Abstract
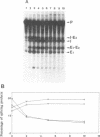
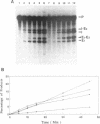
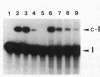
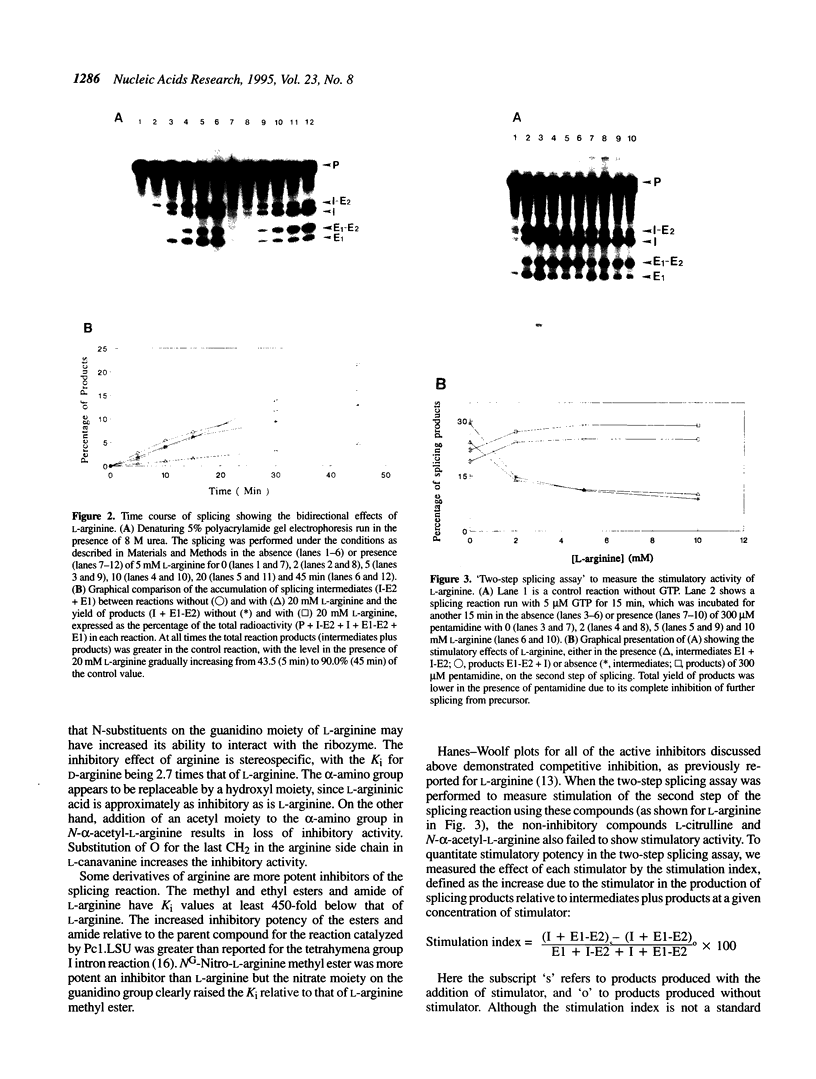
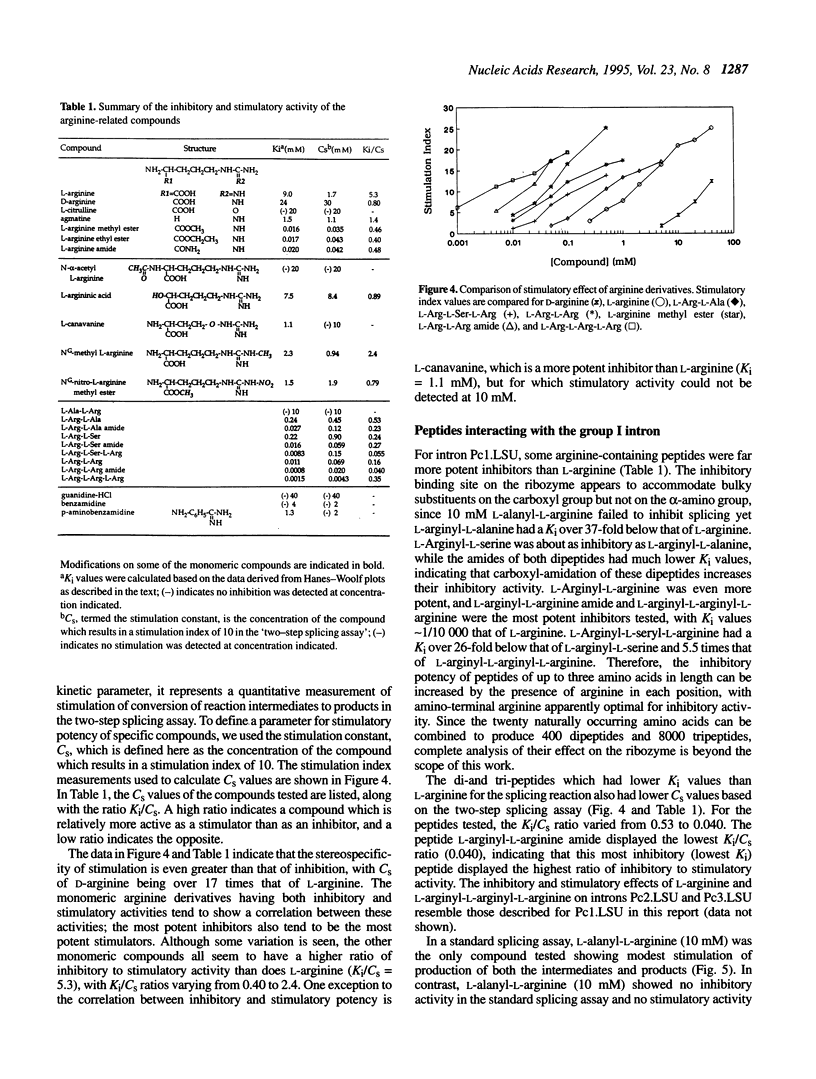
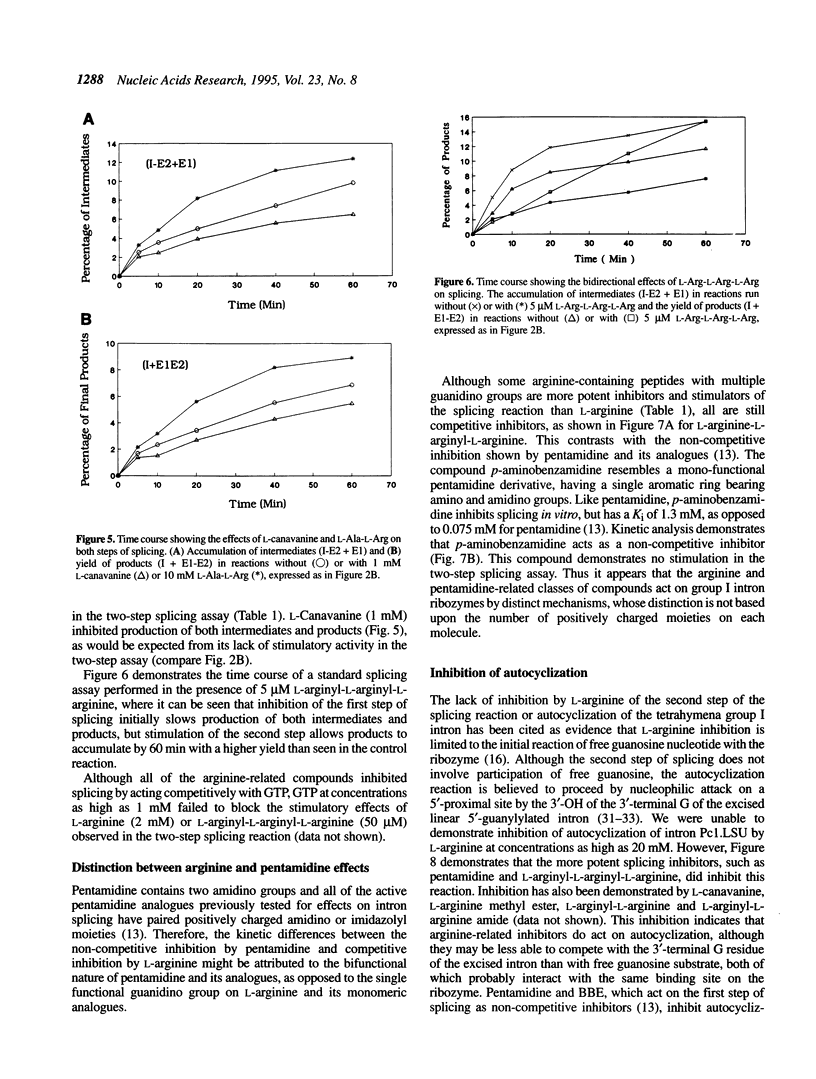
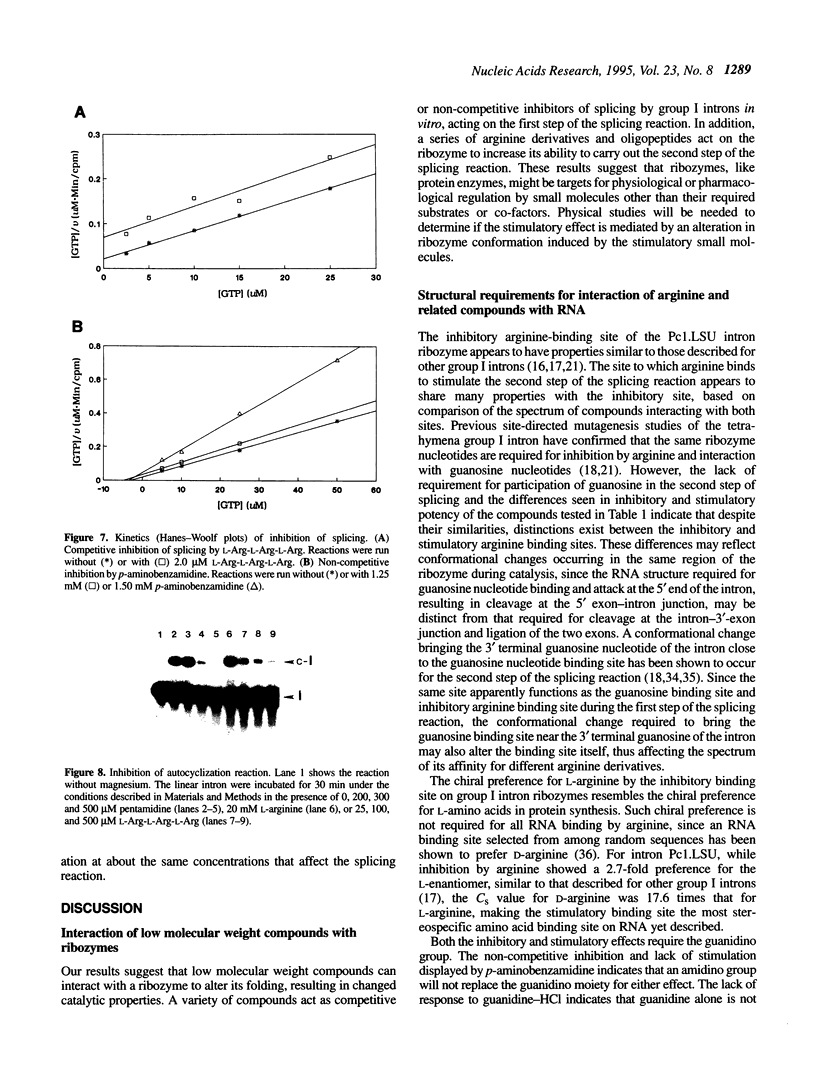
The group I self-splicing introns found in many organisms are competitively inhibited by L-arginine. We have found that L-arginine acts stereoselectively on the Pc1. LSU nuclear group I intron of Pneumocystis carinii, competitively inhibiting the first (cleavage) step of the splicing reaction and stimulating the second (ligation) step. Stimulation of the second step is most clearly demonstrated in reactions whose first step is blocked after 15 min by addition of pentamidine. The guanidine moiety of arginine is required for both effects. L-Canavanine is a more potent inhibitor than L-arginine yet it fails to stimulate. L-Arginine derivatized on its carboxyl group as an amide, ester or peptide is more potent than L-arginine as a stimulator and inhibitor, with di-arginine amide and tri-arginine being the most potent effectors tested. The most potent peptides tested are 10,000 times as effective as L-arginine in inhibiting ribozyme activity, and nearly 400 times as effective as stimulators. Arginine and some of its derivatives apparently bind to site(s) on the ribozyme to alter its conformation to one more active in the second step of splicing while competing with guanosine substrate in the first step. This phenomenon indicates that ribozymes, like protein enzymes, can be inhibited or stimulated by non-substrate low molecular weight compounds, which suggests that such compounds may be developed as pharmacological agents acting on RNA targets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Cech T. R. Specific interaction between the self-splicing RNA of Tetrahymena and its guanosine substrate: implications for biological catalysis by RNA. 1984 Apr 26-May 2Nature. 308(5962):820–826. doi: 10.1038/308820a0. [DOI] [PubMed] [Google Scholar]
