Abstract
Point mutations at m.8993T>C and m.8993T>G of the mtDNA ATPase 6 gene cause the neurogenic weakness, ataxia and retinitis pigmentosa (NARP) syndrome, a mitochondrial disorder characterized by retinal, central and peripheral neurodegeneration. We performed detailed neurological, neuropsychological and ophthalmological phenotyping of a mother and four daughters with NARP syndrome from the mtDNA m.8993T>C ATPase 6 mutation, including 3-T brain MRI, spectral domain optical coherence tomography (SD-OCT), adaptive optics scanning laser ophthalmoscopy (AOSLO), electromyography and nerve conduction studies (EMG-NCS) and formal neuropsychological testing. The degree of mutant heteroplasmy for the m.8993T>C mutation was evaluated by real-time allele refractory mutation system quantitative PCR of mtDNA from hair bulbs (ectoderm) and blood leukocytes (mesoderm). There were marked phenotypic differences between family members, even between individuals with the greatest degrees of ectodermal and mesodermal heteroplasmy. 3-T MRI revealed cerebellar atrophy and cystic and cavitary T2 hyperintensities in the basal ganglia. SD-OCT demonstrated similarly heterogeneous areas of neuronal and axonal loss in inner and outer retinal layers. AOSLO showed increased cone spacing due to photoreceptor loss. EMG-NCS revealed varying degrees of length-dependent sensorimotor axonal polyneuropathy. On formal neuropsychological testing, there were varying deficits in processing speed, visual–spatial functioning and verbal fluency and high rates of severe depression. Many of these cognitive deficits likely localize to cerebellar and/or basal ganglia dysfunction. High-resolution retinal and brain imaging in NARP syndrome revealed analogous patterns of tissue injury characterized by heterogeneous areas of neuronal loss.
Electronic supplementary material
The online version of this article (doi:10.1007/s00415-010-5775-1) contains supplementary material, which is available to authorized users.
Keywords: Mitochondrial disorders, Neuroophthalmology, Neuropsychology, Cerebellar disease, Neuropathy
Introduction
All nucleated cells in the human body contain mitochondria, the organelles responsible for intracellular energy production [1]. Each mitochondrion carries multiple copies of maternally inherited, circular, double-stranded mitochondrial DNA (mtDNA), which encodes for 2 rRNA, 22 tRNA, and 13 protein subunits essential for oxidative phosphorylation. Autosomal genes encode for all other mitochondrial proteins [1]. Mitochondrial DNA mutations are an important cause of neurodegeneration [2–4], and autosomally encoded mitochondrial proteins have also been implicated in a wide range of neurodegenerative conditions [5].
Point mutations at position 8993 of the mtDNA ATPase 6 gene cause neurogenic weakness, ataxia and retinitis pigmentosa (NARP) syndrome, a neurodegenerative disorder of the retina and central and peripheral nervous systems. The syndrome is named for its clinical manifestations: sensorimotor axonal polyneuropathy, ataxia, retinitis pigmentosa (RP), sensorineural hearing loss, seizures and cognitive impairment [6–10]. The same ATPase 6 point mutations that cause NARP syndrome also cause maternally inherited Leigh syndrome (MILS), a subacute necrotizing encephalomyelopathy that is a final common phenotype for a number of mutations associated with impaired energy production [11]. Since many patients with ATPase 6 mtDNA point mutations exhibit phenotypes that overlap with NARP and MILS, these previously discrete syndromes are probably best understood as part of a shared phenotypic spectrum [9].
Phenotypic variability is a cardinal feature of NARP and MILS [6, 9, 12, 13]. Mitochondrial genetic heteroplasmy, the ratio of mutant to wild-type mtDNA, helps to explain some of this variability, as greater degrees of mutant heteroplasmy tend to lead to more severe clinical deficits [1, 3]. In NARP/MILS, the risk of developing severe functional disability increases greatly past a threshold of >60–70% mutant blood heteroplasmy for the m.8993T>G ATPase 6 mutation and >80–90% blood heteroplasmy for the m.8993T>C mutation [12, 14]. Environmental, autosomal and tissue-specific factors may also modulate disease expression [15, 16].
To better characterize the range of disease expression in NARP syndrome from the m.8993T>C mutation, we performed detailed phenotyping of retinal, central and peripheral disease expression in five family members with NARP syndrome from the m.8993T>C mutation.
