS blockade may not be required. Giving L-158,809 before, during, and for only 5 weeks postirradiation ameliorated the cognitive impairment observed at 26 weeks postirradiation, suggesting that AT1R-mediated prevention/amelioration of radiation-induced cognitive impairment may require administration of the drug before, during, and perhaps for only 5 weeks after fWBI [104]. However, additional studies are required to confirm these provocative findings.
We have extended these observations to show that RAS blockade using the ACEI ramipril can similarly prevent fWBI-induced cognitive impairment (Lee et al, unpublished observations). Administering ramipril (15 mg/kg) in the drinking water starting 3 days before, during, and for 28 weeks after fWBI of young adult male F344 rats prevented the radiation-induced decrease in cognitive function observed 26 weeks postirradiation. Chronic ACE inhibition was associated with marked increases in plasma levels of Ang I and Ang-(1–7), again inferring effective RAS blockade. Thus, RAS blockade with either ACEIs or AT1RAs appears effective at treating radiation-induced brain injury. However, the mechanisms involved are unclear.
Conner et al tested whether the cognitive benefits of L-158,809 were associated with amelioration of the sustained neuroinflammation and changes in neurogenesis resulting from fWBI [106]. In rats examined at 28 and 54 weeks after fWBI, L-158,809 treatment did not alter the effects of radiation on the number and activation of microglia in the perirhinal cortex and hippocampus, nor did it prevent the radiation-induced decrease in proliferating cells and immature neurons in the hippocampus. These findings suggest that L-158,809 does not prevent or ameliorate radiation-induced cognitive impairment by modulation of chronic inflammatory mechanisms, but rather may reduce radiation-induced changes that occur earlier and that lead to cognitive dysfunction.
This interpretation may not be applicable to the ACEI ramipril. Analysis of radiation-induced changes in the number of total and activated microglia in the DG of F344 rats treated with fWBI w/wo ramipril indicate that the ramipril-mediated prevention of the radiation-induced cognitive impairment is associated with prevention of the radiation-induced activation of microglia 28 weeks postirradiation (Lee et al, unpublished observations). It is unclear if this difference in the ability of L-158,809 and ramipril to modulate radiation-induced neuroinflammation represents differences in specific biological mechanisms and/or signaling pathways. Nevertheless, these findings clearly indicate that the development of radiation-induced cognitive impairment and its modulation by RAS blockers involves multiple and complex mechanisms that are the focus of ongoing investigations.
THE MECHANISTIC BASIS FOR RAS BLOCKER-MEDIATED MODULATION OF RADIATION-INDUCED BRAIN INJURY
The ability of RAS blockers to prevent/ameliorate radiation-induced brain injury, including cognitive impairment, in the absence of any data indicative of activation of the systemic RAS led to the hypothesis that the blockers are acting via inhibition of Ang II produced within the irradiated brain [98]. Ang II is increasingly recognized as a potent inflammatory peptide, mediating the release of proinflammatory mediators including adhesion molecules, cytokines, and chemokines [107] through activation of the redox-regulated transcription factors activating protein-1 (AP-1) and nuclear factor κB (NFκB) [108]. Although the proinflammatory role of Ang II has been well studied in peripheral tissues [109], recent studies reveal that Ang II and AT1R are involved in LPS-mediated microglial activation and neuroinflammation [110]. Indeed, AT1R activity appears essential for the unrestricted development of full-scale innate immune response in the brain [111]. Blocking the WBI-mediated inflammatory response with a cyclo-oxygenase inhibitor partially attenuates the decline in neurogenesis [112], although its impact on cognitive function was not explored.
Ang II, via binding to the AT1R, enhances the production of ROS through activation of nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase [113]. NADPH oxidase is a multisubunit enzyme localized to cell membranes and consists of membrane-bound components (gp91phox and p22phox) and cytosolic components (p47phox, p67phox, and Rac-1) that translocate to the membrane on activation. Once the multisubunit complex is formed, superoxide (O2•−) is generated by the transfer of a single electron from NADPH to molecular oxygen via the following reaction: NADPH + 2O2 → NADP+ + H++ 2O2•− [114]. The O2•− anion is rapidly dismutated by superoxide dismutase to produce H2O2, itself a signaling molecule that can diffuse across membranes [115]. H2O2 can also activate NADPH oxidase to cause oxidant injury via production of additional oxidant species, implicating the inappropriate activation of NADPH oxidase in chronic oxidative stress [114]. ROS derived from NADPH oxidase have been implicated in a variety of chronic CNS disorders, including Alzheimer’s disease and ischemic stroke [116]. Cell types expressing NADPH oxidase in the brain include microglia, endothelial cells, vascular smooth muscle cells and, to a limited extent, neurons [116;117].