- Been M. D., Perrotta A. T. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991 Apr 19;252(5004):434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Connell G. J., Illangesekare M., Yarus M. Three small ribooligonucleotides with specific arginine sites. Biochemistry. 1993 Jun 1;32(21):5497–5502. doi: 10.1021/bi00072a002. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., LaFemina R. L., Callahan P. L., Condra J. H., Sardana V. V., Graham D. J., Nguyen T. M., LeGrow K., Gotlib L., Schlabach A. J. Sequence-specific interaction of Tat protein and Tat peptides with the transactivation-responsive sequence element of human immunodeficiency virus type 1 in vitro. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8985–8989. doi: 10.1073/pnas.87.22.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Zhang J., Kaselis M., Giuntoli D., Stringer S. L., Stringer J. R. Evidence for two genetic variants of Pneumocystis carinii coinfecting laboratory rats. J Clin Microbiol. 1993 May;31(5):1217–1223. doi: 10.1128/jcm.31.5.1217-1223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling U., Roy S., Sumner-Smith M., Barnett R., Reid L., Rosen C. A., Sonenberg N. The number of positively charged amino acids in the basic domain of Tat is critical for trans-activation and complex formation with TAR RNA. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6234–6238. doi: 10.1073/pnas.88.14.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Group I introns as mobile genetic elements: facts and mechanistic speculations--a review. Gene. 1989 Oct 15;82(1):91–114. doi: 10.1016/0378-1119(89)90034-6. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Ehrenman K., Pedersen-Lane J., West D., Herman R., Maley F., Belfort M. Processing of phage T4 td-encoded RNA is analogous to the eukaryotic group I splicing pathway. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5875–5879. doi: 10.1073/pnas.83.16.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke B. J., Christian E. L., Yarus M. Stereoselective arginine binding is a phylogenetically conserved property of group I self-splicing RNAs. EMBO J. 1989 Dec 1;8(12):3843–3851. doi: 10.1002/j.1460-2075.1989.tb08562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Sullivan F. X., Cech T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J Mol Biol. 1986 May 5;189(1):143–165. doi: 10.1016/0022-2836(86)90387-6. [DOI] [PubMed] [Google Scholar]
- Jenison R. D., Gill S. C., Pardi A., Polisky B. High-resolution molecular discrimination by RNA. Science. 1994 Mar 11;263(5152):1425–1429. doi: 10.1126/science.7510417. [DOI] [PubMed] [Google Scholar]
- Kamine J., Loewenstein P., Green M. Mapping of HIV-1 Tat protein sequences required for binding to Tar RNA. Virology. 1991 Jun;182(2):570–577. doi: 10.1016/0042-6822(91)90598-6. [DOI] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Lin H., Niu M. T., Yoganathan T., Buck G. A. Characterization of the rRNA-encoding genes and transcripts, and a group-I self-splicing intron in Pneumocystis carinii. Gene. 1992 Oct 1;119(2):163–173. doi: 10.1016/0378-1119(92)90268-t. [DOI] [PubMed] [Google Scholar]
- Liu Y., Leibowitz M. J. Variation and in vitro splicing of group I introns in rRNA genes of Pneumocystis carinii. Nucleic Acids Res. 1993 May 25;21(10):2415–2421. doi: 10.1093/nar/21.10.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Rocourt M., Pan S., Liu C., Leibowitz M. J. Sequence and variability of the 5.8S and 26S rRNA genes of Pneumocystis carinii. Nucleic Acids Res. 1992 Jul 25;20(14):3763–3772. doi: 10.1093/nar/20.14.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tidwell R. R., Leibowitz M. J. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J Eukaryot Microbiol. 1994 Jan-Feb;41(1):31–38. doi: 10.1111/j.1550-7408.1994.tb05931.x. [DOI] [PubMed] [Google Scholar]