Methods
Subjects
We evaluated a mother (M1) and four daughters (D1–D4) with the m.8993T>C mtDNA ATPase 6 point mutation. The family was of Spanish and Colombian descent. M1 had one brother with coordination problems, four unaffected siblings, and a maternal aunt who had lost all four children in infancy from unknown causes. The father of D1–D4 suffered from bipolar disease.
All participants provided written informed consent. The Institutional Review Boards of UCSF and UC Berkeley approved the study protocol, and the study was performed in accordance with the Declaration of Helsinki.
Neurological evaluation
A study neurologist (A.G.) performed a comprehensive neurological examination of all five subjects. An electrophysiologist (L.G.) performed electromyography and nerve conduction studies (EMG-NCS) in three subjects.
Neuropsychological evaluations
All subjects underwent a brief interview regarding their cognitive function and approximately 2 h of formal neuropsychological testing using measures of global cognition, intellectual ability, verbal memory, visual-constructional skills and recall, working memory, processing speed, executive function, verbal fluency, language, reading, depression and anxiety (Electronic supplementary material). All results were converted to standard scores based on age-appropriate normative data; education-corrected normative data were also utilized when available.
3-T MRI evaluation
Four subjects had a research-quality brain MRI scan (Tim Trio 3-T MR scanner with a 12-channel head coil; Siemens Medical, Germany) which included T1 MPRAGE, T2 TSE, FLAIR, diffusion, ADC and proton-density sequences; the fifth subject (D3) had a clinical MRI scan at age 18 years, which was also analyzed. A study neuroradiologist interpreted the images.
Ophthalmological evaluation
All subjects underwent a comprehensive ophthalmological evaluation, including slit lamp and dilated fundus examination, best-corrected visual acuity using a standard eye chart, visual field testing, color vision testing, high-resolution spectral domain optical coherence tomography (SD-OCT, Spectralis® HRA + OCT system, Spectralis® 3.1 software; Heidelberg Engineering, Vista, CA) and full-field electroretinography (ERG), as reported previously [17]. Those with stable fixation also underwent multifocal ERG (mfERG, VERIS 5.1.10X; Electro-Diagnostic Imaging, Redwood City, CA) using a Burian-Allen contact lens electrode, following ISCEV standards as previously described [17]. Three subjects underwent adaptive optics scanning laser ophthalmoscopy (AOSLO) imaging with cone spacing analysis using customized equipment and software at the UC Berkeley School of Optometry [17–21].
Mitochondrial DNA point mutation analysis
Twenty different hair bulb samples (ectoderm) from three different scalp locations were obtained from each subject, and the DNA was extracted and amplified using PCR. Total DNA from blood leukocytes was also extracted from whole-blood samples (mesoderm) and amplified with PCR using specific primer pairs. The presence of the heteroplasmic m.8993T>C mutation was detected by allele-specific oligonucleotide dot blot analyses followed by quantification of the degree of heteroplasmy using real-time allele refractory mutation system (ARMS) quantitative PCR [22, 23]. The primers used for real-time ARMS-qPCR were: forward wild-type (ARMS T8993-1m) 5′-TACTCATTCAACCAATAGCCaT-3′, mutant type (ARMST8993C-1 m) 5′-TACTCATTCAACCAATAGCCaC-3′, and shared reverse primer (mtR9046) 5′-TTAGGTGCATGAGTAGGTGGC-3′. The analyses of the testing samples were repeated four times using the pooled DNA samples and quality control samples with known heteroplasmy content. The reaction was run in duplicate for each analysis. The intrarun and interrun differences were less than 20 and 30%, respectively. The final heteroplasmy rate was calculated by averaging the values obtained in duplicate runs [17].
Results
Table 1 summarizes the range of neurological, neuropsychological and ophthalmological disease expression in this family with NARP syndrome.
Table 1.
