Abstract
Objective
To investigate the longitudinal pharmacokinetics, safety and efficacy of lopinavir/ritonavir (LPV/r) in HIV-infected infants initiating combination antiretroviral therapy (cART) between 2 weeks and 6 months of age.
Method
A prospective, open-label, multicenter Phase I/II study of LPV/r-based cART at a dose of 300/75 mg/m2/dose LPV/r twice daily. Intensive pharmacokinetic sampling at 12 months of age and quarterly predose LPV concentrations were collected and safety, virologic and immunologic responses were monitored every 4–12 weeks up to 252 weeks.
Results
Thirty-one HIV-infected infants enrolled into two age cohorts, 14 days to <6 weeks and 6 weeks to <6 months; 29 completed ≥48 weeks of follow-up (median=123 weeks, range 4–252). At 12 months of age, median LPV area under the curve was comparable for both age cohorts and similar to older children and adults. At week 48, 22 of 31 patients (71%) had HIV-1 RNA <400 copies/ml and 11 of 15 (73%) had <50 copies/ml; 29 of 31 achieved HIV-1 RNA <400 copies/ml on study treatment and 19 (66%) remained durably suppressed until the end of study; viral suppression correlated with a higher percentage of predose time points exceeding the LPV target of 1 μg/ml (92 vs. 71%, P=0.002).
Conclusion
LPV/r at 300/75 mg/m2/dose as part of a cART regimen resulted in viral suppression through 96 weeks of treatment in >65% of young infants. Due to initially low LPV exposure in infants <6 weeks of age, frequent dose adjustment for weight gain is advisable and consideration should be given to studying a higher dose for very young infants.
Keywords: AIDS, HIV-1, initiation of antiretroviral therapy in young infants, lopinavir/ritonavir, pharmacokinetics of antiretrovirals
Introduction
Despite tremendous progress in the scale up of prevention of mother-to-child transmission programs in resource-limited countries, up to 1000 HIV-infected infants are born worldwide every day [1]. Multiple studies have demonstrated the benefit of early initiation of combination antiretroviral therapy (cART) resulting in decreased disease progression, immunologic decline, long-term neurologic sequelae and mortality [2-4]. On the basis of the accumulated data, the World Health Organization (WHO), the Pediatric European Network for Treatment of AIDS and the United States Department of Health and Human Services Working Group on Antiretroviral Therapy have recommended initiation of cART as soon as a diagnosis is established for all HIV-infected infants less than 12 months of age [5-7]. Data from South Africa showed that initiation of protease inhibitor-based therapy before 12 weeks of age was associated with a 76% reduction in mortality when compared with deferred therapy [3]; however, only a limited number of potent antiretroviral agents have been well studied in this age group. Our study was designed to investigate the pharmacokinetics, safety and efficacy of cART including the liquid formulation of the protease inhibitor lopinavir/ritonavir (LPV/r) in HIV-infected infants starting therapy between the ages of 2 weeks and 6 months. Pharmacokinetics performed at 2 weeks on treatment and safety and efficacy during the first 24 weeks of treatment was published previously [8,9] and showed that half of the infants who started LPV/r under the age of 6 weeks had significantly lower LPV levels than older children. These results make it important to report longer follow-up to identify subsequent pharmacokinetics data and any enduring consequences of these younger infants’ low drug exposure. This report presents pharmacokinetics results at 12 months of age as well as virologic outcomes and safety data at the conclusion of the study at 48 weeks after the last enrollment, with follow-up ranging up to 252 weeks.
Methods
Pediatric AIDS Clinical Trials Group (PACTG) protocol 1030 was a prospective multicenter, Phase I/II open label trial of a high dose (300 mg LPV/75 mg ritonavir/m2/dose twice daily) of LPV/r-based cART in HIV-1 infected infants <6 months of age. The study was approved by each United States (USA) clinical site’s Institutional Review Board (IRB) and by the National IRB in Brazil (CONEP); written informed consent was obtained from each patient’s legal guardian before enrollment. Two age cohorts were enrolled based on age at study entry (Cohort 1, ≥14 days to <6 weeks and Cohort 2, ≥6 weeks to <6 months of age). Patients were followed until 48 weeks after enrollment of the last patient, except that follow-up discontinued when a patient discontinued study treatment before this time. The study design including evaluations to 24 weeks has been reported previously [8,9]. The following methods pertain to data collected after 24 weeks.
