Abstract
We hypothesized that dysregulation of lactate/pyruvate (monocarboxylate) transporters (MCT) and lactate dehydrogenase (LDH) isoforms contribute to the Warburg effect in cancer. Therefore, we assayed for the expression levels and the localizations of MCT (1, 2, and 4), and LDH (A and B) isoforms in breast cancer cell lines MCF-7 and MDA-MB-231 and compared results with those from a control, untransformed primary breast cell line, HMEC 184. Remarkably, MCT1 is not expressed in MDA-MB-231, but MCT1 is expressed in MCF-7 cells, where its abundance is less than in control HMEC 184 cells. When present in HMEC 184 and MCF-7 cells, MCT1 is localized to the plasma membrane. MCT2 and MCT4 were expressed in all the cell lines studied. MCT4 expression was higher in MDA-MB-231 compared with MCF-7 and HMEC 184 cells, whereas MCT2 abundance was higher in MCF-7 compared with MDA-MB-231 and HMEC 184 cells. Unlike MCT1, MCT2 and MCT4 were localized in mitochondria in addition to the plasma membrane. LDHA and LDHB were expressed in all the cell-lines, but abundances were higher in the two cancer cell lines than in the control cells. MCF-7 cells expressed mainly LDHB, while MDA-MB-231 and control cells expressed mainly LDHA. LDH isoforms were localized in mitochondria in addition to the cytosol. These localization patterns were the same in cancerous and control cell lines. In conclusion, MCT and LDH isoforms have distinct expression patterns in two breast cancer cell lines. These differences may contribute to divergent lactate dynamics and oxidative capacities in these cells, and offer possibilities for targeting cancer cells.
Keywords: glycolysis, Warburg effect, monocarboxylate transporters, lactate dehydrogenase
most cancer cells display a Warburg effect, a state of active glycolysis with lactate production under aerobic conditions (3, 17, 31, 38, 51). Studies of lactate metabolism in humans and rodents have shown that lactate is not only an end product of glycolysis but is an important fuel for active muscles and other tissues and may have hormone-like actions (7–9). The operation of lactate shuttles within and among cells, tissues, and organs such as retina, brain, testis, liver, and cardiac and skeletal muscle under fully aerobic conditions is well established (7–9). In skeletal muscle, the cell-cell lactate shuttle involves the exchange of lactate between glycolytic and oxidative fibers and cardiocytes that actively respire lactate. Moreover, during physical exercise, lactate released from working muscle and other tissues becomes the main precursor for hepatic gluconeogenesis (4). The intracellular lactate shuttle plays an important role in maintaining the redox balance within cells (7, 9). After transport from cytosol to mitochondria, proximal to the inner membrane, lactate is reconverted to pyruvate, a process that generates NADH to be used by the mitochondrial electron transport chain (ETC), as well as pyruvate to be used by the TCA cycle, again to produce reducing equivalents for the ETC (25). Functioning of the intracellular lactate shuttle in muscle may be facilitated by the presence of a mitochondrial lactate oxidation complex (mLOC) comprising monocarboxylate transporter-1 (MCT1), its chaperone basigin (CD147), lactate dehydrogenase (LDH), and cytochrome oxidase (COx) (25). More recent studies suggest that lactate may also be oxidized to pyruvate by an intermembrane space mitochondrial lactate oxidase and produce hydrogen peroxide (13), a reactive oxygen species with second messenger properties implicated in diverse cellular processes (18, 47, 54) including carcinogenesis (6, 11) and metastasis (35, 42).
While lactate accumulation is characteristic of cancer cells, there is no consensus on its cause. Some researchers postulate that lactate production by tumors is due to exaggerated glycolysis, while others suggest that lactate accumulation is due to limited clearance capacity imposed by impaired capability for oxidative phosphorylation (29, 40, 48). Lactate production has been proposed as a marker for malignancy in some human cancers and is associated with poor outcome (56). In normal and patho-physiology, MCTs are the major gateways for lactate trafficking between and within cells (39, 41). The fact that cancer cells also express MCTs like normal cells suggests that these transporters might facilitate lactate exchange and be involved in cancer proliferation. However, little research has been done to detail the roles of MCTs and related proteins in cancer.
Breast tissue expresses lactate transporter proteins, and the plasma membrane abundances of these proteins change significantly in cancer. The MCT4 gene is upregulated in the breast cancer cell line MDA-MB-231 (20), and the MCT1 gene promoter is reported to be hypermethylated in 4 of 19 breast cancer tissues (1). The gene encoding the MCT chaperone, Basigin (CD147), is also upregulated in metastatic breast cancer cells and has been shown to induce extracellular matrix metalloproteinase and play a role with MCT4 in cancer cell invasion (20, 59). The intracellular localization of MCTs may also play a role transducing the changes in lactate concentrations. In healthy slow red oxidative myofibers, MCT1 exists in mitochondrial and plasma membranes (10, 15, 28). In skeletal muscle, peroxisomal membranes express MCT1 and MCT2 (37). In fast-glycolytic fibers, MCT4 and MCT1 are localized to the plasma membrane, and mitochondrial abundance of MCT1 is low as the mitochondrial reticulum is sparse (28). Although MCT1 is the only monocarboxylate transporter in the MitoCarta (mitochondrial proteome list) (45), we have found that, depending on area in mammalian brain, either MCT1 or MCT2, or both, are the mitochondrial MCTs (mMCT) (26). However, little is known about the distribution of lactate transporters in mitochondrial and plasma membranes in cancerous cells.
Given the recent realization of the crucial role of lactate exchange and metabolism in normal physiology and the prevalence of lactate in tumors, lactate shuttling and MCT proteins emerge as possible targets for cancer treatment. In neuroblastoma cells, blocking MCT1 activity was shown to cause acidosis inside those cells, leading to their apoptotic death. The same study showed that lactate transporters facilitate nutrient exchange and thereby facilitate cancer cell growth (16). In a different study, MCT1 inhibition was shown to block lactate transport between glycolytic and oxidative regions within tumors of various cancer types, causing death in the centers of the tumors. Lactate transporters were suspected of playing another permissive role on cancer cells growth, that of transporting lactate from the core cells of tumors to be used as energy substrates by the peripheral tumor cells (53). Knowing the differential roles of lactate transporters in normal and cancer cells could offer opportunities for targeting the latter. Therefore, in the effort to extend knowledge of the role of lactate in cancer growth, we sought to identify differences in the expression of MCT and LDH isoforms in two breast cancer cell lines MCF-7 [a luminal-like breast cell line, estrogen (ER) and progesterone (PGR) receptor positive, and weakly invasivein vitro] and MDA-MB-231 [a mesenchymal-like breast cell line, estrogen (ER) and progesterone (PGR) receptor negative, and highly invasive in vitro] (32), and the control primary breast cell line HMEC 184 (a 10–25% luminal-like and 75–90% basal-like breast cell line, and prestasis with finite lifespan) (21). In addition, we examined available microarray (12) and MassArray data (43) to extend our findings to a larger set of breast cancer cell lines. Results of our study could contribute to efforts to design better strategies for targeting cancer cells based on lactate transporters and LDH isoforms. Knowing the localization and expression patterns of MCT and LDH may help in efforts to target the cancer cells specifically without killing normal cells.
MATERIALS AND METHODS
Tissue culture.
