Abstract
The somatosenosory barrel cortex in the rodent forms during the first postnatal week setting up a periphery related map with each whisker represented as a bundle of thalamocortical axons (TCAs) in layer IV. The centers of each barrel (hollows) contain the densely packed TCAs, while the areas between each barrel (septa) form a boundary between each barrel. NG2 chondroitin sulfate proteoglycan (CSPG) expressing cells (NG2 cells, polydendrocytes) make up a unique population of glial cells that receive synaptic like input and form close contacts with growing axons. In the present study we investigated the developmental distribution of NG2 cells in the barrel cortex to determine if they display preferential septa distribution similar to other extracellular and cell surface CSPGs. Immunohistochemistry for NG2 and platelet-derived growth factor receptor alpha (PDGFRα) in NG2DsRedBAC transgenic mice showed uniform distribution of NG2 cells and processes in barrel hollows and septa at postnatal (P) days 5, 6, 7, 8, 14, and 30. Changes in the barrel pattern formation caused by cauterization of one row of whiskers at P1 resulted in corresponding changes in extracellular and cell surface CSPG distribution at P7 but no detectable changes in NG2 cell bodies and processes. Furthermore, no abnormalities in barrel formation or reorganization were detected in NG2 knockout mice. These observations suggest that NG2 cells are unlikely to play an inhibitory boundary role on TCA growth and that NG2 expression is not necessary for normal barrel formation.
Keywords: NG2, oligodendrocyte progenitor, barrel cortex, chondroitin sulfate proteoglycan
1. Introduction
The somatosensory barrel cortex in the rodent is organized as a topographic map where axons projecting from the ventral posterior medial nucleus (VPM) of the thalamus form bundles that represent individual mystacial vibrissae (Woolsey and Van der Loos, 1970; Petersen, 2007). These projections set up two functional domains: 1) the barrel hollows that are the bundle of axons within each barrel that preferentially respond to individual whiskers and 2) the barrel septa that are the boundaries between each hollow. This pattern forms during the first postnatal week and can be altered during a developmental critical period, by changes in sensory input by whisker removal before postnatal day 3 (Wong-Riley and Welt, 1980).
The end of the critical period for large-scale structural plasticity in the barrel cortex coincides with the unequal distribution of axon growth inhibitory extracellular matrix (ECM) and cell surface molecules at the septa. ECM molecules such as lectins, tenascin, aggrecan, neurocan and other chondroitin sulfate proteoglycans (CSPGs), which are generally known to be repulsive to growing axons (Snow et al., 1990), show increased expression in the septa during the first postnatal week (Cooper and Steindler 1986a; Steindler et al., 1989; Bahia et al., 2008; Nakamura et al., 2009), when the thalamocortical axon (TCA) branches elaborate and locate their targets within each barrel (Erzurumlu and Jhaveri, 1990).
Cells that express the NG2 CSPG molecule on their surface (NG2 cells, polydendrocytes) comprise a unique population of glial cells in the central nervous system (CNS) separate from astrocytes, oligodendrocytes, and microglia (Nishiyama et al., 2009). NG2 cells, also known as oligodendrocyte progenitor cells (OPCs) exist widely in both gray and white matter of developing and mature CNS (Dawson et al., 2003). Furthermore, they receive synaptic input from neurons in both gray (Bergles et al., 2000; Jabs et al., 2005; Ge et al., 2006) and white matter (Zisken et al., 2007; Kuckley et al., 2007) into adulthood. These data indicate that axon terminals interact intimately with NG2 cells possibly influencing axon growth.
Chondroitin sulfate molecules are generally known to be repulsive to growing axons, (Snow et al., 1990). The NG2 CSPG has shown inhibitory action on neurite outgrowth (Dou and Levine, 1994) and increased expression after CNS injury (Levine, 1994). Other studies have demonstrated however that NG2 cells, unlike the NG2 molecule, are conducive to and may even provide “guide posts” for growing axons (Yang et al., 2006, Busch et al., 2010). If NG2 cells were repulsive to growing axons it could be hypothesized that they would be located at the septa of each barrel during somatosensory cortex development. It would be unlikely that axon growth inhibitory cells would be found in the center of a densely packed bundle of axons.
The objective of this study was to determine whether NG2 cells could be localized to septa of the barrel cortex when thalamocortical axons are finding their targets. We performed immunohistochemistry for NG2 glial cells on tangential sections through barrel cortex at different developmental stages in normal, NG2 knockout and whisker deprived mice and rats. Interestingly, unlike extracellular and cell surface chondroitin sulfate proteoglycans, we demonstrate that NG2 cells are uniformly distributed in barrel hollows and septa, and deletion of NG2 had no effect on the formation or reorganization of the barrels.
2. Material and Methods
2.1 Animals
Postnatal day 5, 6, 7, 8, 14, and 30 FVB mice, C57BL/6 mice, NG2DsRedBAC transgenic mice, NG2 knockout mice and P7 Sprague Dawley rats were used in this study. In NG2DsRedBAC transgenic mice DsRed is expressed specifically in the cell bodies and proximal processes of NG2 glial cells and pericytes as previously reported (Zhu et al., 2008). These mice were bred and maintained as heterozygotes in a mixed background of C57Bl/6 and FVB at the University of Connecticut animal research facility. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut.
To generate NG2 knockout mouse, we designed a targeting vector in which Cre gene was placed just behind the translational initiation site of the NG2 (CSPG4) gene in frame. A knock-in vector pNG2CreTV contained a 3.8 kb fragment at the 5′ side, an nlsCre cDNA (Cre with a nuclear localization signal) placed behind the CSPG4 translational start codon, a pgk (phosphoglycerate kinase)-neo-cassette, a 4.0 kb fragment at the 3′ side, and an MC1 promoter-driven diphtheria toxin gene. Linearized pNG2CreTV was electroporated into C57BL/6ES cells (RENKA Line) (Mishina and Sakimura, 2007), and correctly targeted clones were isolated by Southern blotting. To produce germ line chimeras, the cloned ES cells were microinjected into eight cell-stage embryos of CD1 mouse strain, and the NG2CreKI line was established. These mice will be referred to as NG2 knockout mice throughout this manuscript. There was no overt phenotype of the NG2 knockout mice, similar to what had been reported for a previously established NG2 null line (Grako et al., 1995).