Phenotypic characterization of a family with NARP syndrome from the m.8993T>C ATPase 6 mutation
| Mother (Ml) | Daughter 1 (Dl) | Daughter 2 (D2) | Daughter 3 (D3) | Daughter 4 (D4) | |
|---|---|---|---|---|---|
| Age (years) | 48 | 28 | 26 | 22 | 16 |
| Hair-bulb heteroplasmy (%) | 87 | 99 | 54 | 99 | 99 |
| Leukocyte heteroplasmy (%) | 78 | 78 | 42 | 95 | 92 |
| Clinical symptoms | RP, mild numbness, weakness, depression | Severe RP, moderate ataxia, neuropathy, weakness, depression | Remote history of depression with suicidal ideation | Severe ataxia, moderate RP, remote depression | Severe RP, episodic mild ataxia, mild depression |
| Neurological examination | Mild asymmetric weakness, absent triceps and S1 reflexes, decreased pain and vibration sense in the feet | Lingual dysarthria, moderate proximal weakness, mildly decreased vibration sense in the feet, moderate dysmetria, impaired tandem (straight line) gait | Normal | Nystagmus, saccadic breakdown, dysarthria, moderate weakness, spasticity, absent S1 reflex, dysmetria, tremor, impaired tandem (straight line) gait, decreased pain and vibration sense in the feet | Overshot saccades, mild proximal weakness, moderate dysmetria and dysdiadochokinesia, decreased S1 reflexes, decreased pain and vibration sense in the feet |
| EMG-NCS | Sensorimotor axonal polyneuropathy | Not done | Not done | Sensorimotor axonal polyneuropathy | Sensorimotor axonal polyneuropathy |
| MRI | Cystic/cavitary T2 hyperintensities in the bilateral putamina and globus pallidi, mild cerebellar atrophy, mild global volume loss | Cystic/cavitary T2 hyperintensities in bilateral putamina, anterior commissure, frontal gyrus recti, cerebellar atrophy | Mild T2 cystic/cavitary changes in basal ganglia | (Performed at age 18 years) Diffuse atrophy of cortex, cerebellum and cervical cord, T2 cystic/cavitary basal ganglia hyperintensities | Cystic/cavitary T2 hyperintensities in the anterior putamina and caudate heads, mild cerebellar atrophy |
| Neuropsychological testing | MMSE 29/30; selective impairments in information processing speed, set-shifting, and visual-spatial skills | MMSE 27/27 (excluding visual tasks); selective mild deficits in verbal fluency | MMSE 29/30; grossly normal with the exception of slightly variable information processing speed | MMSE 27/30; relative sparing of verbal abilities with impairments in motor speed, information processing speed, visual-spatial skills and memory | MMSE 30/30; selective impairments in motor speed, information processing speed, verbal fluency, and visual recall (despite adequate copy performance) |
| Mood | Moderate depression with suicidal ideation | Severe depression with moderate anxiety, despite antidepressant therapy; remote history of suicide attempt | Remote history of depression with suicidal ideation | Remote history of depression | Mild depression with severe anxiety |
| Best-corrected visual acuity | 20/50 | 5/125 | 20/16 | 20/25 | 20/50 |
| High-resolution optical coherence tomography | Moderate–severe photoreceptor layer thinning, with mild involvement of retinal nerve fiber layer | Severe foveal thinning, including photoreceptor layer, retinal pigment epithelial layer and retinal nerve fiber layer/ganglion cell layer | Not done | Patchy ring of photoreceptor/retinal nerve fiber layer thinning |
Progressive thinning of the temporal photoreceptor/retinal nerve fiber layer |
RP retinitis pigmentosa, MMSE mini-mental state examination
Genotype–phenotype correlation
The four family members with mutant heteroplasmy greater than 78% in the blood and 87% in the hair bulbs suffered from sensorimotor axonal polyneuropathy and RP, and the three daughters with the greatest degree of mutant heteroplasmy (>78% in the blood and 99% in the hair bulbs) also had ataxia and cerebellar degeneration. Heteroplasmy rates were greater in pooled hair bulb samples than in blood. There was marked variability in the types of tissues affected within individuals. For example, one daughter with 99.9% hair bulb and 78% leukocyte heteroplasmy (D1) suffered from moderate ataxia and severe RP, while her sister with 99% hair bulb and 95% leukocyte heteroplasmy (D3) had severe ataxia but only moderate RP.
The age at time of first symptom ranged from ataxia at 13 months in subject D3 to visual impairment at 10, 12 and 34 years in D4, D1 and M1, respectively, which also correlated inversely with heteroplasmy.
Peripheral neurodegeneration
Four of the five subjects (all except D2) had evidence of peripheral neuropathy on clinical examination, most commonly characterized by large-fiber sensory deficits and absent S1 deep-tendon reflexes. Three subjects underwent EMG-NCS, which revealed decreased or absent sural sensory nerve action potential amplitudes, and long-duration, high-amplitude motor unit action potentials and reduced recruitment in the abductor hallucis longus. These findings are consistent with a length-dependent sensorimotor axonal polyneuropathy.