The human breast cancer cell lines (MCF-7, MDA-MB-231) were a gift from Dr. Gary Firestone, UC Berkeley, and the normal primary-human breast cell line (HMEC 184) was a gift from the Human Mammary Epithelial Cell (HMEC) Bank, Lawrence Berkeley National Laboratory (LBNL). The MCF-7 cell line was grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 0.25% penicillin-streptomycin, and (10 μg/ml) insulin. MDA-MB-231 cell line was grown in high-glucose Iscove modified Eagle's medium (IMDM) supplemented with 10% FBS, 1% l-glutamine, and 0.25% penicillin-streptomycin. The HMEC 184 cell line (passage 5–8) was grown in M87A+CT+GFX medium prepared by the HMEC, LBNL. The M87A+CT+GFX medium contained 50% mammary epithelial basal medium (supplemented with 5 mg/ml insulin, 70 μg/ml bovine pituitary extract, 0.5 μg/ml hydrocortisone, 5 ng/ml EGF, 5 μg/ml transferrin, 10−5 M isoproterenol, 2 nM glutamine), and 50% DMEM/F12 (supplemented with 10 μg/ml insulin, 10 nM Tri-iodothyronine, 1 nM β-estradiol, 0.1 μg/ml hydrocortisone, 0.5% fetal calf serum, 5 ng/ml EGF, 2 mM glutamine, and 1 ng/ml cholera toxin), and 0.1 nM oxytocin and 0.1% lipid-rich bovine serum albumin (albuMaxI). Cells were grown in 5% CO2 atmosphere at 37°C.
Materials.
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Tissue culture medium, serum, and reagents were purchased from GIBCO (Carlsbad, CA).
Preparation of subcellular fractions and immunoblots.
Cells were grown in 15 cm dishes until they reached 80–90% confluence, washed with phosphate-buffered saline (PBS), and collected by scraping and brief centrifuging at 700 g for 5 min. The subcellular fractions of whole homogenate and cytosolic-enriched and mitochondrial-enriched fractions were prepared as previously described (25). In brief, cells were homogenized in buffer A (250 mM sucrose, 5 mM NaN3, 2 mM EGTA, 100 μM PMSF, 1 μM pepstatin A, 10 μM leupeptin, 20 mM HEPES-Na, pH 7.4), using a loose-fitting Dunce (Teflon-glass) homogenizer. Homogenates were centrifuged at 600 g for 10 min at 4°C to remove nuclei and unbroken cells. The pellet was discarded and the supernatant [whole homogenate (WH)] was removed, and some was saved for immunoblotting. The rest of the supernatant was centrifuged at 10,000 g for 30 min at 4°C. The supernatant was removed and saved for immunoblotting [cytosolic fraction (Cyto)]. The pellet was washed with buffer A and repelleted by centrifuging at 10,000 g for 30 min at 4°C. This pellet was washed once in buffer C (1 mM EDTA and 10 mM Tris, pH 7.4), then resuspended in buffer C with 1% NP-40 [mitochondrial fraction (MI)]. Protein concentration was determined using a BCA protein assay kit (Pierce Biotechnology, Radford, IL). Western blotting was performed as previously described (25), and the same amount of total protein (30 μg) was loaded in each well. In brief, samples were diluted with LDS sample buffer (Invitrogen, Grand Island, NY) and incubated in a 70°C water bath for 10 min. Samples and the molecular weight standard, MagicMark XP (Invitrogen), were separated on a SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane (GE Healthcare, Amersham, Piscataway, NJ). The membrane was blocked with 10% milk in TTBS buffer (0.1 M NaCl, 0.1 M Tris pH 7.5, 0.1% Tween 20) for 1 h and then incubated with primary antibody with 10% milk in TTBS for 2 h. Next, the membrane was washed three times with TTBS buffer and then incubated with a second antibody in 10% milk in TTBS for 1 h. Finally, the membrane was washed three times with TTBS buffer and then incubated with a chemiluminescence reagent kit (ECL plus kit, GE Healthcare, Amersham) for 5 min, then exposed to X-ray film (GE Healthcare, Amersham). Primary antibodies used were rabbit anti-MCT1, and rabbit anti-MCT4 (Brooks, polyclonal custom antibody), rabbit anti-MCT2 (Chemicon International, Temecula, CA), rabbit and mouse anti-cytochrome oxidase subunit IV, and goat anti-LDH, that reacts with all LDH isoforms (see Fig. 1E; Abcam, Cambridge, MA), rabbit anti-LDHA and rabbit anti-LDHB (Sigma-Aldrich), goat anti-CD147 (Research Diagnosis, Flanders, NJ), rabbit anti-β-actin, mouse anti-GAPDH (Imgenex, San Diego, CA), mouse anti-Na+-K+-ATPase-α (Upstate, Millipore Corporate, MA), rabbit anti-GLUT1, and rabbit anti-TGFβ-R2 (Santa Cruz Biotechnology, Santa Cruz, CA). Band intensity was quantified by Bio-Rad GS-700 Densitometer. The band used for densitometer quantification was marked by underlining the molecular weight standard that corresponded to its size in Fig. 1.
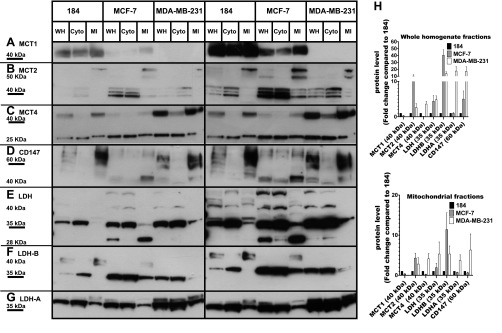
Fig. 1.
Expression of CD147 and monocarboxylate transporter and lactate dehydrogenase (LDH) isoforms detected by immunoblotting. To show relative abundances left- and right-hand plates show unsaturated and saturated autoradiograms, respectively. The expression of MCT1 (A), MCT2 (B), MCT4 (C), CD147 (D), total LDH (E), LDHB (F), and LDHA (G) in whole homogenate (WH), cytosolic fraction (Cyto), and mitochondrial fraction (MI) from 1 control (HMEC 184) and 2 cancerous breast cell lines (MCF-7, MDA-MB-231). All cell lines expressed lactate transporters (MCT1, 2, 4) in WH and MI fractions except MDA-MB-231, which did not express MCT1. An LDH antibody that reacts with all LDH isoforms was used in E. LDH was found in both Cyto and MI fractions of all cell lines. The same amount of total protein was loaded (30 μg) in each well. The fold changes in the expression levels of lactate shuttle proteins in whole homogenate fraction and mitochondrial fraction in the 2 cancerous cell lines were compared with the control cell line (H). The band of interest that was used for densitometer quantification was marked by underlining the molecular weight standard that corresponded to its size in A, B, C, D, E, F, and G and was reported in H. Data are derived from the average of 4 different experiments ± SE.
Confocal laser scanning microscopy.
Cells grown on circular cover slips were washed with PBS and fixed with acetone on ice for 5 min. Cells were washed with PBS and permeabilized with 0.2% Triton X-100 for 5 min. Cells were blocked with 2% FBS for 1 h and then incubated with primary antibodies overnight at 4°C. Cells were washed with PBS and incubated with secondary antibodies for 1 h and then washed with PBS and H2O and mounted using Vectashield (Vector Laboratories, Burlingame, CA). The primary and secondary antibodies were used as previously described (25). Primary antibodies used were rabbit anti-MCT1, rabbit anti-MCT4, rabbit anti-MCT2, rabbit and mouse anti-cytochrome oxidase subunit IV, and goat anti-LDH (the same antibodies as described for Western blotting). The secondary antibodies were anti-rabbit Alexa Fluor 488 conjugate secondary antibody (Molecular Probes), anti-mouse Cy3 (Chemicon International), and anti-goat Alexa Fluor 546 conjugated secondary antibodies (Molecular Probes, Invitrogen, Grand Island, NY). An oil immersion objective on Zeiss 510 META (Zeiss 63x/1.4 numerical aperture) was used. Images represent optical slices of ∼1 μm. Linear adjustments of contrast and brightness were not applied. Hence, images are not contrast enhanced.
Lactate measurements.