2.2 Tissue Processing
Each animal was initially weighed and photographed in order to document its developmental stage (Hoerder-Saubedissen et al., 2008). Animals were anesthetized with isoflurane and transcardially perfused with 4% paraformaldehyde solution in 0.1M sodium phosphate buffer (pH7.4) containing 0.1M L-Lysine and 0.01M sodium meta-periodate (McLean and Nakane, 1974). Brains were removed and postfixed for 1 hour in the same solution followed by four washes in 0.2M sodium phosphate buffer over 2 hours. Brains were then cryoprotected in 30% sucrose in 0.2M sodium phosphate buffer overnight at 4°C.
Tangential sections (Welker and Woolsey, 1974) were prepared by flattening and freezing each cortical hemisphere in Tissue-Tek O.C.T. compound. Thirty-five-μm sections were cut from the dorsal surface of the flattened tissue with a cryostat (Microm HM 500M).
2.3 Cytochrome Oxidase Histochemistry
The mitochondrial enzyme cytochrome oxidase was used to identify the barrel pattern in layer IV. Tangential sections were incubated at 37°C in the dark for 3–4 hours in a solution containing 0.3mg/mL cytochrome C (Sigma C-2506), 0.6mg/mL 3,3′ diaminobenzidine (Sigma D-8001) and 5% sucrose in 0.2M sodium phosphate buffer (pH 7.4) until the barrel pattern could be easily identified (Wong-Riley, 1979).
2.4 Immunohistochemisty and Fluorescence Histochemistry
Tangential sections were prepared for immunohistochemistry by blocking for one hour at room temperature in 5% normal goat serum (NGS) and 0.1% Triton X-100 in phosphate-buffered saline (PBS). Sections were then incubated in primary antibodies in 5% NGS and PBS overnight at 4°C. Primary antibodies used included: rabbit anti-NG2 IgG (1:500, Chemicon), CS-56 mouse anti-chondroitin sulfate IgM (1:300, Sigma Aldrich), rabbit anti-serotonin transporter (5HTT) IgG (1:500, Calbiochem), rabbit anti-Olig2 (1:20,000, Drs. Charles Stiles and John Alberta, Dana Farber Cancer Institute, Boston, MA) and rabbit anti platelet-derived growth factor receptor alpha (PDGFRα) IgG (1:1000, Dr. W. Stallcup). After PBS wash, sections were incubated in corresponding species-specific fluorochrome-conjugated secondary antibodies for 1 hour at room temperature in 5% NGS and PBS. Secondary antibody dilutions used: Alexa 488-conjugated antibodies (1:500, Molecular Probes), Cy3-conjugated antibodies (1:500, Jackson ImmunoResearch), Cy5-conjugated antibodies (1:50, Jackson Immunoresearch). Green Nissl was used at 1:500 dilution (Molecular Probes). Sections were then dried onto Superfrost glass slides (Fisher Scientific) and mounted in Vectashield mounting medium (Vector Laboratories) with the nuclear counterstain DAPI.
2.5 Fluorescence Microscopy and Quantification
Fluorescence images were captured on a Zeiss Axiovert 200M microscope with an ORCA ER camera (Hamamatsu) and Apotome grid confocal system (Zeiss). Sections were also imaged with a Leica TCS SP2 laser scanning confocal microscope to confirm data obtained from the Zeiss Apotome grid confocal system. Images were processed in Zeiss AxioVision 4.6 software, Adobe Photoshop 9.0 (Adobe Photo Systems) and ImageJ (NIH). Image manipulations were limited to gray-scale level adjustment.
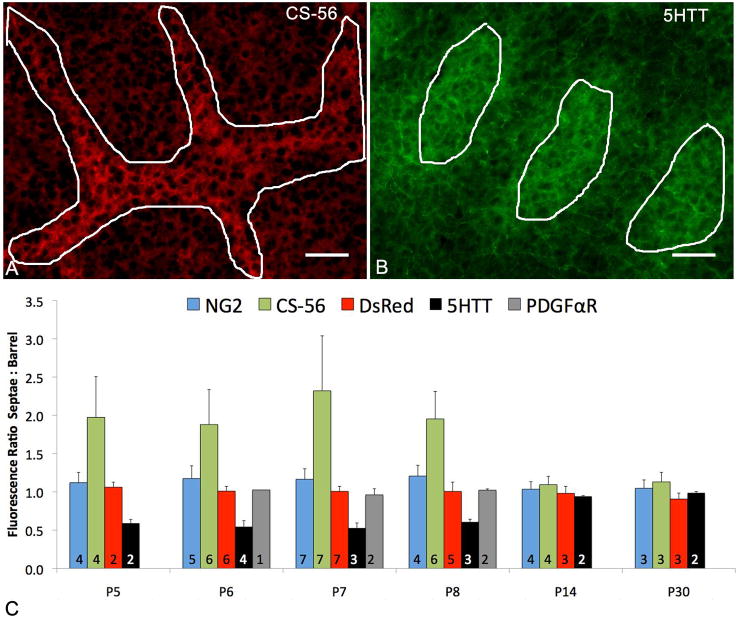
Quantification of fluorescence intensity in barrel hollows and septa was done in ImageJ. Barrel hollows and barrel septa were traced using CS-56, 5HTT, or Nissl staining for P14 and P30 mice and saved as regions of interest (ROIs) (Fig. 4A–B). Mean gray values for corresponding NG2, DsRed, and PDGFRα immunostaining were measured, and the ratio of the gray scale values in the barrel septa and barrel hollows was obtained. Ratios greater than one indicate higher expression levels detected at the barrel septa while values less than one show higher expression levels detected in barrel hollows.
Fig. 4.
Changes in fluorescence intensities during barrel development.
Examples of regions of interest (ROIs) selected for barrel septa (A) using CS-56 immunoreactivity or barrel hollows (B) using 5HTT expression on thalamocortical axons (see materials and methods). Fluorescence intensity quantification (C) shows the ratios of barrel septa to barrel hollows at the different postnatal ages examined. As expected average ratios of barrel septa to hollows are approximately 2:1 for CS-56 from P5–P8 and 0.5:1 for 5HTT. At all ages NG2, DsRed and PDGFRα average ratios remain close to 1:1 indicating uniform distribution in barrel hollows and septa. Numbers at the base of each bar indicate number of animals examined at each age. Scale Bars 50μm in A–B. Error Bars = standard deviation
2.6 Whisker Cauterization
Whisker cauterization on the right whisker pad was performed on postnatal day 1 (P1) NG2DsRedBAC transgenic mice or NG2 knockout mice in order to examine changes in NG2 glial cell distribution associated with barrel cortex structural plasticity. Animals were sacrificed at P7, both hemispheres were flattened and 35μm tangential sections were cut on a cryostat. Adjacent sections were processed for CO cytochemistry and immunolabeled with anti-NG2 and CS-56 antibodies. Changes in row C whisker barrels of the contralateral (left) hemisphere were compared to ipsilateral barrels.