Safety and adverse events
Patients were evaluated every 4 weeks from weeks 24 through 48, then every 6 weeks through week 96, and every 12 weeks thereafter until study closure. Physical examination and nonfasting laboratory evaluations (including electrolytes, glucose, blood urea nitrogen, creatinine, total bilirubin, aspartate aminotransferase, alanine aminotransferase, calcium, phosphorus, triglycerides, cholesterol, total amylase, complete blood count with differential and platelets, and HIV-1 RNA level) were performed during each visit. Lymphocyte surface markers were evaluated every 12 weeks.
Pharmacokinetics
An intensive pharmacokinetic study of LPV/r therapy was performed at the first visit after the patient reached 12 months of age and consisted of blood samples obtained prior to the morning dose and 2, 4, 8 and 12 h following an observed dose. Area under curve (AUC) at the intensive pharmacokinetics visit was determined using the linear trapezoidal method. After 24 weeks, predose samples were also drawn every 12 weeks. All samples were stored at −70°C and batched for analysis.
Virology
The lower limit of HIV-1 RNA detection was 400 copies/ml, except when an ultrasensitive assay was performed, for which it was 50 copies/ml; Roche Amplicor Version 1.5 microwell assay was used for both assays. Durable viral suppression was defined as the absence of confirmed virologic rebound (i.e. not having two consecutive HIV-1 RNA levels >400 copies/ml) after achieving the first HIV-1 RNA <400 copies/ml; missing viral load data at any time point was assigned the value of the next viral load drawn after the missing sample.
Statistics
Wilcoxon’s rank sum test was used for comparing the percentage of LPV predose concentrations exceeding the target value of 1 μg/ml between children achieving vs. not achieving durable viral suppression at week 96. Virologic endpoints were analyzed using an ‘intent-to-treat’ (ITT) approach in which children who discontinued study treatment for any reason were considered to be failures and an ‘as-treated’ approach that only included measurements obtained while a child was taking study treatment. Association between age and LPV predose concentration was evaluated using the Spearman correlation coefficient. Age and sex-specific weight and height Z scores were calculated using CDC Child Growth Standards [10]. Formal analyses of changes in virologic, immunologic and growth parameters are restricted to the first 96 weeks of treatment as the number of patients followed thereafter is small, particularly in Cohort 1.
Results
Study population
Thirty-one children from 17 clinical centers in the USA and Brazil were enrolled and treated with a high dose of LPV/r (300 mg LPV/75 mg ritonavir/m2/dose orally twice daily) between August 2002 and September 2006; baseline demographics by cohort, adverse events, virologic and immunologic responses through 24 weeks and baseline genotypic resistance were previously reported [8,9,11]. The median duration of study treatment and follow-up was 123 weeks (range 4–252 weeks). Because Cohort 1 took longer to enroll, the median follow up for this cohort was shorter (103 weeks, range 43–181 weeks) than for Cohort 2 (124 weeks, range 4–252 weeks). Ten infants (32%) permanently discontinued study medications prior to study closure, including four before 12 months of age: two patients met protocol-defined criteria for discontinuation when their HIV-1 RNA level rebounded to >50 000 copies/ml (weeks 43 and 176), three discontinued after parents’ refusal to attend study visits and/or administer study medications (weeks 2, 42, and 145), three had nontreatment-related confounding conditions (disseminated perinatally acquired cytomegalovirus (CMV) infection resulting in death at week 8, failure to thrive due to severe food allergy at week 70, and severe iron-deficiency anemia at week 120), and two patients were removed from the study because their research sites closed (weeks 73 and 120).