Cells were seeded on 60 mm dishes at 4.6 × 105 cells/dish and allowed to grow for 3 days to reach 80–90% confluence. Cells were washed with PBS and incubated with IMDM without phenol red and supplemented with 10% FBS, 1% l-glutamine, and 0.25% penicillin-streptomycin, and with/without 40 mM oxamate (Oxa, LDH inhibitor) and 50 μM iodoacetate (IAA, glycolysis blocker). The medium (350 μl) was collected and added to 100 μl of 7% perchloric acid (PCA) at time 0, 1, 2, 3, 4, and 5 h. Cells were washed with PBS and collected by scraping and brief centrifuging, and total protein concentration was measured by BCA protein assay kit (Pierce Biotechnology). Lactate concentration (μM) was measured by spectrophotometry (23) and normalized to total protein concentration (μg). Lactate standards were made with sodium l-lactate in IMDM (without phenol red and supplemented as mentioned above). Lactate standards (350 μl) were added to 100 μl of 7% PCA. Samples and lactate standards were centrifuged at 3,000 g for 10 min at 4°C, and the pellets were discarded and the supernatants saved. Samples or lactate standards (25 μl) were incubated with 250 μl of reaction buffer (0.5 M glycine, 2% hydrazine hydrate, pH 9, 2.6 units of l-lactate dehydrogenase, 3.0 mM nicotinamide adenine dinucleotide, NAD+) for 40 min at 37°C in 96-well plate. The plate was allowed to cool for 20 min, and then the absorbance was determined at 340 nm using a SPECTRA MAX spectrophotometer (Molecular Devices, Sunnyvale, CA).
Polarographic measurement of oxygen consumption.
Oxygen consumption of intact MCF-7, MDA-MB-231, and HMEC 184 cells was measured using a Clark-type oxygen electrode (Rank Brothers, Cambridge, UK) and LabVIEW software (National Instruments) was used to record the data. Fresh medium was allowed to equilibrate in the sample chamber at 37°C before adding the trypsinized cells (5–8 × 106 cells) from 10 mm dishes. The chamber was stoppered and endogenous oxygen consumption was recorded for 15 min. Carbonyl cyanide m-chlorophenylhydrazone (CCCP; 10, 5, 2.5 μM final concentration was added to MCF-7, HMEC 184, MDA-MB-231 cells, respectively), and maximum uncoupled oxygen consumption was recorded for an additional 10 min. CCCP was added to MDA-MB-231 cells by titration. Data were normalized to total cell number, which was determined by hemacytometer. One-way analyses of variance were used to compare the means of the groups. If a significant F value (P < 0.05) was obtained, a Dunnett's test was performed using HMEC 184 as the control while maintaining α at 0.05. As well, selected Student's t-tests were used to evaluate significance differences between cancer cell lines (P < 0.05).
LDH separation and analysis by electrophoresis.
LDH isoforms present in Cyto and MI fractions from MCF-7, MDA-MB-231, and HMEC 184 cell lines were separated on 1% agarose gels as described previously (10). In brief, 1% agarose gel was prepared and equilibrated in TAE electrophoresis buffer (40 mM Tris acetate, 1 mM EDTA) for 1 h. Samples containing 12 μg of total protein were diluted in sample buffer (20% glycerol, 0.05% bromphenol blue, 80% TAE buffer). Samples and LDH marker (LDH Isotrol, Sigma) were separated by electrophoresis at 90 V for 30 min. The LDH bands were stained and visualized with colorimetric procedure (Sigma Procedure 705). The gel was fixed in methanol-acetic acid solution (5 parts acetic acid, 75 parts methanol, 20 parts water) for 30 min. The gel was washed with distilled water for 30 min, then dried for 15–30 min in a forced air incubator at 37°C, then scanned using a Bio-Rad GS-700 imaging Densitometer.
RESULTS
CD147, MCT, and LDH isoforms were detected by immunoblotting.
Figure 1 is a montage of images compiled from individual Western blot images of MCT1, MCT2, MCT4, LDHA, LDHB, and CD147 proteins. Relative protein abundances with the predicted MWs, identified by the specified antibodies, are shown to facilitate comparisons. Protein levels in whole homogenate and mitochondrial fractions were normalized to β-actin expression, and their expression levels in the two cancer cell lines (MCF-7 and MDA-MB-231) were compared with those in the control cells HMEC 184 cell line; the fold changes of the examined proteins are reported in Fig. 1H. To show relative abundances left- and right-hand plates show unsaturated and saturated autoradiograms, respectively. The MCT1 blot showed a single band at 40 kDa (Fig. 1A). MCT1 expression was lower in MCF-7 and MDA-MB-231 compared with HMEC 184 (Fig. 1H). The MCT2 blot shows multiple bands around 40 kDa (Fig. 1B). The mitochondrial fractions also showed two major bands of MCT2 at 50 kDa in all three cell lines; these mitochondrial bands were heavier than the MCT2 proteins that were localized to the plasma membrane. MCT2 expression was higher in MCF-7 compared with MDA-MB-231 and HMEC 184 cells (Fig. 1H). MCT4 was expressed in higher amounts in MDA-MB-231 than in MCF-7 and HMEC 184 cells (Fig. 1H). MCT4 showed two unique bands at 40 and 25 kDa (Fig. 1C). CD147 was expressed in three cell lines and had two major bands at 40 and 60 kDa, which represent the core-glycosylated and the fully glycosylated forms, respectively (Fig. 1D). The CD147 protein was more highly expressed in MDA-MB-231 than in HMEC 184 and MCF-7 (Fig. 1H).
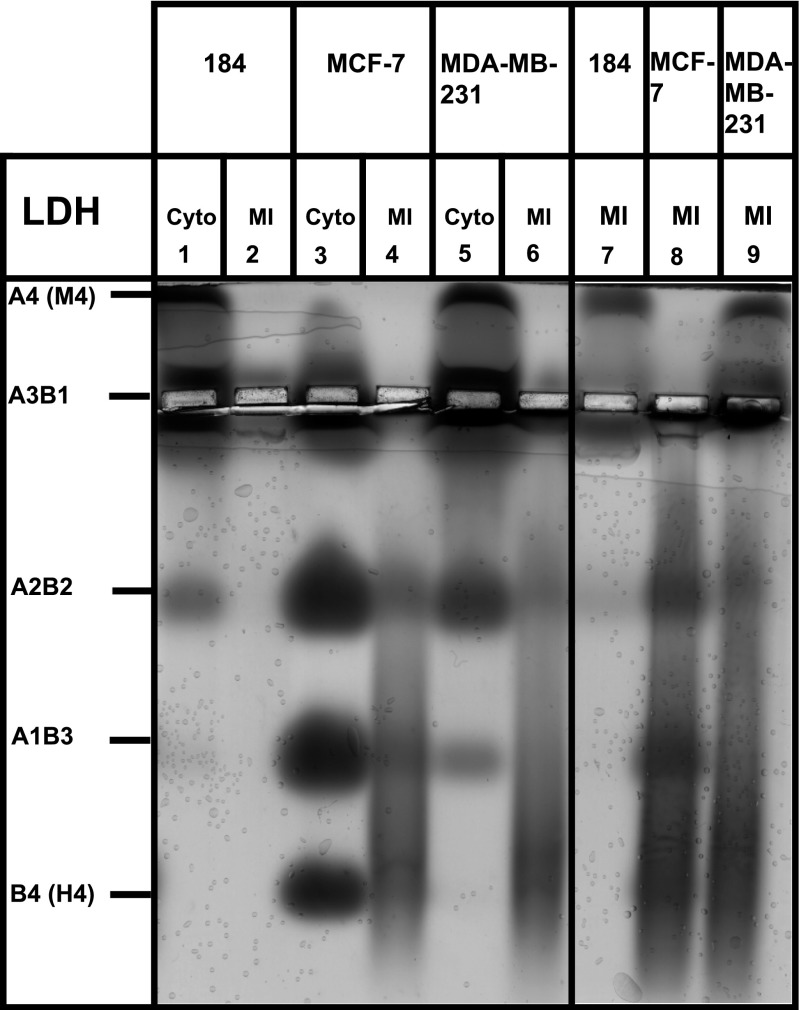
LDH was highly expressed in cancerous cell lines (Fig. 1, E and H), but the LDHA protein was mainly expressed in MDA-MB-231 and HMEC 184 (Figs. 1G and 2), while the LDHB isoform was mainly expressed in MCF-7 (Figs. 1F and 2). LDH isoenzymes separated by electrophoresis on agarose gels confirmed this result (Fig. 2). With an LDH antibody that reacts with all five LDH isoenzymes (Fig. 1E), LDH blots showed unique size bands in mitochondrial fractions of three cell lines, different from the cytosolic LDH, and MCF-7 showed a unique band of LDH at 28 kDa (Fig. 1E).