3. Results
3.1 Chondroitin sulfate proteoglycan expression during barrel development
Histochemical reaction for the mitochondrial enzyme cytochrome oxidase (CO) with diaminobenzidine (DAB) was performed on tangential sections through layer IV of the somatosensory barrel cortex in order to reveal the whisker pattern. The barrel pattern was reliably detected in all animals between the ages P5 and P30 and was used on every other section in order to identify sections that exhibited a clear barrel pattern and could be used for immunohistochemistry (Fig. 1A,D,G,J).
Fig. 1.
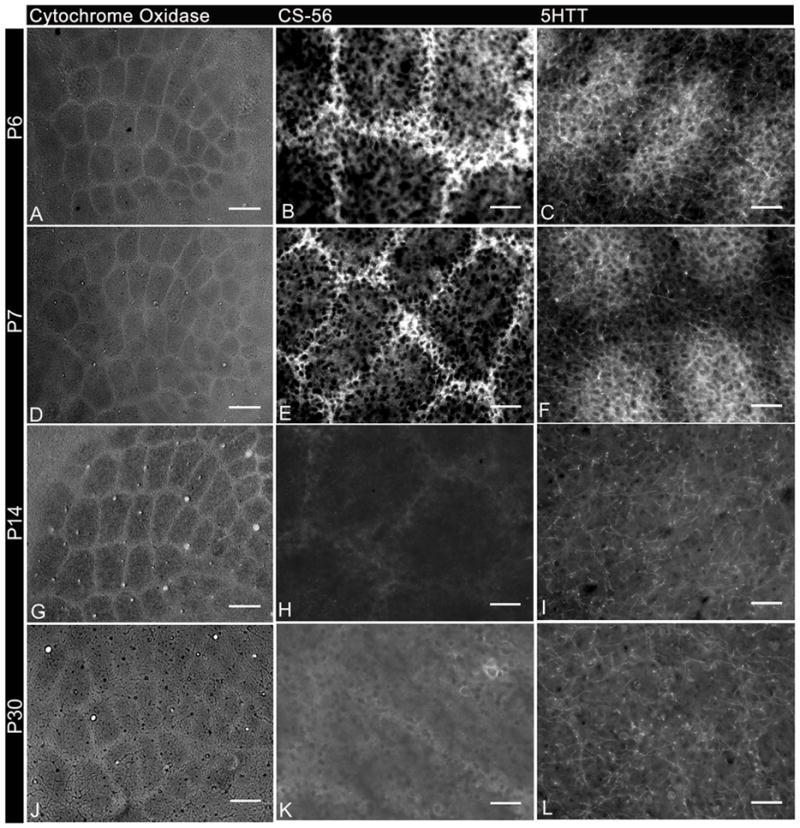
Changes in expression levels of chondroitin sulfate and serotonin transporter in tangential sections during barrel cortex development from P6 to P30.
A,D,G,J: Cytochrome oxidase histochemistry.
B,E,H,K: Immunolabeled with antibody to CS-56.
C,F,I,L: Immunolabeled with antibody to 5-HTT.
Cytochrome Oxidase histochemistry reveals the barrel pattern at all ages examined. Adjacent tangential sections stained for chondroitin sulfate proteoglycans (CS-56) and serotonin transporter (5HTT) show transient expression in barrel septa and hollows respectively from P6–P7. Scale bars 100μm in A–D and 50μm E–L.
Immunohistochemistry for chondroitin sulfate glycosaminoglycan chains using the CS-56 monoclonal antibody was performed on tangential sections through the somatosensory barrel cortex adjacent to those used for CO detection at postnatal days 5, 6, 7, 8, 14 and 30. As previously reported (Miller et al., 1995, Nakamura et al., 2008), more intense CS-56 immunoreactivity was observed in the barrel septa (Fig. 1B and E) compared to the barrel hollows until P7. CS-56 immunoreactivity in the septa declined thereafter, and by P14 and P30 immunoreactivity in the septa was only marginally higher than that in the hollows (Fig. 1H and K). In the present study, CS-56 immunoreactivity was used to identify barrel septa in the early postnatal somatosensory cortex as described below.
3.2 5HTT expression during barrel development
Serotonin has been shown to be important for normal barrel development and both the serotonin receptor 5HT1B and serotonin transporter (5HTT) are transiently expressed on the thalamocortical axons from P4–P10 (Cases et al., 1996; Young-Davies et al., 2000). Immunohistochemistry for 5HTT on tangential sections through layer IV revealed high levels of this transporter on the thalamocortical axons from P5–9 (Fig. 1C and F), which declined to background levels by P14 (Figure 1I and L), consistent with previous reports (Rhoades et al., 1990). Immunoreactivity for 5HTT was used in this study to identify the barrel hollows in the early postnatal mice. For mice older than P10, Green Nissl stain was used to identify the barrel hollows (Figure 2I).
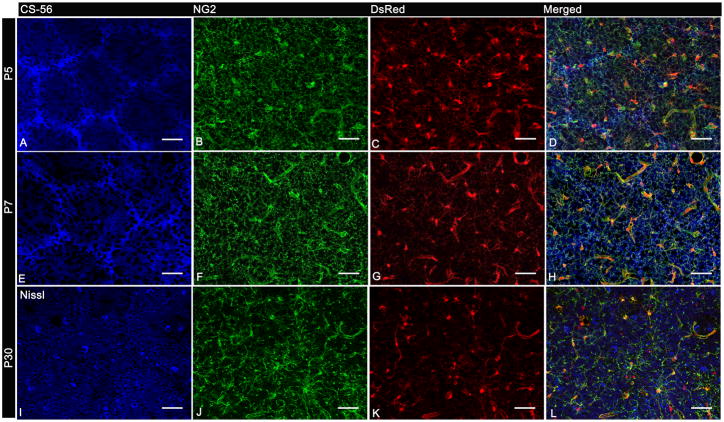
Fig. 2.