Pharmacokinetics
An intensive pharmacokinetics study was performed in the 26 patients taking study medications at 12 months of age and results were evaluable in 20 patients; among the six patients not evaluable, four had samples collected at ≤3 of the five required times, one was nonadherent with medications, and one whose dose was escalated after the week 2 pharmacokinetics evaluation was analyzed separately. For the 20 evaluable patients, the median age at which the 12-month intensive pharmacokinetics study was performed was 12.4 months (range 12.0–15.5) and the median body surface area was 0.43 m2 (range 0.33–0.50). The median AUC of the two cohorts was comparable at 12 months of age (99.1 μg h/ml [interquartile range (IQR) 82.4–124.5] vs. 112 μg h/ml [IQR 95.0–148.8], P=0.93). In addition, a significant association was found between age and LPV predose concentration (P < 0.0001) (Fig. 1).
Fig. 1.
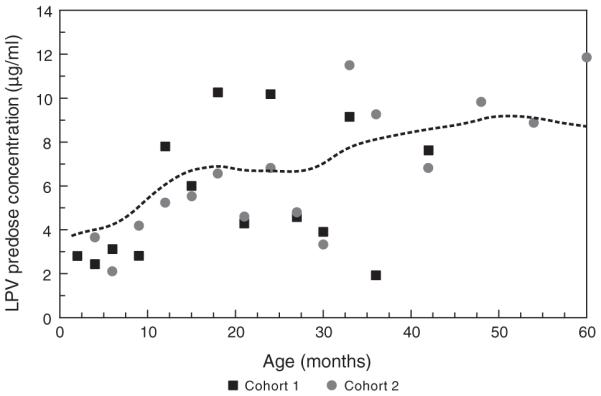
Median lopinavir (LPV) predose concentrations for patients in each cohort, by age at time of measurement. The solid line represents a nonparametric smooth polynomial through the data; there was a significant positive correlation of LPV trough concentration with age (Spearman r=0.30, P < 0.0001).
Virology
Virologic responses through 24 weeks were previously published [8,9]. At week 48, by ITTanalysis, 22/31 (71%) had HIV-RNA <400 copies/ml (6/10 in Cohort 1 and 16/21 in Cohort 2). Among 15 patients from the combined cohorts who were on study treatment at week 48 and evaluated using the ultrasensitive assay, 11 (73%) had HIV-RNA levels <50 copies/ml. Only one of the five patients who had discontinued prior to week 96 did so because of virologic failure. Overall, 29 of 31 patients achieved an HIV-1 RNA <400 copies/ml while on study treatment; one patient discontinued study treatment by 8 weeks due to progressive CMV infection, and the other patient’s mother declined to continue the study at week 2. Among these 29 patients, 19 (66%) patients (6/10 in Cohort 1 and 13/19 in Cohort 2) remained durably suppressed until the end of study at a median of 123 weeks (range 42–252). Of the 10 patients who did not remain durably suppressed, four had periods of poor antiretroviral adherence with confirmed viral rebound, but when adherence improved, these patients re-suppressed and maintained HIV-RNA levels <400 copies/ml for 32–124 weeks in duration before discontinuing the study. The remaining six patients either immediately discontinued study therapy after viral rebound or did not resuppress after confirmed viral rebound. There was no significant difference in the AUC’s obtained at either week 2 of study treatment or at 12 months of age between patients who did or did not have sustained viral suppression. However, those who sustained viral suppression had a higher percentage of time points at which predose concentrations exceeded the LPV target of 1 μg/ml (92 vs. 71%, P=0.002).