Recent finding suggest that activation of NADPH oxidase may play a role in radiation-induced oxidative stress [118]. In vitro irradiation of rat brain microvascular endothelial cells (RBMECs) led to increased i] intracellular ROS generation, ii] activation of NFκB, and iii] expression of the proinflammatory mediators intercellular adhesion molecule-1 (ICAM-1) and plasminogen activator inhibitor-1 (PAI-1). Pharmacologic and genetic inhibition of NADPH oxidase blocked these radiation-induced changes. Irradiating RBMECs was also associated with increased expression of NOX-4, p22phox and p67phox at both the mRNA and protein level, providing a mechanism for continual NADPH oxidase activation and chronic ROS production. Collectively, these data suggest a putative role for NADPH oxidase in the development and progression of radiation-induced brain injury and a mechanism whereby RAS blockers might act to prevent/ameliorate this morbidity through inhibition of NADPH oxidase-mediated oxidative stress/inflammation.
Thus, both Ang II and IR mediate their biological effects through generation of ROS, either by direct actions of the peptide, or indirectly through activation of proinflammatory mediators that contribute to ROS generation. These findings led to the hypothesis that the efficacy of RAS blockers to prevent/ameliorate the severity of radiation-induced brain injury reflected inhibition of an ongoing interaction between radiation and Ang II [98]. However, recognition of the growing complexity of the RAS as well as the functional relevance of novel Ang peptides, particularly Ang-(1–7) [119], point to a need to update this working model.
As discussed previously, Ang-(1–7) is a novel Ang peptide recognized as playing important physiologic roles by binding to the mas receptor [120] and counterbalancing the biological actions of Ang II. Ang-(1–7) is formed from Ang I or Ang II (Figure ) by several endopeptidases and carboxypeptidases, including ACE-2 [119]. During ACE inhibition or AT1R blockade, plasma Ang-(1–7) levels increase, as does cardiac ACE-2 mRNA expression [121], suggesting that the beneficial effects of RAS blockade may be due, in part, to a shift in the RAS from the ACE-Ang II- AT1R axis to the ACE2-Ang-(1–7)-mas receptor axis [122].
There are data to support a similar shift in the RAS axes in the brain following long-term RAS blockade. Analysis of RAS components in the dorsomedial medulla of male F344 rats treated with L-158,809 for one year revealed a significant increase in ACE2 and neprilysin gene expression compared with age-matched controls; AT1b, AT2, and mas receptor mRNA levels were also significantly higher in the L-158,809 treated rats [73]. Thus, long-term RAS blockade activates enzymes and receptors in the brain that would shift the balance from Ang II to Ang-(1–7). Of interest, preliminary studies indicate an fWBI-induced decrease in ACE-2 gene expression in the cortex one year postirradiation in F344 × BN male rats, which was prevented by long-term administration of L-158,809 (Robbins et al, personal communication). These novel, albeit preliminary findings, suggest that WBI may indeed impact RAS component expression, leading to a decrease in the Ang II/Ang-(1–7) balance, resulting in a proinflammatory response in the brain and radiation-induced brain injury. In contrast, RAS blockade would change the balance in favor of Ang-(1–7), and suppression of the proinflammatory response. Indeed, Ang-(1–7) has been shown to increase NO release in ischemia and to modulate COX-2 and NO related to plasticity in the amygdale [95;96]. Along with studies of effects of Ang-(1–7) in the periphery to reduce NFκB and NADPH oxidase activation [123;124], Ang-(1–7) would be expected to counteract the above mentioned pro-inflammatory and pro-oxidative stress actions of Ang II. However, defining the putative role of Ang-(1–7) in the treatment of radiation-induced brain injury and its role as a modulator of inflammation or oxidative stress for the RAS blocker-mediated prevention of radiation-induced cognitive impairment, will require additional experimental investigation.