NG2 cell distribution during barrel cortex development.
A,F: Immunolabeled with CS-56 antibody
I: Labeled with Nissl stain
B,F,J: Immunolabeled with antibody to NG2
C,G,K: DsRed Expression in NG2DsRedBAC transgenic mice
Single tangential sections from NG2DsRedBAC transgenic mice double immunostained for CS-56 and NG2 at P5 (A–D) and P6 (E–H) and double immunostained for Nissl and NG2 at P30 (I–L). These panels demonstrate the uniform distribution of NG2 cells in barrel hollows and septa at all ages in contrast to the higher levels of CS-56 immunoreactivity in barrel septa. Scale Bars 50μm in A–L
3.3 NG2+ glial cell distribution during barrel development
Tangential sections from NG2DsRedBAC mice were double immunostained with a rabbit antibody to NG2 and the CS-56 monoclonal antibody in order to compare the localization of NG2 glial cells and extracellular and cell surface chondroitin sulfate glycosaminoglycans. Since anti-NG2 antibody labels cell processes as well as the cell body, it was sometimes difficult to see where the cell bodies were located. To facilitate localization of NG2 cell bodies, we used NG2DsRedBAC mice, in which DsRed fluorescence is detected mostly in NG2 cell bodies and proximal processes (Zhu et al., 2008).
NG2 immunostaining at postnatal days 5, 6, 7, 8, 14 and 30 on tangential sections revealed a lack of preferential distribution of NG2 glial cell bodies or process in reference to barrel septa or hollows (Fig. 2B,F,J). NG2 glial cells could be found in the center of the barrel hollow along with the barrel septa at all ages examined (Fig. 2B,F,J and Fig. 3 arrows). Similarly coronal sections immunostained for NG2 revealed a lack of preferential distribution of NG2 glial cell bodies or processes in barrel hollows or septa (Fig. 3J–L). A lower level of NG2 immunoreactivity was evident in Layer IV (compared to other cortical layers) but NG2 distribution was uniform with respect to barrel hollows and septa (Fig. 3J–L). The NG2 antibody also stains pericytes but NG2 glial cells were easily distinguishable by their multipolar morphology while pericytes were bipolar and were in close proximity to the vasculature (Fig. 3 arrowheads). Occasionally a slightly higher NG2 immunoreactivity could be observed in the barrel septa compared with barrel hollows. Interestingly this rare phenomenon was most often observed in C57BL/6 mice and in the locations where CS-56 expression was the highest (Supplementary Fig. 1) but could also be observed occasionally in the other animal strains examined. It is possible that there are some differences in NG2 expression between mouse strains (Mangin and Gallo, 2010).
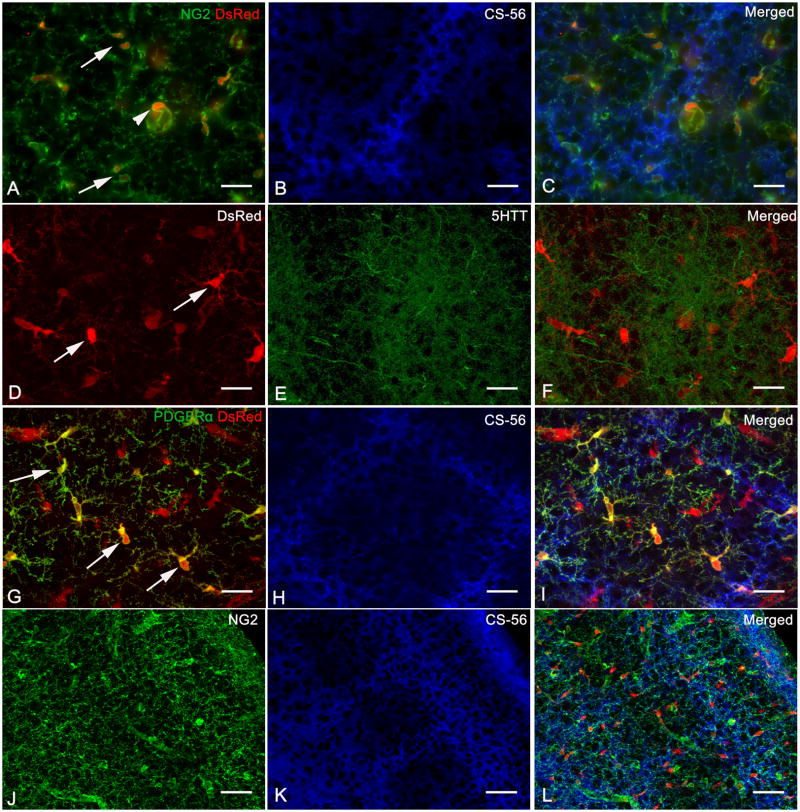
Fig. 3.
NG2 cells are found in barrel hollows and septa at postnatal day 7.
A,J: Immunolabeled with antibody to NG2
A,D,G: DsRed expression in NG2DsRedBAC transgenic mice
B,H,K: Immunolabeled with CS-56 antibody
E: Immunolabeled with antibody to 5-HTT
G: Immunolabeled with antibody to PDGFRα
Single tangential sections from a P7 NG2DsRedBAC transgenic mouse double immunostained for NG2 and CS-56 (A–C), immunostained for 5HTT (D–E) and double immunostained for PDGFRα and CS-56 (G–I) show the presence of NG2 cells in barrel hollows and septa (Arrows). Arrowheads indicate typical morphology of NG2+DsRed+ pericytes. A coronal section immunostained for NG2 and CS-56 similarly shows uniform distribution of NG2 immunoreactivity in barrel hollows and septa (J–L). Scale Bars 25 μm in A–I and 50 μm in J–L
Consistent with NG2 immunostaining, DsRed+ cells in NG2DsRedBAC transgenic mice were distributed uniformly throughout barrel septa and hollows at all ages examined (Fig. 2C,G,K and Fig 3). In order to ensure that the apparent uniform distribution of NG2 cells was not being misinterpreted by the labeling of pericytes by the NG2 antibody and in the NG2DsRed transgenic mice, immunohistochemisty was performed for PDGFRα, which is expressed on NG2 cells but not pericytes and is more concentrated in the cell bodies (Nishiyama et al., 1996; Rivers et al., 2008). The distribution of PDGFRα+ cells was uniform with respect to barrel hollows and septa (Fig. 3G–I) confirming the results demonstrated by NG2 immunostaining and DsRed fluorescence in NG2DsRedBAC transgenic mice. Unlike the occasionally higher NG2 immunostaining seen in the septa of some P5–7 mice (Supplementalry Fig. 1), DsRed+ and PDGFRα+ cells did not reveal any unequal distribution (between barrel hollows and septa) at all ages examined. The DsRed+ PDGFRα-cells in Fig. 3G are likely to be vascular pericytes, which were seen in both barrel hollows and septa. These observations suggest that NG2 cells and their processes are not preferentially distributed in the barrel septa at the end of the critical period for structural plasticity. Additionally, immunostaining for NG2, Olig2 and PDGFRα on P7 rat coronal and tangential sections showed uniform distribution in barrel hollows and septa consistent with data obtained from mouse tissue (Supplementary Fig. 2). Olig2, a basic helix loop helix (bHLH) transcription factor expressed in OPCs and mature oligodendrocytes (Ligon et al., 2006), was used as an additional marker to show the distribution of cells within the oligodendroglial lineage.