Cerebellar degeneration
The three subjects (D1, D3 and D4) with the greatest degrees of blood and hair bulb heteroplasmy suffered from ataxia, with varying combinations of dysmetria, dysdiadochokinesia, tremor, dysarthria, imbalance, saccadic overshoot, end-gaze jerk nystagmus, and impaired tandem gait. Truncal stability was preserved in all subjects. While all patients experienced chronic, progressive worsening of cerebellar symptoms over time, two of the three subjects (D3 and D4) also experienced additional, punctuated episodes of profound worsening of ataxia, which were associated temporally with adolescence, oral contraceptive pills and pregnancy.
3-T MRI: cerebellar and basal ganglia abnormalities
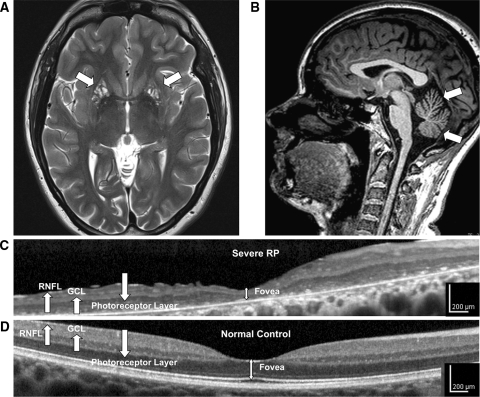
MRI of the brain was abnormal in all five family members, but the severest abnormalities occurred in those with the greatest degrees of mutant heteroplasmy (Table 1; Fig. 1a). The lesions involved the bilateral putamen in all subjects and the anterior commissure, frontal gyrus recti and caudate heads in the most affected subjects. All three daughters with ataxia (D1, D3 and D4) showed cerebellar atrophy on MRI scans (Fig. 1b), and the degree of atrophy correlated with the severity of the clinical deficit. D3 had diffuse cortical, corpus callosal, pontine and cervical cord atrophy, and M1, the oldest subject, also had mild global brain atrophy.
Fig. 1.
High-resolution retinal and brain imaging in NARP syndrome demonstrates analogous patterns of tissue injury. This 28-year-old woman (D1) with NARP syndrome from the mtDNA ATPase 6 m.8993T>C mutation with 78% blood leukocyte and 99% hair-bulb heteroplasmy had severe RP and moderate ataxia. a, b 3-T MRI demonstrates cystic and cavitary T2 hyperintensities in the bilateral putamina (a), likely reflecting neuronal necrosis, and also moderate cerebellar atrophy with T1 imaging (b). c High-resolution OCT image of the macula demonstrates severe retinal thinning, primarily due to degeneration of the photoreceptor and the retinal pigment epithelial cell layers, but also associated thinning of the ganglion cell (GCL) and retinal nerve fiber layer (RNFL). d Macular OCT image from an age-similar normal female shown for comparison
Neuropsychological profiles
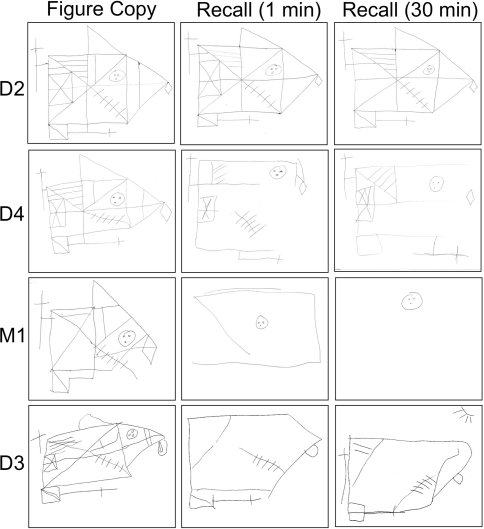
The most consistent cognitive deficits in this family were impairments in processing speed, visual-spatial copy and recall and verbal fluency (see Table 1; Electronic Supplementary Material). D1, who had severe visual deficits from RP, had mild verbal fluency impairment. M1 and D4 had significant deficits in information processing speed and visual-spatial functioning (see Fig. 2). D3, who suffered the severest cerebellar injury, had deficits in processing speed, motor speed, memory and visual-spatial functioning. D2, who was unaffected clinically, tested normally in all cognitive domains. The mini-mental state examination was an insensitive screen for cognitive deficits in this family, as it was near normal in all subjects (range 27/30–30/30).