SUMMARY
Administration of ACEIs and AT1RAs, drugs that target the intrinsic RAS, appear effective in preventing/ameliorating radiation-induced brain injury, including cognitive impairment. Both types of drugs are 1] routinely prescribed for treatment of hypertension [125], 2] well-tolerated, and 3] exhibit antitumor effects, including inhibition of angiogenesis and proliferation [126]. Thus, they appear to be ideal drugs for translational clinical trials. However, there is a need for experimental studies aimed at addressing a number of important and unresolved mechanistic questions, including: 1] how does RAS blockade lead to prevention/amelioration of radiation-induced brain injury? 2] Will combined therapies with ACEIs and AT1RAs or renin inhibitors be more effective than the single RAS blockers alone? 3] Is Ang(1–7) the primary Ang peptide in RAS blocker-mediated treatment of radiation-induced brain injury? 4] What signaling pathways mediate the beneficial effects of Ang-(1–7) versus the detrimental effects of Ang II? Answering these important questions will generate the data required to successfully translate these exciting preclinical observations into future clinical trials, thereby offering the promise of improving the QOL of brain tumor patients who receive fWBI.
Acknowledgments
This work was supported by NIH grants CA122318 and HL51952, as well as an award from Elekta, Inc.
Financial support: CA122318 (MER), HL51952 (DID), and Elekta, Inc (MER)
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. Ca Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Perez C, Brady LW. Principles and practice of radiation oncology. Philadelphia: Lippincott-Raven; 2003. [Google Scholar]
- 3.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 5.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Curr Pharm Des. 2007;13:1317–1325. doi: 10.2174/138161207780618821. [DOI] [PubMed] [Google Scholar]
- 6.Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophages and the septal fibroblast. Int J Radiat Oncol Biol Phys. 1992;24:93–101. doi: 10.1016/0360-3016(92)91027-k. [DOI] [PubMed] [Google Scholar]
- 7.Denham JW, Hauer-Jensen M. The radiotherapeutic injury - a complex “wound”. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 8.Hauer-Jensen M, Wang J, Boerma M, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 9.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Schultheiss TE, Stephens LC. Permanent radiation myelopathy. Br J Radiol. 1992;65:737–753. doi: 10.1259/0007-1285-65-777-737. [DOI] [PubMed] [Google Scholar]
- 12.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1218. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 13.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 14.Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–176. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 15.Giovagnoli AR, Boiardi A. Cognitive impairment and quality of life in long-term survivors of malignant brain tumors. Ital J Neurol Sci. 1994;15:481–488. doi: 10.1007/BF02334609. [DOI] [PubMed] [Google Scholar]
- 16.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 17.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 18.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumors in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 19.Nathan PC, Patel SK, Dilley K, Goldsby R, Harvey J, Jacobseb C, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer. Arch Pediatr Adolesc Med. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 20.Butler RW, Haser JK. Neurocognitive effects of treatment for childhood cancer. MRDD Research Reviews. 2006;12:184–191. doi: 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 22.Brown PD, Asher AL, Farace E. Adjuvant whole brain radiotherapy: strong emotions decide but rational studies are needed. Int J Radiat Oncol Biol Phys. 2008;70:1305–1309. doi: 10.1016/j.ijrobp.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 23.Khuntia D, Brown P, Li J, Mehta MP. Whole brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 24.Shaw EG, Robbins ME. The management of radiation-induced brain injury. Cancer Treat Res. 2006;128:7–22. doi: 10.1007/0-387-25354-8_2. [DOI] [PubMed] [Google Scholar]