In order to semi-quantitatively determine the relative abundance of NG2 cell bodies and processes in the mouse barrel septa and hollows, fluorescence intensity of immunolabeling for NG2, DsRed and PDGFRα was measured. Intensity measurements were obtained from the septa and hollows and compared to measurements taken for CS-56 and 5HTT on the same section. The ratio between barrel septa and hollow was then calculated and used to show the relative spatiotemporal change in expression levels for the different molecules examined. As expected the ratio of barrel septa to hollow was relatively high for CS-56 and low for 5HTT from P5 to P9 (Figure 4C). These data indicate that this type of quantification would be useful to detect unequal distribution of immunofluorescence between barrel septa and hollows. At all ages tested, the ratio of barrel septa to hollow remained close to one for NG2, DsRed, and PDGFRα, as shown in Fig. 4C. These data indicate that at the end of the critical period, NG2+ cell bodies and processes remain evenly distributed between barrel hollows and septa while other extracellular and cell surface CSPGs show unequal distribution.
3.4 Effects of whisker cauterization on CSPG distribution and NG2 cells
Cauterization of one whisker pad in NG2DsRedBAC transgenic mice at P1 revealed changes in the barrel cortex organization when examined at P7 (n=3). Cytochrome oxidase histochemistry revealed a merging of barrel rows and a decrease in overall size (Fig. 5A) for the barrels corresponding to the whiskers that had been removed. Immunostaining of adjacent sections for CS-56 and NG2 revealed a similar change in pattern for chondroitin sulfate expression but no obvious change in NG2+ glial cell distribution (Fig. 5B–E, K, O). A lack of change in NG2 glial cells was also confirmed by examination of DsRed expression (Fig. 5D–E, L, P), suggesting that NG2 cells do not undergo spatial rearrangement under conditions when the thalamocortical axons and extracellular and cell surface CSPG patterns do.
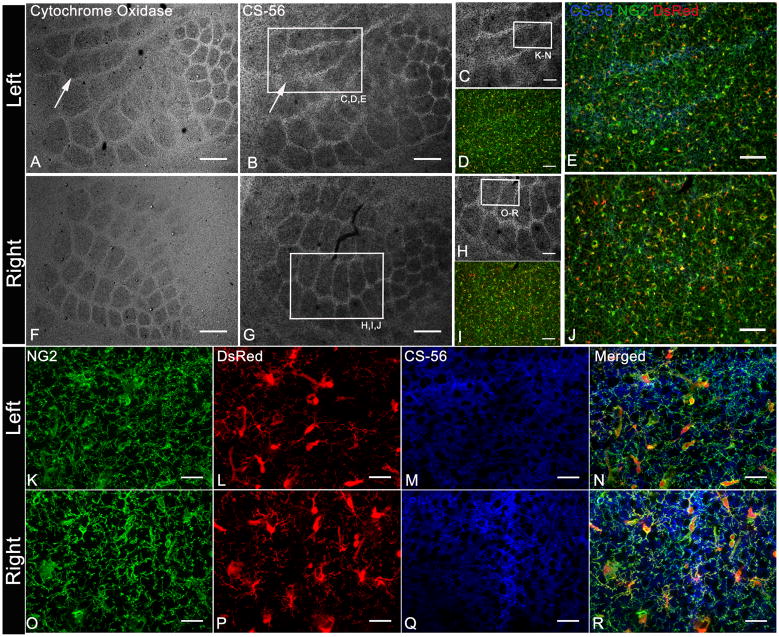
Fig. 5.
Whisker cauterization causes changes in CS-56 but not NG2 distribution.
A,F: Cytochrome oxidase histochemistry
B,C,G,H,M,Q: Immunolabeled with CS-56 antibody
D,I,K,O: Immunolabeled with antibody to NG2
D,I, L,P: DsRed expression in NG2DsRedBAC transgenic mice
Tangential sections from NG2DsRedBAC transgenic mice processed for cytochrome oxidase histochemistry (A,F) revealed changes in barrel pattern in layer IV of the left somatosensory cortex corresponding to cauterized whiskers on right whisker pad (arrow in A). Adjacent tangential sections immunostained for CS-56 (B,G) showed similar changes in barrel pattern (arrow in B). Area indicated by box in B and G is double immunostained for NG2 and CS-56 and shown in C,D,E and H,I,J respectively. Area indicated by boxes in C and H show high magnification images (K–R) of NG2 immunoreactivity and DsRed expression in NG2 cells from cauterized (K–N) and normal (O–R) tangential sections. NG2+DsRed+ cells did not alter their distribution in response to barrel pattern changes unlike CS-56. Scale Bars 100μm in A, B, F, G; 50μm in C–E, H–J and 25 μm in K–R.
