SUMMARY
Our previous studies show that stimulation of adenylyl cyclase in preglomerular vascular smooth muscle cells (PGVSMCs) increases extracellular 3’,5’-cAMP; however, the mechanism by which PGVSMCs transport intracellular 3’,5’-cAMP into the extracellular milieu is unknown.
We hypothesize that multidrug resistance protein 4 (MRP4) is the primary transporter mediating efflux of intracellular 3’,5’-cAMP from PGVSMCs.
Both RT-PCR and real-time PCR detected MRP4 mRNA in PGVSMCs in culture. Moreover, Western blotting using an antibody specific for MRP4 gave rise to a 150 kDa signal consistent with the presence of MRP4 protein in PGVSMCs.
Specifically-designed siRNA reduced MRP4 mRNA expression by 71% (p=0.0075) and MRP4 protein by 80% (p=0.0004).
Isoproterenol (1 μmol/L) increased intracellular 3’,5’-cAMP, which resulted in efflux of 3’,5’-cAMP into the medium. siRNA knockdown of MRP4 significantly reduced basal extracellular 3’,5’-cAMP and nearly abolished isoproterenol-induced increases in extracellular 3’,5’-cAMP (p=0.0143, interaction between isoproterenol and MRP4 siRNA in 2-factor analysis of variance). In isoproterenol-treated cells, MRP4 siRNA decreased the ratio of extracellular 3’,5’-cAMP to intracellular 3’,5’-cAMP by 72% (p=0.0019).
We conclude that MRP4 is the dominant 3’,5’-cAMP transporter in PGVSMCs.
INTRODUCTION
Our previous work supports the existence of a biochemical mechanism that contributes to extracellular levels of adenosine, i.e., the extracellular cAMP-adenosine pathway (1-3), which entails: 1) receptor-induced intracellular production of 3’,5’-cAMP (cAMP); 2) transport of cAMP to the cell surface; 3) extracellular metabolism of cAMP to 5’-AMP; and 4) extracellular conversion of 5’-AMP to adenosine. Independent laboratories confirm the extracellular cAMP-adenosine pathway in pial microvessels (4), skeletal muscle (5) and the gut (6).
Adenylyl cyclase catalyzes the biosynthesis of cAMP, and receptor-mediated stimulation of adenylyl cylcase activates the extracellular cAMP-adenosine pathway (7). CD73 is an ecto-5’-nucleotidase (8), and inhibition of CD73 attenuates the extracellular cAMP-adenosine pathway (9-15). Therefore, steps 1 and 4 of the extracellular cAMP-adenosine pathway are mediated by adenylyl cyclase and CD73, respectively. However, the molecular identities of mediators of step 2 (the cAMP transporter(s)) and step 3 (the enzyme(s) that convert cAMP to AMP, i.e., the ecto-phosphodiesterase(s)) remain unclear.
With regard to the identity of the cAMP transporter(s), as reviewed by Dean et al. (16) and Deeley et al. (17), over-expression studies of multidrug resistance protein 4 (MRP4), a member of the ATP-binding cassette transporter superfamily (17-19), indicate that MRP4 can transport cAMP. Moreover, studies by Li et al. demonstrate that MK571, an inhibitor of MRP4, decreases cAMP transport in gut epithelial HT29-CL19A cells and raises intracellular cAMP levels in gut epithelial T84 cells (20). Therefore, it is conceivable that MRP4 participates in step 2 of the extracellular cAMP-adenosine pathway. Accordingly, we hypothesized that MRP4 mediates in part transport of endogenous cAMP following receptor-induced stimulation of adenylyl cyclase, and the goal of the present study was to test this hypothesis. We chose as our model system cultured rat preglomerular vascular smooth muscle cells (PGVSMCs) because our previous studies in intact rat kidneys, isolated renal microvessels and PGVSMCs demonstrated the existence of an extracellular cAMP-adenosine pathway in the rat renal microcirculation (9, 11, 21, 22).
METHODS
Culture of PGVSMCs
PGVSMCs were cultured by explant from preglomerular microvessels obtained from adult rats as previously described (22).
RT-PCR
RNA was isolated (TRIZOL Reagent; Invitrogen, Carlsbad, CA), reverse transcribed and amplified (Titanium One-step RT-PCR kit; Clontech, Mountain View, CA; forward primer, ggatcctcatacccctggtt; reverse primer, tgcatcaaacagctcctgac; 200 base-pair (bp) amplification product). PCR cycles (total 30) consisted of denaturing (94°C, 30 seconds), annealing (65°C, 30 seconds), and extension (68°C, 60 seconds). RT-PCR products were separated on a 1.5 % agarose gel and visualized with ethidium bromide.