- 25.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Rev Res. 2008;14:238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 26.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson PS, Perfilieva E, Björk-Erikkson T, Alborn AM, Nordberg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 28.Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosc Res. 2002;6:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 30.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 32.Monje ML, Mizumatsu S, Fike JR, Palmer T. Irradiation induced neural precursor-cell dysfunction. Nature Med. 2002;8:955–961. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 33.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 34.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 36.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 37.Atwood T, Payne VS, Zhao W, Brown WR, Wheeler KT, Zhu J-M, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168:574–581. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- 38.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, et al. Spatial learning and memory deficits following whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 39.Villasana L, Acevado S, Poage C, Raber J. Sex and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 40.Noel F, Gumin GJ, Raju U, Tofilon PJ. Increased expression of prohormone convertase-2 in the irradiated rat brain. FASEB J. 1998;12:1725–1730. doi: 10.1096/fasebj.12.15.1725. [DOI] [PubMed] [Google Scholar]
- 41.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 43.Robbins MEC, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 44.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen species and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connor JR, Menzies SL. Cellular management of iron in the brain. J Neurol Sci. 1995;134:33–44. doi: 10.1016/0022-510x(95)00206-h. [DOI] [PubMed] [Google Scholar]
- 46.Bast A, Haenen GRMM, Doelman CJA. Oxidants and antioxidants: State of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 47.Peuchen S, Bolanos JP, Heales SJR, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 48.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 50.Fukuda H, Fukuda A, Zhu C, Korhonen L, Swanpalmer J, Hertzman S, et al. Irradiation-induced progenitor cell death in the developing brain is resistant to erythropoietin treatment and caspase inhibition. Cell Death Differ. 2004;11:1166–1178. doi: 10.1038/sj.cdd.4401472. [DOI] [PubMed] [Google Scholar]
- 51.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang T-T, Fike JR. Cell-density-dependent regulation of neural precursor cells function. PNAS. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, Limoli CL, et al. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res. 2005;164:556–560. doi: 10.1667/rr3412.1. [DOI] [PubMed] [Google Scholar]
- 53.Gerard C, Rollins BJ. Chemokines and disease. Nature Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 54.Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zale B, Rostene W, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- 55.Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diz D. Approaches to establishing angiotensin II as a neurotransmitter revisited. Hypertension. 2006;47:334–336. doi: 10.1161/01.HYP.0000203146.72879.c3. [DOI] [PubMed] [Google Scholar]
- 57.Brosnihan KB, Diz DI, Schiavone MT, Averill DB, Ferrario CM. In: Brain Peptides and Catecholamines in Cardiovascular Regulation. Buckley JP, Ferrario CM, editors. New York: Raven Press; 1987. pp. 313–328. [Google Scholar]
- 58.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, et al. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. PNAS. 1999;96:3975–3980. doi: 10.1073/pnas.96.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diz DI. Approaches to establishing angiotensin II as a neurotransmitter revisited. Hypertension. 2006;47:334–336. doi: 10.1161/01.HYP.0000203146.72879.c3. [DOI] [PubMed] [Google Scholar]
- 60.Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension. 1986;8:544–548. doi: 10.1161/01.hyp.8.6.544. [DOI] [PubMed] [Google Scholar]
- 61.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- 62.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension. 2004;43:1116–1119. doi: 10.1161/01.HYP.0000125143.73301.94. [DOI] [PubMed] [Google Scholar]
- 63.Krob HA, Vinsant SL, Ferrario CM, Friedman DP. Angiotensin-(1–7) immunoreactivity in the hypothalamus of the (mRen-2d)27 transgenic rat. Brain Res. 1998;798:36–45. doi: 10.1016/s0006-8993(98)00384-9. [DOI] [PubMed] [Google Scholar]
- 64.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 65.Chappell MC, Westwood BM, Pendergrass KD, Jessup JA, Ferrario CM. Distinct processing pathways for the novel peptides angiotensin-(1–12) in the serum and kidney of the hypertensive mRen2. Lewis rat. Hypertension. 2007;50:E139. [Google Scholar]
- 66.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, et al. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R111–R115. doi: 10.1152/ajpregu.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isa K, Garcia-Espinosa MA, Shaltout HA, Arnold AC, Chappell MC, Ferrario CM, et al. Intracerebroventricular anti-Ang-(1–12)-IgG improves baroreflex sensitivity and heart rate variability in transgenic (mRen2)27 hypertensive rats. FASEB J. 2009;2:612.3. [Google Scholar]