3.5 Barrel formation and Reorganization in NG2 knockout mice
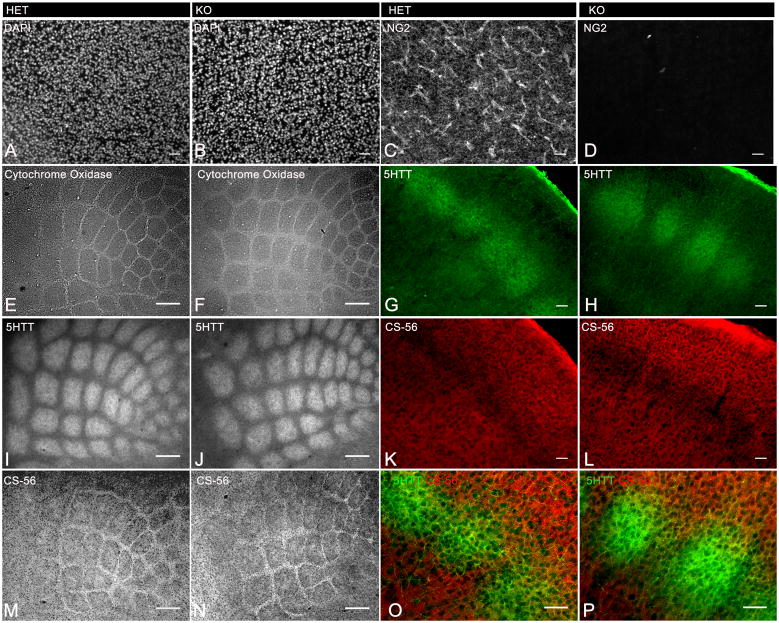
Tangential and coronal sections were analyzed from NG2 knockout (KO) mice to determine if lack of NG2 CSPG expression in the cortex resulted in disrupted axonal targeting and/or barrel formation. NG2 immunoreactivity was not detected in the cortex of NG2 KO mice (Fig. 6C–D, Fig. 7A). Cytochrome oxidase histochemistry, revealed normal barrel pattern at P7 (n=4) in NG2 KO mice (Fig. 6F) which was also evident on adjacent tangential sections using immunostaining for 5HTT and CS-56 (n=4) (Fig. 6I–J and M–N). Coronal sections that were stained for 5HTT and CS-56 showed no detectable abnormalities in layer specific thalamocortical axon targeting (Fig. 6G–H) (n=4). Furthermore, immunostaining for Olig2 and PDGFRα on coronal sections from NG2 KO mice demonstrated the presence of oligodendroctye lineage cells even in the absence of NG2 CSPG and uniform distribution in barrel hollows and septa in contrast to (the segregated distribution of) CS-56 and 5-HTT immunoreactivity (Figure 7).
Fig. 6.
Barrels develop normally in NG2 knockout mice
A–B: DAPI nuclear stain
C–D: Immunolabelled with antibody to NG2
E–F: Cytochrome oxidase histochemistry
G–H, I–J: Immunolabelled with antibody to 5-HTT
K–L, M–N: Immunolabelled with CS-56 antibody
Single tangential sections taken from heterozygous (A,C) and NG2 knockout (B,D) mice at P7 show the lack of NG2 immunoreactivity in the cortex in the knockout. Tangential sections taken from NG2 knockout mice revealed normal barrel pattern formation demonstrated with cytochrome oxidase histochemistry (B), 5HTT immunohistochemistry (F) and CS-56 immunohistochemistry (J). Single coronal sections double immunostained for CS-56 and 5HTT from heterozygous (G,K,O) and knockout mice (H,L,P) show normal barrel formation and layer specific projections. Scale bars 100μm in E–F, I–J, M–N and 50μm in A–D, G–H, K–L, O–P.
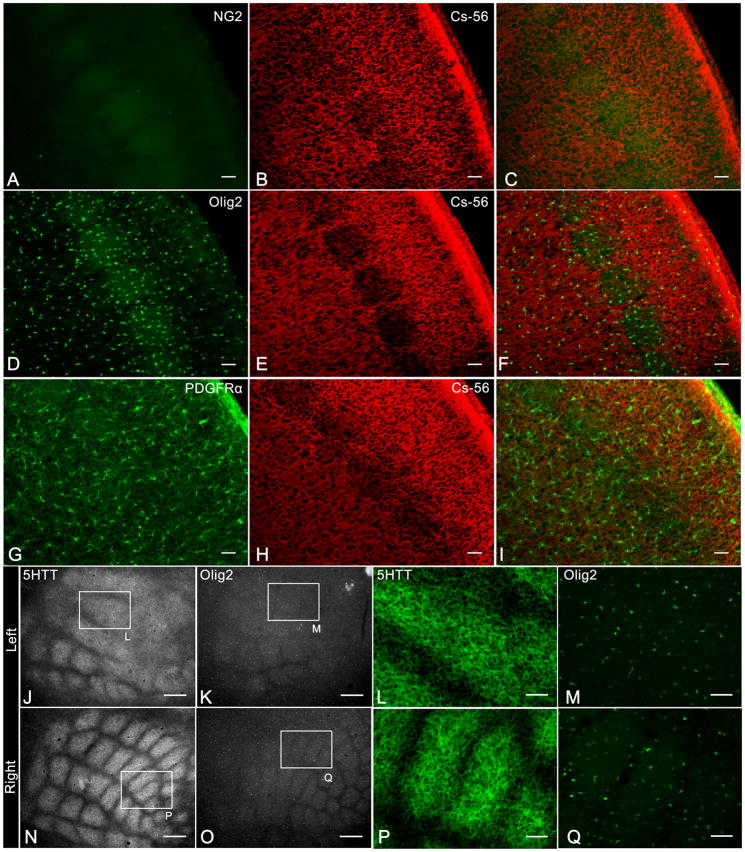
Fig 7.
Uniform distribution of Olig2 and PDGFRα positive cells in normal and whisker cauterized NG2 knockout mice.
A: Immunolabelled with antibody to NG2
B,E,H: Immunolabelled with CS-56 antibody
D,K,M,O,Q: Immunolabelled with antibody to Olig2
G: Immunolabelled with antibody to PDGFRα
J,L,N,P: Immunolabelled with antibody to 5-HTT
Coronal sections (A–I) from NG2 knockout mice show the lack of NG2 immunoreactivity (A) and uniform distribution of Olig2+ cells (D) and PDGFRα+ cells (G) in barrel hollows and septa in contrast to higher CS-56 immunoreactivity in barrel septa (B,E,H). Tangential sections from whisker cauterized (left J–M) and normal (right N–Q) NG2 knockout mice show changes in barrel pattern demonstrated with 5-HTT immunoreactivity but no changes in the distribution of Olig2+ cells in adjacent sections. Scale bars 100μm in J–K, N–O and 50 μm in A–I, L–M, P–Q.
Finally, cauterization of the right whisker pad of NG2 KO mice resulted in redistribution of left barrel pattern demonstrated with 5-HTT immunoreactivity (Fig. 7J,L) but no detectable changes in the distribution of Olig2+ cells (Fig. 7K,M). Thus, lack of NG2 had no apparent effect on the formation of the barrel pattern or restructuring after whisker cauterization.