Immunology
Measurements were obtained in patients taking study medications. The median CD4 percentage prior to starting study treatment was 35% (n=31, range 11–59%). Twenty-four patients with data available at 48 weeks had a median increase from baseline of 4% (95% confidence interval (CI) −4 to +13%, P=0.12), and 23 (96%) CD4 percentage >25%. The median entry CD8 percentage was 23% (range 14–57%) and at week 48, the median change in CD8 percentage in 24 patients was −3% (95% CI follow-up −5 to −1%, P=0.03). Among patients with follow-up through 96 weeks (n=19), the median CD4 percentage increased 8% (95% CI −2 to 13%, P=0.15), and the CD8 percentage changed by −2% (95% CI −9 to 3%, P=0.21).
Adverse events
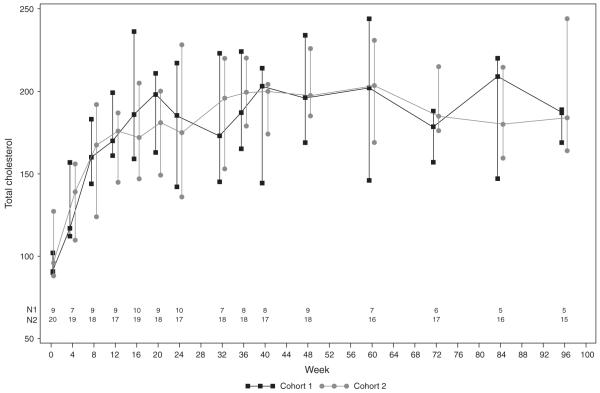
There were no new symptoms, signs or diagnoses after 24 weeks of study that were considered related or possibly related to study treatment and no patient experienced clinical progression of disease after 24 weeks of study. Nonfasting lipid values by cohort are shown in Fig. 2. The median total cholesterol for the combined cohorts was 95 mg/dl at baseline (95% CI 90–118 mg/dl, n=29) and increased to 170 mg/dl (95% CI 130–186 mg/dl) in paired samples from 26 patients at 8weeks (P<0.001); thereafter it remained higher than baseline in both age cohorts through 96 weeks (n=20, P<0.001). The median triglycerides levels were 138 mg/dl (95% CI 86–221 mg/dl, n=28) at baseline (n=28), and 130 mg/dl at 96 weeks (95% CI 79–194 mg/dl, n=20), showing no significant change from baseline at any time point.
Fig. 2.
Median nonfasting total cholesterol over time on study, by cohort. The bars represent interquartile range.
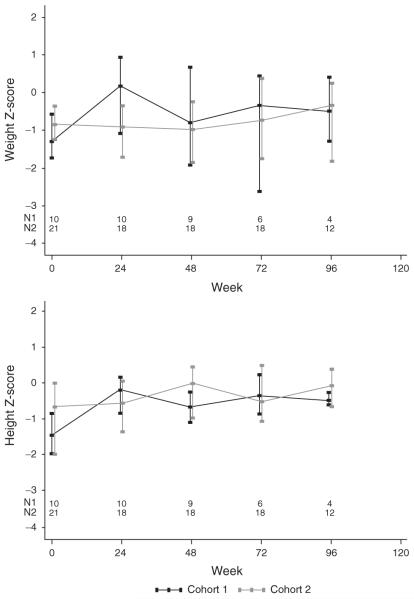
Growth measurements were obtained in patients taking study medications. Across the combined cohorts, the median change in height Z score showed significant increases at nearly all measurement times. The median increase in height Z score among children still on study treatment was 0.88 at week 48 (P=0.004) and 0.82 at week 96 (P<0.001), and was of similar magnitude in both cohorts. (Fig. 3) In contrast, there was a modest median increase in weight Z score during the first 12–16 weeks on study, particularly in the younger cohort, but no significant increase thereafter.
Fig. 3.
Median weight for age/sex Z score and height-for-age/sex Z score, by cohort over time. The bars represent interquartile range.