- Michel F., Hanna M., Green R., Bartel D. P., Szostak J. W. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989 Nov 23;342(6248):391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Smulian A. G., Walzer P. D. The biology of Pneumocystis carinii. Crit Rev Microbiol. 1992;18(3):191–216. doi: 10.3109/10408419209114558. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Edman J. C. A self-splicing intron in the small subunit rRNA gene of Pneumocystis carinii. Nucleic Acids Res. 1989 Jul 11;17(13):5349–5359. doi: 10.1093/nar/17.13.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R., Stringer S. L., Zhang J., Baughman R., Smulian A. G., Cushion M. T. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993 Nov-Dec;40(6):733–741. doi: 10.1111/j.1550-7408.1993.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Stringer S. L., Stringer J. R., Blase M. A., Walzer P. D., Cushion M. T. Pneumocystis carinii: sequence from ribosomal RNA implies a close relationship with fungi. Exp Parasitol. 1989 May;68(4):450–461. doi: 10.1016/0014-4894(89)90130-6. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Van der Horst G., Kamps A. M., Arnberg A. C. Interlocked RNA circle formation by a self-splicing yeast mitochondrial group I intron. Cell. 1987 Jan 16;48(1):101–110. doi: 10.1016/0092-8674(87)90360-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. M. The structure and replication of hepatitis delta virus. Annu Rev Microbiol. 1992;46:253–276. doi: 10.1146/annurev.mi.46.100192.001345. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank H., Rogers J., Davies J., Schroeder R. Peptide antibiotics of the tuberactinomycin family as inhibitors of group I intron RNA splicing. J Mol Biol. 1994 Mar 4;236(4):1001–1010. doi: 10.1016/0022-2836(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Ampe C., Schultz S. C., Steitz T. A., Crothers D. M. Fragments of the HIV-1 Tat protein specifically bind TAR RNA. Science. 1990 Sep 14;249(4974):1281–1285. doi: 10.1126/science.2205002. [DOI] [PubMed] [Google Scholar]