4. Discussion
Unlike extracellular and cell surface chondroitin sulfate glycosaminoglycans, the NG2 CSPG and NG2 glial cells did not delineate the whisker pattern in layer IV during development of the barrel cortex. Using immunohistochemistry and NG2DsRedBAC transgenic mice we have demonstrated that NG2 cells are uniformly distributed in barrel hollows and septa at the end of the critical period for large-scale structural plasticity in both mice and rats. Changes in sensory input, which resulted in altered organization of barrel structure, did not change the distribution of NG2 cells. Furthermore, using NG2 knockout mice we demonstrated that NG2 CSPG expression in the cortex is not necessary for normal barrel development.
4.1 CSPGs in barrel cortex development
Extracellular and cell surface CSPGs were initially found to be elevated in the barrel septa compared to barrel hollows (Cooper and Steindler 1986a) coincident with preferential distribution of GFAP+ astrocytes in the barrel septa (Cooper and Steindler 1986b). This observation suggested that these axon growth inhibitory molecules might play a role in the formation of the whisker pattern in the cortex by guiding the thalamocortical axons (TCAs) into the corresponding barrel hollow and preventing them from crossing into adjacent barrels. Consistent with these observations, CS-56 monoclonal antibody against chondroitin sulfate chains revealed a high level of expression in the barrel septa relative to the hollows at the end of the critical period between P5 and P9. In contrast, NG2 immunoreactivity was detected uniformly throughout the septa and hollows.
The NG2 core protein has two serine-glycine pairs in the middle portion of its extracellular domain that exist in the context of consensus sequence for chondroitin sulfate attachment (Nishiyama et al., 1991). Mutation analysis revealed that chondroitin sulfate is attached through a single serine residue at amino acid position 999 (Stallcup and Dahlin-Huppe, 2001). Furthermore, NG2 proteoglycan on glial cells isolated from early postnatal brain contains little chondroitin sulfate compared to NG2 from the B49 cell line, and the majority of it appears to exist as the bare core glycoprotein (Nishiyama et al., 1996), as was the case for NG2 in adult brain tissue (Bu et al., 2001). Thus NG2 is not likely to be a major CSPG detected by the CS-56 antibody.
Besides NG2 CSPG, NG2 cells also express at least two members of the lectican family of proteoglycans, versican and neuroglycan-C, (Asher et al., 2002; Cahoy et al., 2008), which carry a large number of CS chains (Yamaguchi, 2000). Additional members of the lectican family of CSPGs in the brain include neurocan and aggrecan synthesized primarily in neurons (Engel et al., 1996; Carulli et al., 2006) and phosphacan and brevican produced primarily in astrocytes (Engel et al., 1996; Carulli et al., 2006; Galtrey and Fawcett 2007). However, it is not known which of these CSPG core proteins is carrying the epitope recognized by the CS-56 antibody detected in the septa. It is also possible that the enrichment of CSPGs in the septa may be a secondary effect caused by the secretion of proteases by the TCAs that enter the barrel hollows and break down the extracellular matrix in the hollows before septa (Steindler et al., 1995).
Our study revealed for the first time, to our knowledge, a detailed developmental distribution of NG2 CSPG and NG2 cells in the barrel cortex. The uniform distribution of NG2 cells and their processes across development in barrel hollow and septa implies that these cells are unlikely to play a major role in guiding thalamocortical axons to navigate into the target barrel without crossing over the boundaries to adjacent barrels. Furthermore, the lack of detectable changes in NG2 CSPG or NG2 cell distribution following whisker removal suggests that NG2 cells do not play a major role in guiding axons as they rearrange to find new targets in response to the altered field of sensory input.
4.2 NG2 molecule and NG2 cells in axon growth
If the NG2 CSPG were acting as an axon growth inhibitory molecule, then aberrant TCA targeting to upper cortical layers might be expected in NG2 knockouts, as previously shown in oligodendrocyte myelin glycoprotein knockout mice (Gil et al., 2009). However, we did not detect any abnormalities in barrel formation and patterning in the NG2 knockout mouse. This suggests that NG2 does not play a major inhibitory role in axon guidance and is consistent with lack of evidence showing altered axon growth or regeneration after injury in NG2 knockout mice (de Castro et al., 2005; Hossain-Ibrahim et al., 2007).
The role of the NG2 proteoglycan in neurite and axon growth and regeneration after injury has become a highly debated topic. In vitro studies primarily from Levine and colleagues demonstrated inhibitory action on neurite and axon outgrowth by the NG2 CSPG and core protein (Dou and Levine, 1994; Chen et al., 2003; Ughrin et al., 2003). An vivo study from this group in which anti-NG2 antibody was infused into the lesioned spinal cord also suggested that NG2 CSPG acts as a barrier to axon regeneration (Tan et al., 2006).
There are other reports, however, that suggest the contrary. We have demonstrated that NG2 cells do not inhibit axon growth in vitro and found evidence for extensive contacts with growing axons in vivo (Yang et al., 2006). A similar close spatial relationship between NG2 cell processes and severed axons has been found in the contused spinal cord (McTigue et al., 2006), where the level of NG2 is greatly increased (Jones et al., 2002). Furthermore, observations of regenerating axons after spinal cord injury showed that NG2 cells might stabilize and support their re-growth through the glial scar (Busch et al., 2010). Given that neurons form synaptic like inputs onto most if not all NG2 cells (Bergles et al., 2000, De Biase et al., 2010) it is unlikely that NG2 cells would repel axons.
The precise mechanism by which NG2 cells interact with growing axons remains unknown. It is possible that NG2 cells express molecules on their surface or secrete molecules that are permissive to axonal growth and counter the inhibitory effects of the NG2 CSPG. For example, brain-derived neurotrophic factor has been shown to be secreted by NG2 cells (Tanaka et al., 2009). In addition to secreted molecules, there may be contact-mediated mechanisms that promote survival and extension of axons. Given the lack of evidence for preferential distribution of NG2 cells and their processes to distinct regions of the barrel cortex and the uniform distribution of NG2 cells in other brain regions, one could speculate that NG2 cells play a more general trophic role for multiple axonal populations. Further studies are needed to define the specific role NG2 cells play in axon growth and survival.