Fig. 2.
Visual-spatial deficits in NARP syndrome reflect impaired cognitive functioning, not just poor vision. Subject D4, despite moderate RP causing impaired vision, had normal visual-spatial constructional skills using the Rey-Osterrieth complex figure, but exhibited deficits in visual-spatial recall. On the other hand, M1 and D3, who both also suffered from moderate to severe RP, had difficulty with both visual copy and visual recall (D1 could not perform either of the tasks due to severe visual loss). D2, who was clinically unaffected, performed normally on both tasks
Affective symptoms were prominent in this family, with all five family members having a significant history of depression, many with suicidal ideation. D3 subsequently developed severe psychosis and depression requiring psychiatric hospitalization.
Retinal neurodegeneration
Four of the five subjects demonstrated varying degrees of RP. The best-corrected visual acuity (BCVA) ranged from 20/25 in mild RP (D3) to 5/125 in severe RP (D1). Retinal vascular attenuation was present in all affected subjects, while bone-spicule pigmentary changes and retinal pigment epithelium atrophy were observed in those with severe RP. Full-field ERG demonstrated varying degrees of decreased rod-mediated, cone-mediated and mixed rod- and cone-mediated amplitudes, while multifocal ERG revealed that the macular photoreceptors had the greatest functional impairment, with relative sparing of more peripheral photoreceptors. SD-OCT (Figs. 1 and 3) revealed macular thinning, especially of the photoreceptor (RPE) layer, but also thinning of the retinal nerve fiber and ganglion cell layers in proportion to the severity of RP. AOSLO in the four subjects capable of stable fixation revealed increased cone spacing with variable degrees of patchy cone loss and abnormally enlarged foveal cones (Fig. 3d). There was normal cone spacing on AOSLO in subject D2, who had no visual symptoms (Fig. 3e) [17].
Fig. 3.
Patterns of retinal injury in NARP syndrome. Infrared fundus image (a) and OCT of the macula (a, b) in a 22-year-old woman with NARP syndrome (D3) demonstrate moderate thinning of the photoreceptor layer, as well as associated thinning of the retinal pigment epithelial, retinal nerve fiber and ganglion cell layers. c Macular OCT image of an age-similar normal female shown for comparison. d AOSLO image of the fovea (black dot) reveals a contiguous pattern of decreased cone density in the sampled area due to photoreceptor loss (which makes it easier to resolve the individual photoreceptors in the image). e In contrast, AOSLO image of the fovea in her sister (D2), who had normal vision, shows a normal pattern of high-density, closely packed cones (black scale bars angular distance 0.5°, approximately 150 µm)
Discussion
We describe the range of retinal, peripheral and central nervous system disease expression in a single family with NARP syndrome from the ATPase 6 m.8993T>C mtDNA point mutation. Even amongst family members with the greatest degrees of ectodermal and mesodermal heteroplasmy, there was great variability in tissue types affected and severity of injury within those tissues.
All subjects in our series had greater degrees of hair-bulb heteroplasmy than blood leukocyte heteroplasmy, but the overall trends were similar. Family members with the greatest degrees of hair-bulb and leukocyte heteroplasmy suffered the severest neurological and ophthalmological deficits, but neither hair-bulb nor leukocyte heteroplasmy uniformly predicted which tissues would be affected in a given individual or the severity of deficits within a given tissue (Table 1).
There were analogous patterns of tissue injury on high-resolution retinal and brain imaging characterized by heterogeneous, patchy areas of neuronal loss (Figs. 1c, d, and 3d, e). With 3-T MRI, we were able to appreciate that the basal ganglia hyperintensities typical of NARP-MILS spectrum disease [12, 13, 24] are not homogeneous, but rather consist of multiple, small cystic and cavitary lesions. These basal ganglia hyperintensities likely reflect discrete foci of tissue loss from mitochondria-related energy failure [13]. Since basal ganglia necrosis is also a defining feature of MILS, the prominence of this finding in our study reinforces the concept that NARP and MILS reflect overlapping phenotypes of ATPase 6 dysfunction [3, 6, 9, 12, 13].