- Yarus M. A specific amino acid binding site composed of RNA. Science. 1988 Jun 24;240(4860):1751–1758. doi: 10.1126/science.3381099. [DOI] [PubMed] [Google Scholar]
- Yarus M. An RNA-amino acid complex and the origin of the genetic code. New Biol. 1991 Feb;3(2):183–189. [PubMed] [Google Scholar]
- Yarus M., Christian E. L. Genetic code origins. Nature. 1989 Nov 23;342(6248):349–350. doi: 10.1038/342349b0. [DOI] [PubMed] [Google Scholar]
- Yarus M., Majerfeld I. Co-optimization of ribozyme substrate stacking and L-arginine binding. J Mol Biol. 1992 Jun 20;225(4):945–949. doi: 10.1016/0022-2836(92)90095-2. [DOI] [PubMed] [Google Scholar]
- Yarus M. Specificity of arginine binding by the Tetrahymena intron. Biochemistry. 1989 Feb 7;28(3):980–988. doi: 10.1021/bi00429a010. [DOI] [PubMed] [Google Scholar]
- Zapp M. L., Stern S., Green M. R. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Grabowski P. J., Cech T. R. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983 Feb 17;301(5901):578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Davies J., Schroeder R. Antibiotic inhibition of group I ribozyme function. Nature. 1991 Sep 26;353(6342):368–370. doi: 10.1038/353368a0. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Davies J., Schroeder R. Non-competitive inhibition of group I intron RNA self-splicing by aminoglycoside antibiotics. J Mol Biol. 1992 Aug 20;226(4):935–941. doi: 10.1016/0022-2836(92)91043-o. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Schroeder R. Streptomycin inhibits splicing of group I introns by competition with the guanosine substrate. Nucleic Acids Res. 1991 May 11;19(9):2261–2265. doi: 10.1093/nar/19.9.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]