Fig. 2.
Agarose gel electrophoresis analysis of LDH isoforms. LDH isoforms from Cyto and MI in control primary breast cell line (HMEC 184), and breast cancer cell lines (MCF-7, MDA-MB-231) were separated by agarose gel electrophoresis. Total protein of 12 μg was loaded in wells (1, 2, 3, 4, 5, and 6), and 48 μg in wells (7, 8, and 9). The MCF-7 cell line expressed mainly LDHB, an LDH isoform found in oxidative cell lines. The HMEC 184 and MDA-MB-231 cell lines expressed mainly LDHA, an LDH isoform found in glycolytic cell lines. Data are from 2 different experiments.
Subcellular assessments show mitochondrial fractions to contain Na+-K+-ATPase.
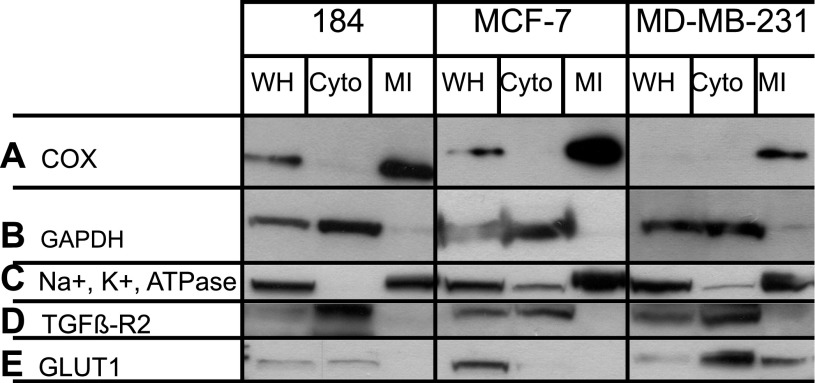
WH, Cyto, and MI cell fractions were isolated from human breast cancer cell lines (MCF-7, MDA-MB-231) and normal primary human breast cell line (HMEC 184). Figure 3 shows that the mitochondrial fractions were rich with mitochondrial protein COxIV. Despite our best efforts at mitochondrial isolation, probing mitochondrial fractions showed strong signals for the plasma membrane maker, Na+-K+-ATPase-α, a small, but observable signal for GLUT1 protein in the mitochondrial fraction of MDA-MB-231 cells, but no signal for TGFβ-R2. Importantly, mitochondrial preparations were negative for the cytosol marker GAPDH. The absence of TGFβ-R2, but variable presence of Na+-K+-ATPase and GLUT1 proteins in the mitochondrial fraction of MDA-MB-231 cells may have resulted from small and variable levels of plasma membrane fragments that coalesce with mitochondrial membranes during isolation. Alternatively, those plasma membrane marker proteins may be functionally associated with the mitochondrial reticulum, in vivo. We previously reported the presence of Na+-K+-ATPase in mitochondrial fractions of primary neuronal cultures (26) and concluded that, rather than indicating contamination, Na+-K+-ATPase may be connected to outer mitochondrial membranes. The same association was also reported by others (57). Seemingly, it would be an advantage for the endergonic Na+-K+-ATPase system, which maintains the plasma membrane cationic gradients, to be associated with the system for maintaining cellular ATP homeostasis. The contamination of mitochondrial fractions by other cell compartments was not tested for because MCT and LDH were mainly found in plasma membrane, mitochondria, and cytosol.
Fig. 3.
Assessment of subcellular contamination. Representative immunoblots showing expressions of COx (A), GAPDH (B), Na+-K+-ATPase-α (C), TGFβ-R2 (D), and GLUT1 (E) in WH, Cyto, and MI from normal primary breast cell line (HMEC 184) and breast cancer cell lines (MCF-7, MDA-MB-231). MI were rich with the mitochondrial marker COx-IV and showed the presence of plasma membrane marker Na+-K+-ATPase-α in all mitochondrial fractions and the presence of plasma membrane marker GLUT1 in the mitochondrial fraction of MDA-MB-231 cells, but no signal of plasma membrane marker TGFβ-R2. There were undetectable amounts of the cytosolic marker GAPDH in mitochondrial fractions.