We observed similar patterns of heterogeneous, focal and destructive injury to neuronal layers of the inner and outer retina. On SD-OCT, the most prominent abnormality was thinning of the photoreceptor (RPE) layer with scattered areas of severe neuronal loss causing a disruption to normal retinal architecture. There was also associated thinning of the retinal nerve fiber and ganglion cell layers in proportion to the degree of RPE injury. Since RNFL thinning is common in other types of retinitis pigmentosa [25] and the severest injury was to the outer retina, we suspect that the primary retinal injury in NARP syndrome is to energy-dependent photoreceptors followed by secondary transsynaptic degeneration of neighboring retinal layers [26]. The abnormalities observed on SD-OCT and AOSLO are also consistent with findings on retinal pathology from a child who died from MILS from the m.8993T>G mutation [27], and illustrate how high-resolution retinal imaging can detect neurodegenerative injury in a mitochondrial disorder.
Our study demonstrates that cognitive impairment in NARP-MILS is characterized by selective dysfunction of specific functional domains, especially in information processing speed, visual-spatial copy and memory and verbal fluency. The visual-spatial deficits were independent of the degree of primary visual loss from RP (see Fig. 2). Visual-spatial and executive function impairment are characteristic of other mitochondrial cytopathies, such as chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome, and the localization of these deficits has been attributed to the prefrontal, parietal and occipital cortex [28, 29]. However, cerebellar dysfunction also causes deficits in executive functioning, verbal fluency, memory and visual-spatial processing [30], and striatal networks also modulate many cognitive processes, including language, and executive and visual-spatial functioning [31, 32]. Given the extensive cerebellar and basal ganglia injury seen in NARP syndrome with relative sparing of cortical structures, we propose that some of the cognitive deficits characteristic of NARP-MILS spectrum disease may be due to cerebellar and basal ganglia dysfunction. Our results also suggest that the mini-mental state examination is not an adequate screening test for cognitive dysfunction in NARP syndrome, and that clinicians should pursue more sensitive evaluations focused on information processing speed, verbal fluency and visual-spatial function.
Two main factors argue against depression confounding the neuropsychological testing results [33]. First, all subjects showed similar patterns of deficits, despite variability in affective symptoms. Second, verbal and working memory tend to be impaired when depression causes cognitive deficits [34], but these domains were not significantly affected in this family, even in those subjects with the severest depression. Depression has not traditionally been recognized as part of the NARP-MILS phenotypic spectrum, but the prominence of affective symptoms in this family raises the question as to whether mitochondrial dysfunction may be involved in its pathogenesis [35]. Depression is common in mitochondrial cytopathies [36, 37], and is also prominent in other neurodegenerative disorders that affect striatal networks, including Huntington’s and Parkinson’s diseases [38, 39]. In our analysis, however, we were not able to account for other factors that might have influenced depression risk in this family, including the history of bipolar disease in the father of D1–D4 or shared environmental exposures. More research is needed to explore this possible association between depression and mitochondrial disease further.
There are several limitations to this study. We characterized disease expression in a single family, which risks overemphasizing shared autosomal or environmental factors that can influence mitochondrial disease expression or confound phenotypes. It is also difficult in this kind of analysis to account for the effects of age on mitochondrial disease progression. We also did not measure heteroplasmy using the urinary epithelium, which has been recently reported to predict neurological involvement in MELAS [40]. Nevertheless, we believe that this study adds to our understanding of the range of phenotypic expression seen in this prototypical mitochondrial neurodegenerative disorder.
In summary, this study characterized patterns of disease expression in NARP syndrome from the m.8993T>C ATPase 6 mtDNA mutation and illustrated how neurodegeneration in the retina, brain and peripheral nervous system can share common mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We acknowledge the support of the following organizations: American Academy of Neurology Foundation/National MS Society Early Clinician Scientist Award (A.J.G.); NIH-NCRR KL2RR024130 (A.J.G., L.A.G.); Career Development Award, Physician Scientist Award and Unrestricted Grant from the Research to Prevent Blindness (J.L.D.); Career Development Award and Clinical Center Grant from the Foundation Fighting Blindness (J.L.D., A.R.); NIH-NEI grants EY002162 (J.L.D.) and EY014375 (A.R.); That Man May See, Inc. (J.L.D.); The Bernard A. Newcomb Macular Degeneration Fund (J.L.D.); Hope for Vision (J.L.D.); the Karl Kirchgessner Foundation (J.L.D.); and NSF Science and Technology Center for Adaptive Optics, managed by the University of California at Santa Cruz under cooperative agreement #AST-9876783 (A.R.).