- 68.Elased KM, Cunha TS, Marcondes FK, Morris M. Brain angiotensin-converting enzymes: role of angiotensin-converting enzyme 2 in processing angiotensin II in mice. Exptl Physiol. 2008;93:665–675. doi: 10.1113/expphysiol.2007.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Tallant EA, et al. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii redcue baroreceptor reflex sensitivity for heart rate control in rats. Exptl Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diz DI, Kasper SO, Sakima A, Ferrario CM. Aging and the brain renin-angiotensin system: Insights from studies in transgenic rats. Cleveland Clinic J Med. 2007;74(Suppl 1):S95–S98. doi: 10.3949/ccjm.74.suppl_1.s95. [DOI] [PubMed] [Google Scholar]
- 71.Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- 72.Gilliam-Davis S, Payne VS, Kasper SO, Tommasi EN, Robbins ME, Diz DI. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–H1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 73.Gilliam-Davis S, Gallagher PE, Payne V, Kasper SO, Tommasi EN, Westwood B, et al. Long-term systemic renin-angiotensin system blockade alters expression of renin-angiotensin system components in dorsomedial medulla of Fischer 344 rats. Hypertension. 2008;52:E61. doi: 10.1152/physiolgenomics.00167.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phillips MI. Antisense inhibition and adeno-associated viral vector delivery for reducing hypertension. Hypertension. 1997;29(part 2):177–187. doi: 10.1161/01.hyp.29.1.177. [DOI] [PubMed] [Google Scholar]
- 75.Paton J, Wang S, Polson J, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 76.Naveri L, Stromberg C, Saavedra JM. Angiotensin IV reverses the acute cerebral blood flow reduction after experimental subarachnoid hemorrhage in the rat. J Cereb Blood Flow Metab. 1994;14:1096–1099. doi: 10.1038/jcbfm.1994.143. [DOI] [PubMed] [Google Scholar]
- 77.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 78.Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Molec Neurobiol. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belcheva I, Ternianov A, Georgiev V. Lateralized learning and memory effects of angiotensin II microinjected into the rat CA1 hippocampal area. Peptides. 2000;21(3):407–411. doi: 10.1016/s0196-9781(00)00163-7. [DOI] [PubMed] [Google Scholar]
- 80.Young D, O'Neill K, Jessel T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. PNAS. 1988;85:5339–5342. doi: 10.1073/pnas.85.14.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, et al. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hremorrhage. J Pharmacol Exp Ther. 2007;322:1051–1058. doi: 10.1124/jpet.107.120097. [DOI] [PubMed] [Google Scholar]
- 82.Makino I, Shibata K, Ohgami Y, Fujiwara M, Furukawa T. Transient upregulation of the AT2 receptor mRNA level after global ischemia in the rat brain. Neuropeptides. 1996;30:596–601. doi: 10.1016/s0143-4179(96)90043-8. [DOI] [PubMed] [Google Scholar]
- 83.Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, et al. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 84.Thone-Reineke C, Zimmerman M, Neumann C, Krikov M, Li J, Gerova N, et al. Are angiotensin receptor blockers neuroprotective? Curr Hypertens Rep. 2004;6:257–266. doi: 10.1007/s11906-004-0019-3. [DOI] [PubMed] [Google Scholar]
- 85.Logan E, Diz DI, Ferrario CM, Ganten D, Averill DB. Inhibition of P13 kinase pathway in the nTS of hypertensive rats improves baroreceptor reflex sensitivity. Hypertension. 2008;52:E80, 122. [Google Scholar]
- 86.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38(5):1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- 87.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, et al. Crosstalk between the insulin and leptin signaling systems in rat hypothalamus 1. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- 88.Camps M, Nicols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 89.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 90.Guthridge MA, Lopez AF. Phosphotyrosine/phosphoserine binary switches: a new paradigm for the regulation of PI3K signalling and growth factor pleiotropy? Biochem Soc Trans. 2007;35:250–252. doi: 10.1042/BST0350250. [DOI] [PubMed] [Google Scholar]
- 91.Teng CH, Huang WN, Meng TC. Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. Curr Hypertens Rep. 2007;6:257–266. doi: 10.1074/jbc.M705142200. [DOI] [PubMed] [Google Scholar]
- 92.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Protein phosphatase 1B activity in the solitary tract nucleus is necessary for normal baroreflex function. Hypertension. 2009;54:e100. [Google Scholar]
- 93.Gallagher PE, Ferrario CM, Tallant EA. MAP Kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calka J, Block CH. Angiotensin-(1–7) and nitric oxide synthase in the hypothalamo-neuropophysial system. Brain Res Bull. 1993;30:677–685. doi: 10.1016/0361-9230(93)90099-w. [DOI] [PubMed] [Google Scholar]