MCTs and LDH localization by confocal laser scanning microscopy.
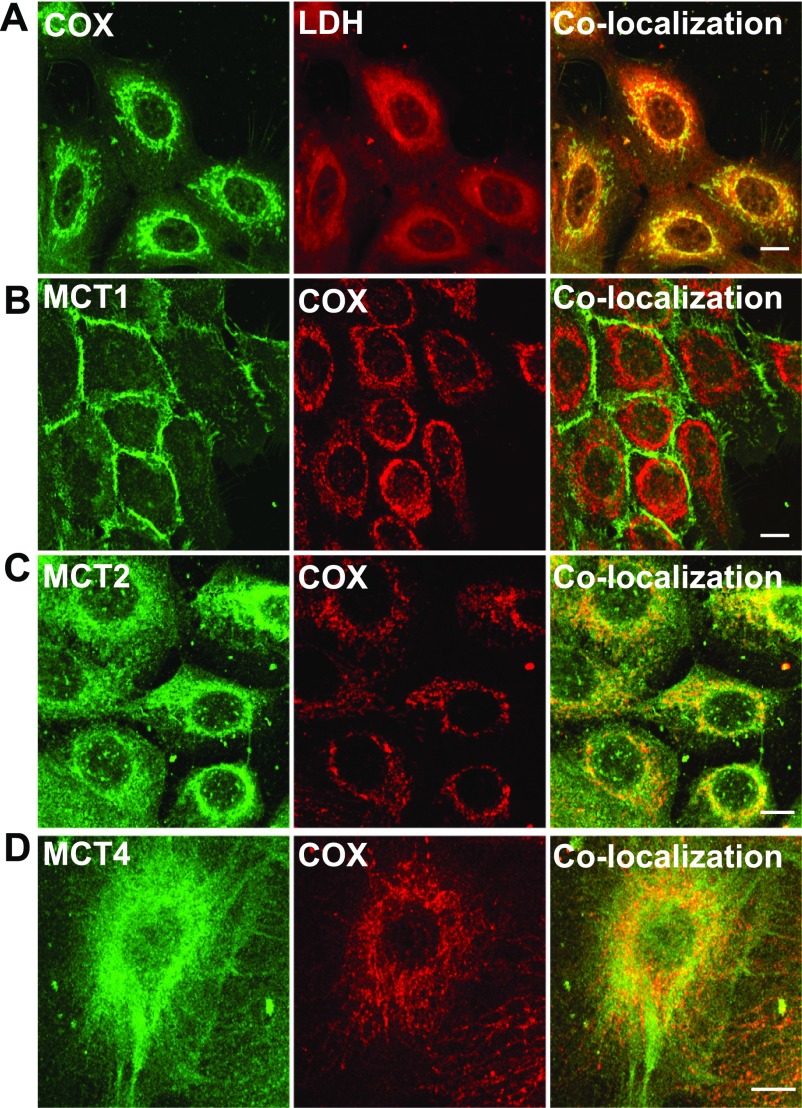
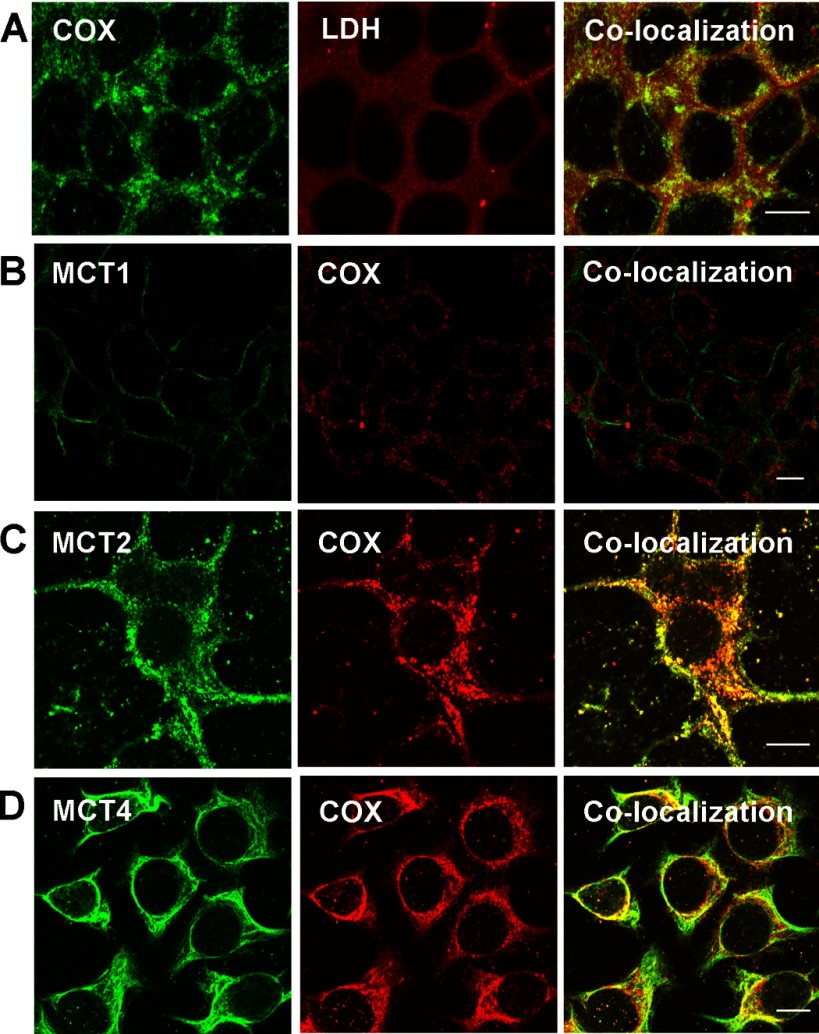
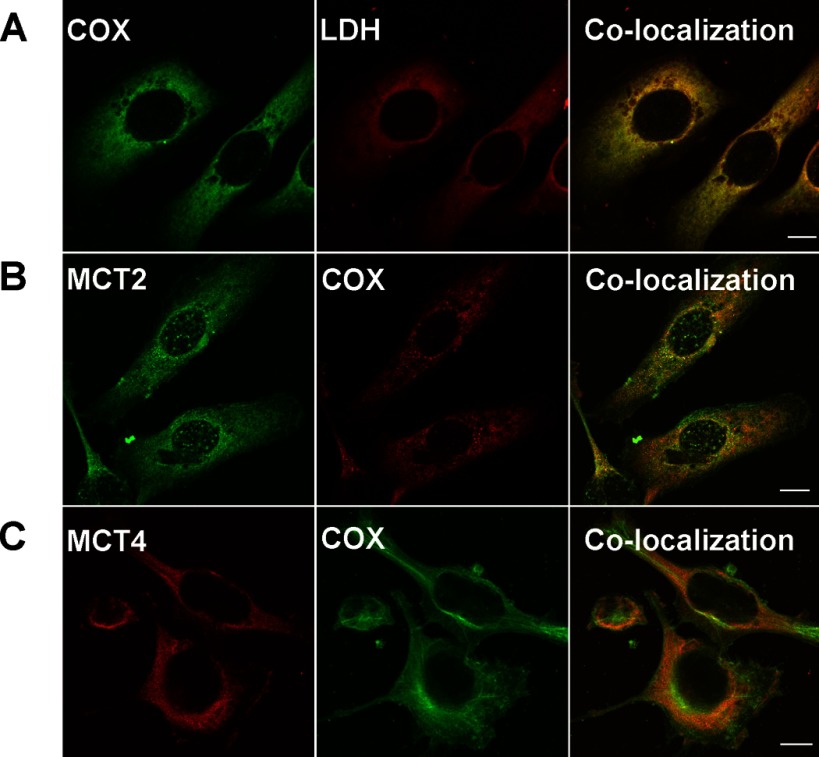
Using confocal laser scanning microscopy, we found that MCT1 was mainly expressed in the plasma membrane in HMEC 184 and MCF-7 cell lines (Figs. 4B and 5B). MCT2, MCT4, and LDH were found to be colocalized with COxIV antibody in the mitochondria of the three cell lines that were tested (Figs. 4–6). The localizations of MCT1, MCT2, MCT4, and LDH in cancerous cells were similar to those in control cells (Fig. 4–6).
Fig. 4.
Immunohistochemical detection of MCT, LDH isoforms, and COx in control breast cell line HMEC 184. LDH isoforms, MCT2, and MCT4 were colocalized with mitochondrial protein marker COx (A, C, D), but MCT1 was not and was localized mainly in the plasma membrane (B). The thickness of the optical section ∼1 μm, scale bar = 10 μm.
Fig. 5.
Immunohistochemical detection of MCT, LDH isoforms, and COx in breast cancer cell line MCF-7. LDH isoforms, MCT2, and MCT4 were colocalized with mitochondrial protein marker COx (A, C, D), but MCT1 was not and was localized to the plasma membrane (B). The thickness of the optical section ∼1 μm, scale bar = 10 μm.
Fig. 6.
Immunohistochemical detection of MCT, LDH isoforms, and COx in breast cancer cell line MDA-MB-231. LDH isoforms, MCT2, and MCT4 were colocalized with mitochondrial protein marker COx (A, B, C). MCT 1 was not expressed in MDA-MB-231 cells. The thickness of the optical section ∼1 μm, scale bar = 10 μm.
Lactate accumulation is higher in cancer cells, and the oxygen consumption is higher in control cells.
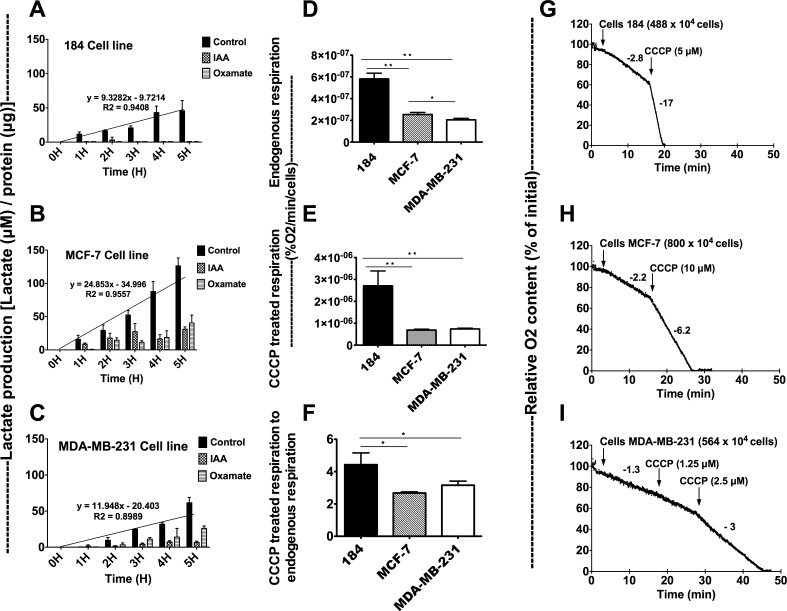
Lactate accumulation in HMEC 184, MCF-7, and MDA-MB-231 cell lines was measured (Fig. 7, A–C). Cells were incubated with IMDM media with or without 40 mM Oxa, an LDH inhibitor, and 50 μM IAA, a blocker of glycolysis. Lactate accumulation was significantly lower in dishes that were incubated with IAA or Oxa compared with control, but higher in cancerous cell lines than control cell lines. Lactate accumulation was highest in the MCF-7 cell line and lowest in the HMEC 184 cell line. Oxygen consumption measurements showed that the three cell lines have different basal (endogenous) and maximum (max) respiratory rates (Fig. 7, D–F). Interestingly, MCF-7 had a higher endogenous respiratory rate than did MDA-MB-231. The HMEC 184 cell line had the highest endogenous and max respiration rates. In aggregate, results suggest that cancer cells display both increased rates of lactate production and reduced capacities for oxidative disposal.