Conflict of interest
Dr. Roorda reports that he holds a patent for the adaptive optics scanning laser ophthalmoscope used in this study. The other authors have no disclosures to report.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6(5):389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63(1):35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 3.Thorburn DR, Dahl HH. Mitochondrial disorders: genetics, counseling, prenatal diagnosis and reproductive options. Am J Med Genet. 2001;106(1):102–114. doi: 10.1002/ajmg.1380. [DOI] [PubMed] [Google Scholar]
- 4.Abramov AY, Smulders-Srinivasan TK, Kirby DM, Acin-Perez R, Enriquez JA, Lightowlers RN, Duchen MR, Turnbull DM. Mechanism of neurodegeneration of neurons with mitochondrial DNA mutations. Brain. 2010;133(Pt 3):797–807. doi: 10.1093/brain/awq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 6.Santorelli FM, Tanji K, Shanske S, DiMauro S. Heterogeneous clinical presentation of the mtDNA NARP/t8993G mutation. Neurology. 1997;49(1):270–273. doi: 10.1212/wnl.49.1.270. [DOI] [PubMed] [Google Scholar]
- 7.Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- 8.Morava E, Rodenburg RJ, Hol F, de Vries M, Janssen A, van den Heuvel L, Nijtmans L, Smeitink J. Clinical and biochemical characteristics in patients with a high mutant load of the mitochondrial t8993G/C mutations. Am J Med Genet A. 2006;140(8):863–868. doi: 10.1002/ajmg.a.31194. [DOI] [PubMed] [Google Scholar]
- 9.Sciacco M, Prelle A, D’Adda E, Lamperti C, Bordoni A, Rango M, Crimi M, Comi GP, Bresolin N, Moggio M. Familial mtDNA T8993C transition causing both the NARP and the MILS phenotype in the same generation. A morphological, genetic and spectroscopic study. J Neurol. 2003;250(12):1498–1500. doi: 10.1007/s00415-003-0246-6. [DOI] [PubMed] [Google Scholar]
- 10.Keranen T, Kuusisto H. NARP syndrome and adult-onset generalised seizures. Epileptic Disord. 2006;8(3):200–203. [PubMed] [Google Scholar]
- 11.Santorelli FM, Shanske S, Macaya A, DeVivo DC, DiMauro S. The mutation at nt 8993 of mitochondrial DNA is a common cause of Leigh’s syndrome. Ann Neurol. 1993;34(6):827–834. doi: 10.1002/ana.410340612. [DOI] [PubMed] [Google Scholar]
- 12.Uziel G, Moroni I, Lamantea E, Fratta GM, Ciceri E, Carrara F, Zeviani M. Mitochondrial disease associated with the T8993G mutation of the mitochondrial ATPase 6 gene: a clinical, biochemical, and molecular study in six families. J Neurol Neurosurg Psychiatry. 1997;63(1):16–22. doi: 10.1136/jnnp.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojo A, Campos Y, Sanchez JM, Bonaventura I, Aguilar M, Garcia A, Gonzalez L, Rey MJ, Arenas J, Olive M, Ferrer I. NARP-MILS syndrome caused by 8993 T>G mitochondrial DNA mutation: a clinical, genetic and neuropathological study. Acta Neuropathol. 2006;111(6):610–616. doi: 10.1007/s00401-006-0040-5. [DOI] [PubMed] [Google Scholar]
- 14.White SL, Collins VR, Wolfe R, Cleary MA, Shanske S, DiMauro S, Dahl HH, Thorburn DR. Genetic counseling, prenatal diagnosis for the mitochondrial DNA mutations at nucleotide 8993. Am J Hum Genet. 1999;65(2):474–482. doi: 10.1086/302488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenuth JP, Peterson AC, Shoubridge EA. Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat Genet. 1997;16(1):93–95. doi: 10.1038/ng0597-93. [DOI] [PubMed] [Google Scholar]
- 16.Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, Klopstock T, Chinnery PF. Gene-environment interactions in Leber hereditary optic neuropathy. Brain. 2009;132(Pt 9):2317–2326. doi: 10.1093/brain/awp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon MK, Roorda A, Zhang Y, Nakanishi C, Wong LJ, Zhang Q, Gillum L, Green A, Duncan JL. Adaptive optics scanning laser ophthalmoscopy images in a family with the mitochondrial DNA T8993C mutation. Invest Ophthalmol Vis Sci. 2009;50(4):1838–1847. doi: 10.1167/iovs.08-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14(11):2884–2892. doi: 10.1364/JOSAA.14.002884. [DOI] [PubMed] [Google Scholar]