Discussion
This study demonstrates that 94% of infants <6 months of age initiating combination antiretroviral therapy with a high dose of LPV/r (300/75 mg/m2 per dose twice daily) were able to achieve HIV-RNA <400 copies/ml and 66% maintained viral suppression for a median of 123 weeks on cART. This occurred despite low LPV levels at 2 weeks of therapy, with the lowest exposures in infants <6 weeks of age, in whom the median AUC was approximately half of that seen in children >6 months [8,9,12]. However, by 12 months of age, the younger cohort caught up with the older cohort, with comparable median LPV AUCs and similar to the mean (±standard deviation) values for children 6 months to 12 years of age taking 300/75 mg/m2/dose of LPV/r (116.4±57.1 μg h/ml) and adults taking 400/100 mg/dose LPV/r (92.6±36.7 μg h/ml) [12,13]. Longitudinal median predose LPV levels, which were also initially low, increased throughout the study, meeting or exceeding levels similar to mean values reported for older children (6.53±4.57) and adults (7.1±2.9 μg/ml) [12,13]. It should be noted, however, that = the LPV dose of 300 mg/m2 used for the duration of the study is higher than the currently recommended dose for children over 6 months of age.
Despite the initially low LPV exposure, the overall virologic efficacy was good with 71% (by ITT analysis) of the combined cohorts having HIV-1 RNA <400 copies/ml at 48 weeks; of note, 80% of the younger cohort achieved virologic suppression during the time when the LPV concentrations were lowest [8]. This compares favorably with other cohorts of early initiated cART regimens: The Italian Register for HIV Infection in Children reported that 22 of 30 (73%) infants treated under 6 months of age had virologic suppression with a variety of cART regimens, five of whom received LPV/r [14]. Prendergast and colleagues initiated treatment of 49 South African infants at a median of 42 days of age (range 7–397) with a nelfinavir-based cART regimen and found that approximately 90% had achieved a viral load <400 copies/ml by 24 weeks [15]. In contrast, more than half of our enrolled patients did not achieve viral loads <400 copies/ml until after 24 weeks. This delay may be due in part to the high median baseline viral load of 5.8 log10 copies/ml, but the slow viral load decline may also be related to initially low LPV concentrations, leaving the two NRTI’s responsible for most of the antiviral activity. This concept may be supported by the observation that patients with a higher percentage of predose concentrations over the target level of 1 μg/ml throughout the study were more likely to have durable suppression, but this correlation could also be explained purely by better medication adherence. Almost all infants in this trial were naive to protease inhibitors and 21 of 21 with baseline genotypes performed had LPV-sensitive HIV strains [11]. It is unknown whether similar virologic efficacy will be achieved in infants born to heavily pretreated mothers who transmit resistant virus.
Among patients treated for longer than 8 weeks, all achieved HIV-RNA <400 copies/ml and 66% maintained viral suppression for the duration of the study; however, several other infants had periods of confirmed poor adherence with viral rebound to >400 copies/ml. This is consistent with an observational study of 112 HIV-infected children by Giannattasio et al. [16] who noted that adherence to antiretroviral therapy is a dynamic phenomenon with individual children gaining and losing adherence over time. In addition, patients taking LPV/r therapy had a significantly higher rate of nonadherence in their cohort than patients taking other agents. Notably, even with periods of poor adherence, our patients’ ability to re-suppress the viral load when adherence improved is encouraging and suggests that LPV/r has a high barrier to resistance.
The median CD4+ T-lymphocyte percentage was stable throughout the study, spanning an age range during which infants generally experience a natural decline in both CD4 percentage and number. Among patients still on therapy, 96% had CD4 >25% at 48 weeks, similar to the Italian cohort of infants treated before 6 months of age, in which 97.5% of patients had >25% CD4+ T-lymphocytes at a median follow-up of 5.96 years [17]. Furthermore, the median CD8+ T-lymphocyte percentage in our patients remained stably low throughout the study; this finding has been reported in other infants treated before 6 months of age, in contrast to elevated CD8 percentages in patients whose therapy was deferred [14].