Fig. 7.
Lactate production is higher in cancer cells, and the oxygen consumption is higher in control cells. Lactate production (μM) per hour in HMEC 184 (A), MCF-7 (B), and MDA-MB-231 (C) cell lines was normalized to total protein concentration (μg). Lactate production rates are represented by histogram bars and diagonal slope. Cells were incubated with media with and without 40 mM oxamate (Oxa), an LDH inhibitor, and 50 μM iodoacetate (IAA), a glycolysis blocker. Lactate production was higher in control, IAA, and Oxa dishes of cancerous cell lines compared with the control cell line. The respiration in control and cancerous cell lines were measured (A, B, C). The endogenous respiration (D), CCCP (uncoupler)-treated respiration (E), and CCCP-treated respiration to endogenous respiration ratio (F) were calculated by measuring the oxygen consumption in HMEC 184 (G), MCF-7 (H), and MDA-MB-231 (I) cell lines. The MCF-7 cell line had higher endogenous respiration rate than did MDA-MB-231. The HMEC 184 cell line has the highest endogenous and max respiration rates. Data are means ± SE. Significantly different between groups: *P < 0.05, **P < 0.001.
Available microarray databases.
To expand our findings to a larger set of breast cancer cell lines, we examined the microarray data of Charafe-Jauffret et al. (12) for the expression of MCT and LDH isoforms, as well as the MassArray data of Novak et al. (43) for MCT methylation status in cancer cells. Twofold changes were taken to be significant. Charafe-Jauffret et al. studied 31 breast cell lines, in which all but three were cancerous: MCF-10A, which is derived from a fibrocystic disease, and HME-1 and 184B5, which were derived from normal mammary tissue. The expression profiles of MCT1, MCT4, LDHA, and LDHB were reported in their Supplementary Table 1, but MCT2 and CD147 were not annotated. MCT1 was identified as one of 1,233 marker genes, which are differentially expressed between the luminal-like and the basal-like breast cell lines. MCT1 was highly expressed in basal-like breast cell lines. By comparing the MCT1 mRNA expression levels of all cancerous cell lines to the three normal cell lines in the data of Charafe-Jauffret et al., we found a significant decrease in MCT1 mRNA expression level in cancer cell lines compared with the normal cell lines. MCT4 also showed a trend of lower expression in cancerous cell lines in the data of Charafe-Jauffret et al., but there were some exceptions such as MDA-MB-231 and five other cell lines, which showed increased mRNA expression levels. MCT2 was not found in Supplemental Table 1 of Charafe-Jauffret et al., but MCT2 was one of the marker genes that were highly expressed in mesenchymal-like cell lines compared with luminal-like cell lines. LDHA showed little change in cancerous cells that did not pass the twofold limit set to assess significance. LDHB expression was lower in many cancerous cells compared with the three normal cell lines and appeared to be a marker gene that was highly expressed in the mesenchymal breast cell lines compared with luminal-like cancer cell lines.
Novak et al. (43) exposed three primary breast cell lines (48RT, 184D, 240LD) to different treatments or genetic manipulations that allow them to pass stasis and telomere dysfunction barriers to acquire immortality. By examining MassArray data in their Supplemental Table 2, we saw that MCT1 promoter hypermethylation appeared in an early stage of cancer development after passing the stasis barrier in some cell lines (48RS and 184B), and MCT1 promoter hypermethylation was significant after passing the telomere dysfunction barrier (184B5, 184AA2, 184A1-RF, 184B5ME, 184ZNMY3, 184ZNMY3-N). No change in MCT2 and MCT4 methylation status was seen in any of the cell lines used in the MassArray data of Novak et al.
DISCUSSION
In this study, the expression and the localization of MCT and LDH isoforms in two breast cancer cell lines, MCF-7 and MDA-MB-231, and the control primary breast cell line, HMEC 184, were examined. We show that MCT (1, 2, and 4), and LDH isoforms (A and B) are expressed in both control and cancerous breast cells occupying both mitochondrial and extra-mitochondrial cell compartments. Our results generated three main conclusions that are discussed in more detail below: 1) breast cancer appears to change the expression of lactate shuttle proteins, but not their subcellular localizations; 2) changes in the expression lactate shuttle proteins are associated with decreased oxidative capacity and an increased lactate accumulation within breast cancer cells; and 3) our data and those of others indicate that MCT1 expression is downregulated in breast cancer cells in general.
Breast cancer appears to change the expression of lactate shuttle proteins, but not their subcellular localizations.
The existence of MCT and LDH proteins in mitochondria in different healthy tissues was reported previously by us (10, 25) and others (2, 5, 34) and supported by the MitoCarta list (45). However, for first time we report on the presence of MCT and LDH isoforms in the mitochondrial reticula of breast cell lines. Our data show that MCT (1, 2, and 4), and LDH (A and B) isoforms are expressed in both control and cancerous breast cells occupying both mitochondrial and extra-mitochondrial cell compartments. An exception is that MDA-MB-231 did not express MCT1 as determined by either of two methods (Figs. 1A and 6). The expression of MCT and LDH isoforms in each cell line was unique. MCT1 was highly expressed in the primary-human breast cell line HMEC 184 (Fig. 1A), MCT2 was highly expressed in MCF-7 cell lines (Fig. 1B), MCT4 was highly expressed in MDA-MB-231 cell lines (Fig. 1C), and LDH was highly expressed in both cancerous cell lines (Fig. 1E). The localization of MCT and LDH isoforms in cancerous and normal cell lines was the same. MCT2, MCT4, and LDH were localized in mitochondria in addition to their localization in plasma membrane and cytosol, respectively (Figs. 4–6), whereas MCT1 was mainly localized in plasma membrane.
The mechanism of the translocation of MCT and LDH proteins to mitochondria is unclear. MCT1 is the only candidate lactate/pyruvate transporter in the MitoCarta list; furthermore, of the LDH isoforms annotated in the MitoCarta, only LDHAL6B and LDH-D have a mitochondrial TargetP signal. While we know of no report showing MCT1 or MCT4 splice variants, our data show that splicing variants might explain the existence of LDH and MCT2 proteins in mitochondria (Fig. 1, E and B). In skeletal muscle (25) and neurons of some brain areas (26), we have found MCT1 to be expressed in both plasma and mitochondrial membranes. Our new result is that MCT1 is either not found at all in breast cancer cells (e.g., MDA-MB-231), or if found, MCT1 is localized mainly to the plasma membrane as in MCF-7 cells, with only trace amounts in the mitochondrial web. The unique localization of MCT1 mainly in plasma membrane may make it the main facilitator of lactate flow between tumor and stroma cells in breast tissue.
The three cell lines expressed MCT2 and MCT4, which were localized in the mitochondria (Figs. 1, 4, 5, and 6). The localizations of MCT1 mainly to plasma membrane, and MCT2 and MCT4 to plasma as well as mitochondrial membranes, is unique for breast cells. MCT2, which was first found in the liver, has a high affinity for substrates (Km of 0.1 mM for pyruvate, and Km of 0.7 mM for lactate), while MCT4, which is highly expressed in highly glycolytic cells, has a low affinity for substrates (Km of 150 mM for pyruvate, and Km of 28 mM for lactate) (24). It may be that MCT2 and MCT4 serve to import lactate and pyruvate into mitochondria for oxidation, whereas one or both transporters act to extrude substrate from mitochondria for use in other pathways. In this context, DeBerardinis et al. (14), who incubated glioblastoma cells with [1,6-13C2]glucose and [3-13C]glutamate, showed that entries of lactate and pyruvate into the TCA cycle were accompanied by export of labeled citrate, presumably to support fatty acid synthesis, and glutamine was used to replenish the TCA cycle compensating for citrate export. Such a pathway in breast cells may exist and therefore requires further investigation.