Real-Time PCR
RNA was isolated as described above. cDNA was synthesized using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). MRP4 primers were as described for RT-PCR. β-actin primers were: forward, actcttccagccttccttc; reverse, atctccttctgcatcctgtc; 171 bp amplification product. Real-time PCR was performed (SYBR Green PCR Master Mix; Applied Biosystems, Foster City, CA) in the AB 7300 Real-Time PCR System. Threshold cycle (Ct) for β-actin was subtracted from Ct for target to calculate 2ΔCt.
Western Blotting
Protein was extracted (Mammalian Protein Extraction Reagent; Pierce Biotechnology Inc., Rockford, IL), measured (BCA assay; Pierce), and boiled (5 minutes in Laemmli buffer). SDS-polyacrylamide-gel electrophoresis was performed on polyacrylamide gels (8-16%) with 40 μg of protein per lane. Proteins were transferred to polyvinylidene difluoride membranes. Membranes were blocked in TBST containing 5% milk and probed with mouse MRP4 primary antibody (1:500; Novus Biologicals Littleton, CO; expected size of signal, 150 kDa). Membranes were exposed to horseradish-peroxidase-conjugated-goat anti-mouse antibody (1:4000; Pierce) and visualized with X-ray film using luminal-based enhanced chemiluminescence substrate (Supersignal West Dura Extended Duration Substrate; Pierce).
MRP4 siRNA Knockdown
PGVSMCs were trypsinized (0.25%) and diluted in growth medium to 100,000 cells/ml. 1.5 μl of siPORT NeoFX (Ambion, Austin, TX) was diluted to 25 μl in Opti-MEM I medium and incubated for 10 minutes at room temperature. 3 μl of 5 μmol/L of MRP4 siRNA (Sense: CCGUGAGGAGCAAUAUUUUtt; Antisense: AAAAUAUUGCUCCUCACGGtt) or the negative control siRNA (provided by Ambion) was diluted to 25 μl in OPTI-MEM I medium to give a 30 nmol/L concentration in the transfection reaction. Diluted siPORT NeoFX and siRNA were combined, incubated for 10 minutes at room temperature, dispensed into culture wells (24-well plate), overlaid with cell suspensions (45,000 cells) and incubated at 37°C. 72 hours after transfection, RNA and protein were obtained and analyzed as described above.
Effects of MRP4 Knockdown on cAMP Induced by Isoproterenol
PGVSMCs were treated with MRP4 siRNA or negative control siRNA as described above. After 72 hours, cells were washed twice (1 ml of PBS), and incubated for 1 hour in 0.5 ml PBS containing either no addition or isoproterenol (1 μmol/L). The medium was collected for analysis of extracellular cAMP. Intracellular cAMP was extracted from cells with 1 ml of 1-propanol (30 minutes at 4°C).
Measurement of cAMP
1-Propanol samples were evaporated and resuspended in mobile phase, whereas samples in PBS were assayed directly. cAMP was measured using liquid chromatography-tandem mass spectrometry with a TSQ Quantum Ultra mass spectrometer (Thermo Electron Corporation, Waltham, MA) as described (23).
Statistics
Multiple groups were compared by 2-factor analysis of variance followed by the Fisher's LSD test, and the unpaired Student's t test was used for single comparisons.
RESULTS
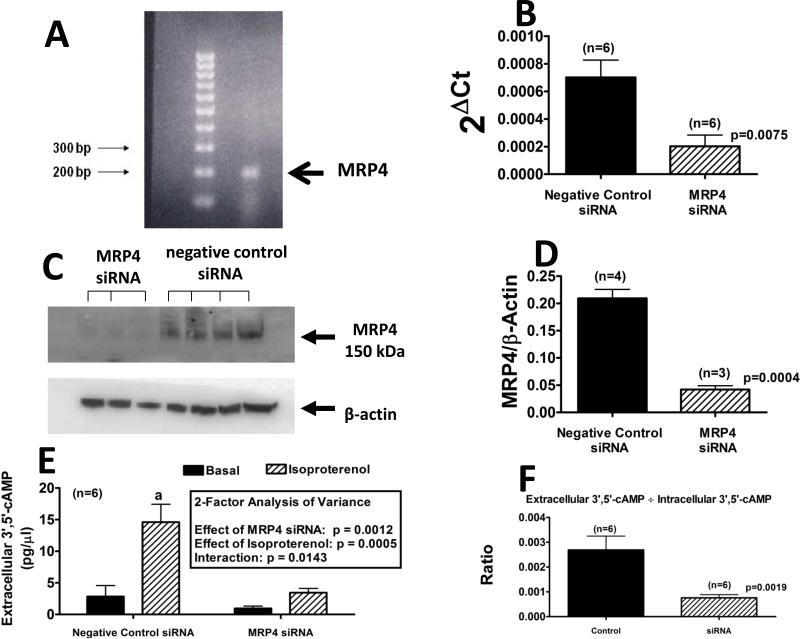
RT-PCR detected the expected 200 bp MRP4 mRNA amplification product (Figure 1A). MRP4 mRNA was also detected by real-time PCR (Figure 1B), and MRP4 siRNA diminished this signal by 71% (p=0.0075; Figure 1B). Western blotting detected MRP protein in untreated cells (data not shown) as well as in cells treated with negative control siRNA (Figure 1C). MRP4 siRNA reduced MRP protein expression by 80% (Figure 1C/D). In cells treated with negative control siRNA, isoproterenol caused a 5-fold increase in the concentration of extracellular cAMP (Figure 1E). Treatment of PGVSMCs with MRP4 siRNA decreased basal levels of extracellular cAMP and nearly abolished isoproterenol-induced increases in extracellular concentrations of cAMP. Two-factor analysis of variance demonstrated a significant effect of MRP4 siRNA on extracellular cAMP (p = 0.0012) and of isoproterenol on extracellular cAMP (p = 0.0005). Also, a significant interaction between MRP4 siRNA and isoproterenol (p = 0.0142) on extracellular cAMP was observed. MRP4 siRNA reduced the extracellular-to-intracellular ratio of cAMP in isoproterenol-treated cells by 72% (p=0.0019; Figure 1F).