LPV/r was generally well tolerated with only one patient discontinuing treatment due to complaints of mild vomiting and loose stools [9]. Patients’ linear growth improved during the course of the study whereas weight Z scores remained relatively stable. None of the infants in our study reported symptoms of immune reconstitution. In contrast, one-third of a South African cohort of 48 infants initiating therapy <6 months of age developed the immune reconstitution inflammatory syndrome (IRIS); however, all had WHO stage III or IV disease or CD4 <25%, thereby representing a more severely affected group of patients [18].
Our patients had a significant increase in nonfasting total cholesterol by 8 weeks which persisted through 96 weeks; these nonfasting values fall into the 75–90 percentiles of fasting serum total cholesterols in US children between 0 and 4 years of age reported in the Lipid Research Clinics Prevalence Study [19]. Data from normal infants show that cholesterol levels rapidly rise from approximately 70 mg/dl at birth to between 100 and 150 mg/dl during the first weeks of life; thereafter, levels slowly increase to an average of 160–165 mg/dl at 2 years of age [19]. Our patients progressed through this age range during the course of the study, making it difficult to differentiate the expected rise in cholesterol and the effect of antiretroviral therapy. The National Cholesterol Education Program of the National Heart, Lung, and Blood Institute established abnormal cut-points of total cholesterol for children 2–18 years of age in 1992 which have been adopted by the American Academy of Pediatrics; a total cholesterol level at the 75–95% (170–199 mg/dl) is considered borderline, whereas cholesterol at the 95%, or >200 mg/dl, is considered elevated and should be treated. Although it is widely accepted that atherosclerotic cardiovascular disease (CVD) begins early in life and has both genetic and environmental factors which determine the disease course, data do not exist which correlate a particular level of childhood cholesterol that predicts adult CVD [20]. The long-term implications of nonfasting cholesterol levels in the range of 170-200 mg/dl in young infants will require more study and focused follow-up. It is interesting that there was no elevation in triglycerides throughout the study, in contrast to that observed in adults treated with LPV/r [21].
Recent publications substantiating the benefit of early initiation of antiviral therapy including protease inhibitors combined with superior efficacy among infants exposed to nevirapine for prevention of mother to child transmission suggests that LPV/r will be used more commonly in very young infants [3,4,21,22]. In many countries, LPV/r is now the standard protease inhibitor used as the initial antiretroviral regimen for infants with a previous exposure to nevirapine [5,22,23]. As the largest database of pharmacokinetics and efficacy among infants treated within the first 6 months of life, this study will help guide dosing and use of LPV/r in this age group. Our study found that the high dose of 300/75 mg/m2/dose of LPV/r seems appropriate for most infants <6 months of age, especially those who are treatment-naive, however, the lower LPV concentrations observed in the first months of life suggest that studying a higher dose of LPV/r in very young infants should be considered. Although improved drug exposure cannot be guaranteed with higher dosing, especially if there is limited absorption of LPV/r in very young infants, this could also allow investigation of whether more rapid decay of virus could be achieved and have an impact on longevity of viral suppression. Frequent monitoring of young infants receiving LPV/r is advisable, to provide both guidance to the family on successful drug administration techniques and to adjust the dose for weight gain to maintain maximal drug exposure during periods of rapid growth. Careful attention to these details should optimize the early efficacy of LPV/r-based antiretroviral therapy for young infants and build the foundation for prolonged virologic suppression.
Acknowledgements
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-HD-8-0001/HHSN267200800001C). Abbott Laboratories supplied the study drug.
Conflicts of interest: E.V.C. has served as a consultant to GlaxoSmithKlein, Bristol-Meyers Squibb and Johnson & Johnson. E.G.C. has had consultancies with Pfizer and Bristol-Meyers Squibb, and has owned stock+/-stock options in Abbott Labs, GlaxoSmithKlein, Merck Inc., Bristol-Meyers Squibb and Schering Plough. M.D.H. received grant support from Roche, honoraria or consultancies with Abbott Labs, Boehringer Ingelheim, Bristol-Meyers Squibb, Chiron, Medicines Development, Roche, Pfizer, Tibotec and Vironyx. R.Y. has served on the Speaker’s Bureau for Merck Inc. and GlaxoSmithKlein. All other authors have no conflicts.