Supplementary Material
Slightly higher NG2-immunoreactivity in septa of C57BL/6 mice.
A,D: Immunolabeled with CS-56 antibody
B,E: Immunolabeled with antibody to NG2
Single tangential sections double immunostained for NG2 and CS-56 from a P6
FvB:C57Bl6:SJL mixed background mouse (A–C) and a P6 C57BL/6 mouse (D–F).
Arrowheads in B indicate barrel septa without elevated NG2 expression in the mixed background while arrows in E indicate higher levels of NG2 expression in septa of the C57BL/6 mouse. This phenomenon was rarely observed, and in the majority of the brains analyzed NG2 expression did not coincide with the barrel pattern. Scale Bars 50μm
NG2 cells are uniformly distributed in P7 rat barrel cortex.
A,J: Immunolabelled with antibody to NG2
B,E,H,K: Immunolabelled with CS-56 antibody
D: Immunolabelled with antibody to Olig2
G: Immunolabelled with antibody to PDGFRα
Coronal sections double immunostained for CS-56 and NG2 (A–C), Olig2 (D–F) or PDGFRα (G–I) from a P7 rat demonstrate uniform distribution of NG2 glial cells in contrast to higher CS-56 immunoreactivity in barrel septa. A single tangential section double immunostained for NG2 and CS-56 (J–L) similarly shows uniform distribution of NG2 immunoreactivity in barrel hollows and septa.
Acknowledgments
This work was funded by grants from the National Multiple Sclerosis Society (A.N.), the National Institutes of Health (A.N.), Grants-in-Aid for Scientific Research 21300118 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K.S.). We thank Youfen Sun for her assistance in maintaining the transgenic mouse colony.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia CP, Houzel JC, Picanco-Diniz CW, Pereira A., Jr Spatiotemporal distribution of proteoglycans in the developing rat’s barrel field and the effects of early deafferentation. J Comp Neurol. 2008;510:145–157. doi: 10.1002/cne.21781. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Cuascut FX, et al. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Brown DJ, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Cooper NG, Steindler DA. Monoclonal antibody to glial fibrillary acidic protein reveals a parcellation of individual barrels in the early postnatal mouse somatosensory cortex. Brain Res. 1986;380:341–348. doi: 10.1016/0006-8993(86)90232-5. [DOI] [PubMed] [Google Scholar]
- Cooper NG, Steindler DA. Lectins demarcate the barrel subfield in the somatosensory cortex of the early postnatal mouse. J Comp Neurol. 1986;249:157–169. doi: 10.1002/cne.902490204. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro R, Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M, Maurel P, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. I. cellular sites of synthesis of neurocan and phosphacan. J Comp Neurol. 1996;366:34–43. doi: 10.1002/(SICI)1096-9861(19960226)366:1<34::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, et al. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Gil V, Bichler Z, Lee JK, et al. Developmental Expression of the Oligodendrocyte Myelin Glycoprotein in the Mouse Telencephalon. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112(Pt 6):905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Paulsen O, Molnar Z. Thalamocortical maturation in mice is influenced by body weight. J Comp Neurol. 2008;511:415–420. doi: 10.1002/cne.21853. [DOI] [PubMed] [Google Scholar]
- Hossain-Ibrahim MK, Rezajooi K, Stallcup WB, Lieberman AR, Anderson PN. Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse. BMC Neurosci. 2007;8:80. doi: 10.1186/1471-2202-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Huttmann K, et al. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–3803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. JNeurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Gallo V. Interactions between thalamocortical fibers and NG2-expressing oligodendrocyte progenitor during the formation of the mouse barrel cortex. Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2010. [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65:406–420. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- Miller B, Sheppard AM, Bicknese AR, Pearlman AL. Chondroitin sulfate proteoglycans in the developing cerebral cortex: the distribution of neurocan distinguishes forming afferent and efferent axonal pathways. J Comp Neurol. 1995;355:615–628. doi: 10.1002/cne.903550410. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nakano K, Morita S, Nakashima T, Oohira A, Miyata S. Expression of chondroitin sulfate proteoglycans in barrel field of mouse and rat somatosensory cortex. Brain Res. 2009;1252:117–129. doi: 10.1016/j.brainres.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Chiaia NL, Bennett-Clarke CA. Development and plasticity of the serotoninergic projection to the hamster’s superior colliculus. J Comp Neurol. 1990;299:151–166. doi: 10.1002/cne.902990203. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Steindler DA, Cooper NG, Faissner A, Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol. 1989;131:243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- Steindler DA, Settles D, Erickson HP, et al. Tenascin knockout mice: barrels, boundary molecules, and glial scars. J Neurosci. 1995;15:1971–1983. doi: 10.1523/JNEUROSCI.15-03-01971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, et al. Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex. 2009;19:2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Davies CL, Bennett-Clarke CA, Lane RD, Rhoades RW. Selective facilitation of the serotonin(1B) receptor causes disorganization of thalamic afferents and barrels in somatosensory cortex of rat. J Comp Neurol. 2000;425:130–138. doi: 10.1002/1096-9861(20000911)425:1<130::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Slightly higher NG2-immunoreactivity in septa of C57BL/6 mice.
A,D: Immunolabeled with CS-56 antibody
B,E: Immunolabeled with antibody to NG2
Single tangential sections double immunostained for NG2 and CS-56 from a P6
FvB:C57Bl6:SJL mixed background mouse (A–C) and a P6 C57BL/6 mouse (D–F).
Arrowheads in B indicate barrel septa without elevated NG2 expression in the mixed background while arrows in E indicate higher levels of NG2 expression in septa of the C57BL/6 mouse. This phenomenon was rarely observed, and in the majority of the brains analyzed NG2 expression did not coincide with the barrel pattern. Scale Bars 50μm
NG2 cells are uniformly distributed in P7 rat barrel cortex.
A,J: Immunolabelled with antibody to NG2
B,E,H,K: Immunolabelled with CS-56 antibody
D: Immunolabelled with antibody to Olig2
G: Immunolabelled with antibody to PDGFRα
Coronal sections double immunostained for CS-56 and NG2 (A–C), Olig2 (D–F) or PDGFRα (G–I) from a P7 rat demonstrate uniform distribution of NG2 glial cells in contrast to higher CS-56 immunoreactivity in barrel septa. A single tangential section double immunostained for NG2 and CS-56 (J–L) similarly shows uniform distribution of NG2 immunoreactivity in barrel hollows and septa.