- 19.Roorda A, Romero-Borja F, Donnelly W, III, Queener H, Hebert T, Campbell M. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10(9):405–412. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Poonja S, Roorda A. MEMS-based adaptive optics scanning laser ophthalmoscopy. Opt Lett. 2006;31(9):1268–1270. doi: 10.1364/OL.31.001268. [DOI] [PubMed] [Google Scholar]
- 21.Duncan JL, Zhang Y, Gandhi J, Nakanishi C, Othman M, Branham KE, Swaroop A, Roorda A. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3283–3291. doi: 10.1167/iovs.06-1422. [DOI] [PubMed] [Google Scholar]
- 22.Wong LJ, Lam CW. Alternative, noninvasive tissues for quantitative screening of mutant mitochondrial DNA. Clin Chem. 1997;43(7):1241–1243. [PubMed] [Google Scholar]
- 23.Wong LJ, Bai RK. Real-time quantitative polymerase chain reaction analysis of mitochondrial DNA point mutation. Methods Mol Biol. 2006;335:187–200. doi: 10.1385/1-59745-069-3:187. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Wada T, Sakai T, Ishikawa Y, Minami R, Tachi N, Saitoh S. Phenotypic variability in a family with a mitochondrial DNA T8993C mutation. Pediatr Neurol. 1998;19(4):283–286. doi: 10.1016/S0887-8994(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 25.Walia S, Fishman GA, Edward DP, Lindeman M. Retinal nerve fiber layer defects in RP patients. Invest Ophthalmol Vis Sci. 2007;48(10):4748–4752. doi: 10.1167/iovs.07-0404. [DOI] [PubMed] [Google Scholar]
- 26.Vanburen JM. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry. 1963;26:402–409. doi: 10.1136/jnnp.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi N, Geraghty MT, Green WR. Ocular histopathologic study of a patient with the T 8993-G point mutation in Leigh’s syndrome. Ophthalmology. 2000;107(7):1397–1402. doi: 10.1016/S0161-6420(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 28.Bosbach S, Kornblum C, Schroder R, Wagner M. Executive and visuospatial deficits in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Brain. 2003;126(Pt 5):1231–1240. doi: 10.1093/brain/awg101. [DOI] [PubMed] [Google Scholar]
- 29.Finsterer J. Mitochondrial disorders, cognitive impairment and dementia. J Neurol Sci. 2009;283(1–2):143–148. doi: 10.1016/j.jns.2009.02.347. [DOI] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 31.De Diego-Balaguer R, Couette M, Dolbeau G, Durr A, Youssov K, Bachoud-Levi AC. Striatal degeneration impairs language learning: evidence from Huntington’s disease. Brain. 2008;131(Pt 11):2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence AD, Watkins LH, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington’s disease: Implications for information processing in corticostriatal circuits. Brain. 2000;123(Pt 7):1349–1364. doi: 10.1093/brain/123.7.1349. [DOI] [PubMed] [Google Scholar]
- 33.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1–2):1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18(2):217–225. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- 35.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, Kato T (2006) Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry 11 (6):577–593, 523 [DOI] [PubMed]
- 36.Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007;12(6):429–438. doi: 10.1017/s1092852900015303. [DOI] [PubMed] [Google Scholar]
- 37.Koene S, Kozicz TL, Rodenburg RJ, Verhaak CM, de Vries MC, Wortmann S, van de Heuvel L, Smeitink JA, Morava E. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord. 2008;114(1–3):327–332. doi: 10.1016/j.jad.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblatt A, Leroi I. Neuropsychiatry of Huntington’s disease and other basal ganglia disorders. Psychosomatics. 2000;41(1):24–30. doi: 10.1016/S0033-3182(00)71170-4. [DOI] [PubMed] [Google Scholar]
- 39.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24(15):2175–2186. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittaker RG, Blackwood JK, Alston CL, Blakely EL, Elson JL, McFarland R, Chinnery PF, Turnbull DM, Taylor RW. Urine heteroplasmy is the best predictor of clinical outcome in the m3243A>G mtDNA mutation. Neurology. 2009;72(6):568–569. doi: 10.1212/01.wnl.0000342121.91336.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.