- 95.Albrecht D. Angiotensin-(1–7)-induced plasticity changes in the lateral amygdala are mediated by COX-2 and NO. Learn Mem. 2007;14:177–184. doi: 10.1101/lm.425907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, et al. Central administration of angiotensin-(1–7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Wright JW, Harding The brain angiotensin system and extracellular matrix molecules in neuralplasticity, learning, and memory. Prog Neurobiol. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 99.Kehoe AD, Nikiforov AM, Alexanin SS, Neronov EG, Tikhomirova OV, Shunkov VB, et al. Angiotensin-converting enzyme genotype and encephalopathy in Chernobyl cleanup workers. Eur J Neurol. 2009;16:95–100. doi: 10.1111/j.1468-1331.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- 100.Kim JH, Brown SL, Kolozsvary A, Jenrow KA, Ryu S, Rosenblum ML, et al. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137–142. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- 101.Jackson EK, Garrison JC. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. Hardman JG, Limbird LE, editors. New York: McGraw-Hill; 2001. p. 744. [Google Scholar]
- 102.Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–124. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 103.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, et al. Capillary loss precedes cognitive impairment induced by fractionated whole-brain irradiation. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 104.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu F-C, Brown WR, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kasper SO, Basso N, Kurnjek ML, Paglia N, Ferrario CM, Ferder LF, et al. Divergent regulation of circulating and intrarenal renin-angiotensin systems in response to long-term blockade. Am J Nephrol. 2005;25:335–341. doi: 10.1159/000086571. [DOI] [PubMed] [Google Scholar]
- 106.Conner KR, Payne VS, Forbes EM, Robbins ME, Riddle DR. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. doi: 10.1667/RR1821.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 108.Blume A, Herdegen T, Unger T. Angiotensin peptides and inducible transcription factors. J Mol Med. 1999;77:339–357. doi: 10.1007/s001090050360. [DOI] [PubMed] [Google Scholar]
- 109.Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:194–205. [PubMed] [Google Scholar]
- 110.Shimizu H, Miyoshi M, Matsumoto K, Goto O, Imoto T, Watanabe T. The effect of central injection of angiotensin-converting enzyme inhibitor and the angiotensin type 1 receptor antagonist on the induction by lipopolysaccharide of fever and brain interleukin-1β response in rats. J Pharmacol Exp Ther. 2004;308:865–873. doi: 10.1124/jpet.103.060392. [DOI] [PubMed] [Google Scholar]
- 111.Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Molec Neurobiol. 2009;29:781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 113.Hanna IR, Taniyama Y, Szöcs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 114.Li WG, Miller E, Zhang H, Spitz D, Oberley L, Weintraub N. H2O2-induced O2•− production by a non-phagocytic NADPH oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 116.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 117.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 118.Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med. 2008;86:663–671. doi: 10.1007/s00109-008-0340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-angiotensin system: angiotensin-(1–7), ACE2 and blood pressure regulation. Contrib Nephrol. 2004;143:77–89. doi: 10.1159/000078713. [DOI] [PubMed] [Google Scholar]
- 121.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1–7) unmasked during combined treatment with linopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 122.Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertens Res. 2009;32:533–536. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Maghrebi M, Benter IF, Diz D. Endogenous angiotensin-(1–7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res. 2009;59:263–268. doi: 10.1016/j.phrs.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benter IF, Yousif MHM, Dhaunsi GS, Kaur K, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 125.Ribeiro AB. Angiotensin II antagonists-therapeutic benefits spanning the cardiovascular disease cintinuum from hypertension to heart failure and diabetic nephropathy. Curr Med Res Opin. 2006;22:1–16. doi: 10.1185/030079905X75041. [DOI] [PubMed] [Google Scholar]
- 126.Molteni A, Ward WF, Ts'ao C, Taylor J, Small W, Jr, Brizio-Molteni L, et al. Cytostatic properties of some angiotensin I converting enzyme inhibitors and of angiotensin II type I receptor antagonists. Curr Pharm Des. 2003;9:751–761. doi: 10.2174/1381612033455396. [DOI] [PubMed] [Google Scholar]