Contrary to our expectations, our data do not show changes in LDH or MCT isoform expression or intracellular distribution unique to cancer; rather, the changes we observed appear consistent with the glycolytic and oxidative capacities of cells (25). We discuss our findings within the context of targeting and killing cancer cells in vivo.
Changes in the expression of lactate shuttle proteins are associated with decreased oxidative capacity and an increased lactate accumulation within breast cancer cells.
The three breast cell lines we studied had different basal endogenous and maximum respiration rates (Fig. 7, D–F), with the control HMEC 184 cell line having the highest endogenous and maximum (uncoupler-stimulated) respiration rates, and with MCF-7 having a higher endogenous respiration than MDA-MB-231. Cells of all types responded to CCCP, indicating coupling of oxidative phosphorylation; however, respiration was less well-coupled in both cancer cell lines than the HMEC 184 control cell line (Fig. 7F). As expected from their high LDH expression levels, the two cancer cell lines accumulated more lactate and used less oxygen than did the control cell line HMEC 184. In contrast, the control cell line HMEC 184 had lower LDH and higher MCT1 expression levels than the cancer cell lines. Lactate accumulation in the two cancer cell lines is indicative of high lactate production, low oxidative capacity, or some combination of both factors. Lactate production was also significantly lower in media when cells were incubated with IAA or oxamate compared with control, but higher in cancer than normal cell lines (Fig. 7, A–C). This may mean that lactate production in all the cell lines was mainly generated through glycolysis but that there were downstream, i.e., mitochondrial, defects that disrupt oxidative disposal of lactate. Alternatively, pathways other than glycolysis contribute to high lactate production in cancerous cells lines. For example, enhanced glutamine metabolism has been associated with increased lactate production in cancerous cell lines (14). However, the mechanism of such an effect is undefined.
Our data may be useful in explaining some of the observations of Sonveaux et al. (53), who showed that cancer cells express different MCT isoforms and substrate use patterns depending on their localization in a tumor mass. Our result of a lower expression of MCT1 in breast cancerous cells compared with normal cells may be an early-programmed cancer strategy, allowing hypoxic tumor cells to utilize glucose without competing with normal cells. The MCT1 inhibitor, α-cyano-4-hydroxycinnamate, used by us previously to block sarcolemmal lactate transport (49, 50), was used by Sonveaux et al. (53) to reduce tumor volume. We suspect that by blocking MCT1 in normal control cells, Sonveaux et al. forced control cells peripheral to the tumor mass to become glycolytic and compete with cancer cells for glucose. We suspect that by virtue of their peripheral location close to the microcirculation, control cells out-competed transformed cancer cells in the tumor core. Our data suggest the possible hypothesis that the high amount of lactate produced by cancer cells acts as a signaling molecule that increases the expression of MCT1 and causes mitochondrial biogenesis in stroma cells surrounding tumors. As with endurance training of muscle, the increased MCT1 expression and oxidative capacity of stroma cells would encourage them to use lactate as their energy source and spare the glucose for the tumor cells. MCT4 expression in the tumor cells would be upregulated by hypoxia (55), which increases lactate transport from the cancer cells to the stroma cells. An increase of 10–15% in pyruvate and lactate uptake was seen in the cancer cell line MDA-MB-231 when transfected with plasmid containing MCT1 gene (22), and this uptake was accelerated at acidic pH and with an increase in lactate or pyruvate concentration in the incubation media (22). In this way, lactate contributes to the seed and soil hypothesis of Paget (44), by optimizing the microenvironment for tumor growth. That lactate acts as a paracrine signal in carcinogenesis was also proposed by others (29), and the idea is supported by the results of the experiments we now describe. We showed that incubation of rat muscle cell line L6 with lactate increased the expression of MCT1 and COx 4, and 100 genes involved in reactive oxygen species signaling and mitochondrial biogenesis (27). Lu et al. (36) showed that lactate and pyruvate stimulated the accumulation of HIF-1α and increased the expression of Aldo-A, VEGF, and GLUT-3 in many cancerous cell lines. Fukumura et al. (19) showed that VEGF expression increased with acidosis in brain tumor cells and that this increase was independent of hypoxia.
Our data and micro- and MassArray data from much larger sets of breast cell lines indicate that MCT1 expression is downregulated in breast cancer cells in general.
To expand our findings to a larger set of breast cancer cell lines, we examined the available microarray data in Charafe-Jauffret et al. (12). Charafe-Jauffret et al. used 31 breast cell lines, all of which were cancerous except three: MCF-10A, which is derived from a fibrocystic disease, and HME-1 and 184B5, which were derived from normal mammary tissue. We found that the MCT1 mRNA expression levels in cancer cell lines are lower than those of three control cell lines (184B5, HME-1, MCF-10A). This finding is consistent with our protein expression data, which compared the control primary breast cell line HMEC 184 to the two cancerous cell lines MDA-MB-231 and MCF-7 (Fig. 1H). One limitation of this comparison is that the three normal control breast cell lines used in microarray comparisons were basal-like breast cell lines, which may exaggerate this reduction, as MCT1 is identified by Charafe-Jauffret et al. (12) and others (46) as a basal-like breast cell line marker.
MCT4 also showed a trend of lower expression in most cancerous cell lines in Charafe-Jauffret et al., but there were some exceptions, such as the MDA-MB-231 cell line and five other cell lines that showed an increase in mRNA expression levels. This result was consistent with the MCT4 protein expression levels we observed in MDA-MB-231 (Fig. 1H) and in other studies (20). MCT4 expression was reported to be upregulated by hypoxia through HIF-1α (55), which may explain its increased expression in some cancer cells lines. MCT2 was not found in the Supplemental Table 1 of Charafe-Jauffret et al., but surprisingly MCT2 was one of the marker genes that were highly expressed in the mesenchymal cell line compared with luminal cells in their Supplemental Table 3. Our results show that in the luminal-like cell line MCF-7, MCT2 was highly expressed compared with HMEC 184 and MDA-MB-231 (Fig. 1H). In this respect our results are different from those reported by Charafe-Jauffret et al.
Our results with two cancerous cell lines showed differences in LDH isoform expression (Fig. 1H), with LDHA mainly expressed in MDA-MB-231 and LDHB mainly expressed in MCF-7 (Fig. 2). These results were inconsistent with those of Charafe-Jauffret et al. (12), who showed no significant (i.e., >2-fold) change in LDHA but instead observed a reduction in LDHB expression. Transcriptional and posttranscriptional regulation of LDHA mRNA has been previously reported (30, 52) and may explain the differences in LDHA expression observed by Charafe-Jauffret et al. and by us. In addition, Charafe-Jauffret et al. reported high expression of LDHB in the mesenchymal compared with luminal-like cell lines, which is inconsistent with our data. LDHA was proposed as a targeting therapy in many cancerous cells (33). However, the data of Charafe-Jauffret et al. show that lower expression of LDHB is more characteristic of cancer cells than is the increase in LDHA.
The MCT1 gene is silenced by hypermethylation in MDA-MB-231 cells as well as in 4 of 19 other breast cancer tissues (1). We asked the question whether this hypermethylation occurred in an early or a late stage in cancer development. Novak et al. (43) had exposed three primary breast cell lines (48RT, 184D, 240LD) to different treatments or genetic manipulations that allowed them to pass stasis and telomere dysfunction barriers to acquire immortality. By examining their MassArray data in their Supplemental Table 2, we found that MCT1 promoter hypermethylation appeared in an early stage of cancer development after passing the stasis barrier in two of seven manipulated cell lines (48RS, 184B), and MCT1 hypermethylation was clearer after cells passed the telomere dysfunction barrier in six of seven manipulated cell lines (184B5, 184AA2, 184A1-RF, 184B5ME, 184ZNMY3, 184ZNMY3-N). However, this early-stage hypermethylation was not associated with MCT1 silencing, as is seen in the 184B5 cell line in the data of Charafe-Jauffret et al. That result tells us that MCT1 silencing or reduction is an early programmed step in many breast cancer cells, and not due to random events.