The study team wishes to thank Kimberly Hudgens MSHCA, MBA; Marisol Martinez-Tristani, MD; Katherine Luzuriaga, MD; Mary Elizabeth Smith, MD; Lynette Purdue, PharmD; Leslie Serchuck, MD; Adam Manzella, MA; and John Rodman, PharmD for their contributions to the performance of the study, and Abbott Laboratories for donation of the study drug, and the patients and families who participated in this study.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS): AIDS Epidemic Update. 2009 December; http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. [PubMed]
- 2.Faye A, Le Chenadec J, Dollfus C, Thuret I, Douard D, Firtion G, et al. French Perinatal Study Group Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39:1692–1698. doi: 10.1086/425739. [DOI] [PubMed] [Google Scholar]
- 3.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. CHer Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. European Infant Collaboration Group Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization antiretroviral therapy of HIV infection in infants and children: recommendations for a public health approach. 2006 http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html.
- 6.US Department of Health and Human Services [Accessed 1 July 2010];Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children: guidelines for the use of antiretroviral agents in pediatric HIV infection. 2009 February 23;:1–139. http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 7.Penazzato M, Donà D, Wool PS, Rampon O, Giaquinto C. Update on antiretroviral therapy in paediatrics. Antiviral Res. 2010;85:266–275. doi: 10.1016/j.antiviral.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick EG, Pinto J, Yogev R, Alvero CG, Hughes MD, Palumbo P, et al. International Maternal Pediatric Adolescent Clinical Trials Group (IMPAACT) P1030 Team Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J. 2009;28:215–219. doi: 10.1097/INF.0b013e31818cc053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadwick EG, Capparelli EV, Yogev R, Pinto JA, Robbins B, Rodman JH, et al. P1030 Team Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–255. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Growth Charts http://www.cdc.gov/growthcharts.
- 11.Persaud D, Palumbo P, Ziemniak C, Chen J, Ray SC, Hughes M, et al. Pediatric AIDS Clinical Trials Group P1030 Team Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. JInfect Dis. 2007;195:1402–1410. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 12.Sáez-Llorens X, Violari A, Deetz CO, Rode RA, Gomez P, Handelsman E, et al. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:216–224. doi: 10.1097/01.inf.0000055061.97567.34. [DOI] [PubMed] [Google Scholar]
- 13.Abbott Laboratories, Abbott Park, IL: Product information. Kaletra (lopinavir/ritonavir) Available at http://www.rxabbott.com/pdf/kaletratabpi.pdf.
- 14.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Italian Register for HIV Infection in Children. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20:207–215. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 15.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 16.Giannattasio A, Albano F, Giacomet V, Guarino A. The changing pattern of adherence to antiretroviral therapy assessed at two time points, 12 months apart, in a cohort of HIV-infected children. Expert Opin Pharmacother. 2009;10:2773–2778. doi: 10.1517/14656560903376178. [DOI] [PubMed] [Google Scholar]
- 17.Chiappini E, Galli L, Tovo PA, Gabiano C, Lisi C, Bernardi S, et al. Five-year follow-up of children with perinatal HIV-1 infection receiving early highly active antiretroviral therapy. BMC Infect Dis. 2009;9:140. doi: 10.1186/1471-2334-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K, Kuhn L, Coovadia A, Meyers T, Hu CC, Reitz C, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program Expert Panel on Blood Cholesterol Levels in Children and Adolescents: Rationale for attention to cholesterol levels in children and adolescents. Pediatrics. 1992;89(Suppl):528–536. [PubMed] [Google Scholar]
- 20.Daniels SR, Greer FR, The AAP Committee on Nutrition Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services [Accessed 1 July 2010];Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2009 December;:1–161. 156. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Appendix B, Table 3.
- 22.Palumbo P, Lindsey J, Hughes M, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Department of Health, South Africa . Guidelines for the management of HIV in Children. 2nd ed. 2010. http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf. [Google Scholar]