The three cell lines used in this study along with those used by Charafe-Jauffret et al. (12), and Novak et al. (43), were grown in optimized culture media. However, those culture conditions may not reflect those in situ, so a concern is that the ex vivo conditions used in research may have affected the observed morphological and genetic changes. In this respect however, Wistuba et al. (58) compared the properties of 18 human breast cancer cell lines and their corresponding tumor tissue and showed a strong correlation between the two groups after 25 mo of culture (12).
MCT inhibition provides a promising treatment for cancer. Nevertheless, we caution that inhibition of either MCT or LDH isoforms needs further investigation and should be regarded with caution because all cells in the body express MCT and LDH isoforms. Importantly also, our data show that MCT and LDH isoforms are localized in mitochondrial fraction in both cancer and normal cells and that this localization is not changed in cancer. These results are consistent with our previous findings (25, 26), which showed that MCT and LDH exist in mitochondria of muscle and brain cells. Hence, inhibiting lactate shuttle proteins would effect the normal function of mitochondria of may cell types including neurons. As far as targeting lactate shuttle proteins as a means to disrupt tumor cells is concerned, we conclude that development of effective means to kill cancer cells by interfering with lactate shuttling in vivo will require better understanding of the unique roles of MCTs and other mLOC proteins in cancer.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-050459 and a gift from CytoSport, Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Kishorchandra Gohil and Xiying Fan for comments on our paper. We thank our undergraduate students Tina Xu and Michael Krugly for help with tissue culture work. We thank Dr. Gary Firestone for the gift of cancer cell lines and Drs. Martha Stampfer and James Garbe for advice on culturing primary-human breast cell lines. We thank Holly Aaron for assistance in microscopy.
Images were taken at the Molecular Imaging Center facility in the Cancer Research Laboratory at UC Berkeley.
REFERENCES
- 1. Asada K, Miyamoto K, Fukutomi T, Tsuda H, Yagi Y, Wakazono K, Oishi S, Fukui H, Sugimura T, Ushijima T. Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology 64: 380–388, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Baba N, Sharma HM. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol 51: 621–635, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayley JP, Devilee P. Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr Opin Genet Dev 20: 324–329, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278: E244–E251, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Brandt RB, Laux JE, Spainhour SE, Kline ES. Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys 259: 412–422, 1987. [DOI] [PubMed] [Google Scholar]
- 6. Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790: 1555–1568, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol 587: 5591–5600, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks GA. Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 32: 790–799, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Brooks GA. Lactate shuttles in nature. Biochem Soc Trans 30: 258–264, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci USA 96: 1129–1134, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cerutti PA. Prooxidant states and tumor promotion. Science 227: 375–381, 1985. [DOI] [PubMed] [Google Scholar]
- 12. Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, Bertucci F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene 25: 2273–2284, 2006. [DOI] [PubMed] [Google Scholar]
- 13. de Bari L, Valenti D, Atlante A, Passarella S. l-lactate generates hydrogen peroxide in purified rat liver mitochondria due to the putative l-lactate oxidase localized in the intermembrane space. FEBS Lett 584: 2285–2290, 2010. [DOI] [PubMed] [Google Scholar]
- 14. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104: 19345–19350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278: E571–E579, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Fang J, Quinones QJ, Holman TL, Morowitz MJ, Wang Q, Zhao H, Sivo F, Maris JM, Wahl ML. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol 70: 2108–2115, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 92: 329–333, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry 49: 835–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61: 6020–6024, 2001. [PubMed] [Google Scholar]
- 20. Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res 67: 4182–4189, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Garbe JC, Bhattacharya S, Merchant B, Bassett E, Swisshelm K, Feiler HS, Wyrobek AJ, Stampfer MR. Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells. Cancer Res 69: 7557–7568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76: 865–873, 1994. [DOI] [PubMed] [Google Scholar]
- 23. Gutmann I, Wahlefeld A. l-(+)-lactate determination with lactate dehydrogenase and NAD. In: Methods of Enzymatic Analysis. Edited by Bergmeyer HU. New York: Academic, 1974. [Google Scholar]
- 24. Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: 619–628, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab 290: E1237–E1244, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One 3: e2915, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21: 2602–2612, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol 567: 121–129, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 134: 703–707, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Jungmann RA, Kiryukhina O. Cyclic AMP and AKAP-mediated targeting of protein kinase A regulates lactate dehydrogenase subunit A mRNA stability. J Biol Chem 280: 25170–25177, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Kuhn KS, Muscaritoli M, Wischmeyer P, Stehle P. Glutamine as indispensable nutrient in oncology: experimental and clinical evidence. Eur J Nutr 49: 197–210, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat 83: 249–289, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107: 2037–2042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemire J, Mailloux RJ, Appanna VD. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1). PLoS One 3: e1550, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez-Lazaro M. Why do tumors metastasize? Cancer Biol Ther 6: 141–144, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 277: 23111–23115, 2002. [DOI] [PubMed] [Google Scholar]
- 37. McClelland GB, Khanna S, Gonzalez GF, Butz CE, Brooks GA. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun 304: 130–135, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta 1801: 381–391, 2010. [DOI] [PubMed] [Google Scholar]
- 39. Merezhinskaya N, Fishbein WN. Monocarboxylate transporters: past, present, future. Histol Histopathol 24: 243–264, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J 274: 1393–1418, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J 10: 311–321, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishikawa M, Hashida M. Inhibition of tumour metastasis by targeted delivery of antioxidant enzymes. Expert Opin Drug Deliv 3: 355–369, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Novak P, Jensen TJ, Garbe JC, Stampfer MR, Futscher BW. Stepwise DNA methylation changes are linked to escape from defined proliferation barriers and mammary epithelial cell immortalization. Cancer Res 69: 5251–5258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8: 98–101, 1989. [PubMed] [Google Scholar]
- 45. Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, Schmitt F, Baltazar F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 56: 860–867, 2010. [DOI] [PubMed] [Google Scholar]
- 47. Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183–189, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nutr Metab Care 9: 339–345, 2006. [DOI] [PubMed] [Google Scholar]
- 49. Roth DA, Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279: 386–394, 1990. [DOI] [PubMed] [Google Scholar]
- 50. Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279: 377–385, 1990. [DOI] [PubMed] [Google Scholar]
- 51. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 7: 7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Short S, Tian D, Short ML, Jungmann RA. Structural determinants for post-transcriptional stabilization of lactate dehydrogenase A mRNA by the protein kinase C signal pathway. J Biol Chem 275: 12963–12969, 2000. [DOI] [PubMed] [Google Scholar]
- 53. Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118: 3930–3942, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281: 9030–9037, 2006. [DOI] [PubMed] [Google Scholar]
- 56. Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60: 916–921, 2000. [PubMed] [Google Scholar]
- 57. Wimmer VC, Horstmann H, Groh A, Kuner T. Donut-like topology of synaptic vesicles with a central cluster of mitochondria wrapped into membrane protrusions: a novel structure-function module of the adult calyx of Held. J Neurosci 26: 109–116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wistuba II, Behrens C, Milchgrub S, Syed S, Ahmadian M, Virmani AK, Kurvari V, Cunningham TH, Ashfaq R, Minna JD, Gazdar AF. Comparison of features of human breast cancer cell lines and their corresponding tumors. Clin Cancer Res 4: 2931–2938, 1998. [PubMed] [Google Scholar]
- 59. Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93: 199–204, 2005. [DOI] [PubMed] [Google Scholar]