Figure 1.
Detection in PGVSMCs of MRP4 mRNA by RT-PCR (Panel A) and real time PCR (Panel B) and of MRP4 protein by Western blotting (Panel C); Knockdown of MRP4 mRNA (Panel B) and MRP4 protein (Panels C and D); Extracellular cAMP levels in culture medium from PGVSMCs pretreated with negative control siRNA or with MRP4 siRNA and stimulated or not with isoproterenol (1 μmol/L; Panel E); Ratio of extracellular to intracellular cAMP in PGVSMCs pretreated with negative control siRNA or with MRP4 siRNA and stimulated with isoproterenol (Panel F). Values represent mean ± SEM for the indicated number of experiments; aindicates significantly different from all other groups.
DISCUSSION
Our results demonstrated strong expression of MRP4 message and protein in PGVSMCs. Using MRP4 siRNA we achieved a meaningful knockdown of MRP4 expression (both message and protein) in PGVSMCs. Therefore, we investigated the ability of MRP4 siRNA to modify the increase in extracellular cAMP in response to isoproterenol in PGVSMCs. We selected isoproterenol as the stimulus because this drug is an agonist for β-adrenoceptors, and β-adrenoceptors are positively coupled to adenylyl cyclase. Moreover, in previous studies, we observed that isoproterenol robustly increased cAMP levels in PGVSMCs (24, 25). In the present study, isoproterenol caused a large increase in extracellular levels of cAMP, and this increase was strongly suppressed, indeed nearly totally inhibited, by MRP4 siRNA. In addition, MRP4 siRNA significantly inhibited basal levels of extracellular cAMP. These findings confirm an important role for MRP4 as a cAMP transporter in PGVSMCs, and suggest that MRP4 is the dominant cAMP transporter in the extracellular cAMP-adenosine pathway, at least in PGVSMCs. However, it is possible that the relative contribution of MRP4 versus other transport proteins, such as MRP5, to the cAMP-adenosine pathway may vary depending on the cell type.
Adenosine is an important autocrine and paracrine hormone that via specific extracellular receptors plays an important role in regulating renal function. For example, extracellular adenosine inhibits renin release, attenuates proliferation of renal mesangial cells, reduces glomerular filtration rate, mediates tubuloglomerular feedback, stimulates sodium reabsorption in the proximal tubule, increases renal medullary blood flow and protects against renal ischemia/reperfusion injury (2, 26). Extracellular adenosine also regulates many physiological processes in organ systems other than the kidney, for example the heart (27), brain (28) and immune system (29). The physiological significance of the present finding is that because MRP4 transports intracellular cAMP to the extracellular compartment and because extracellular cAMP can be converted to adenosine, MRP4 may importantly regulate organ function by participating in the production of extracellular adenosine.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institutes of Health (HL069846 and DK068575).
REFERENCES
- 1.Jackson EK. Adenosine: a physiological brake on renin release. Annual Review of Pharmacology & Toxicology. 1991;31:1–35. doi: 10.1146/annurev.pa.31.040191.000245. [DOI] [PubMed] [Google Scholar]
- 2.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annual Review of Physiology. 2004;66:571–99. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- 3.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. American Journal of Physiology - Renal Physiology. 2001;281:F597–612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- 4.Hong KW, Shin HK, Kim HH, Choi JM, Rhim BY, Lee WS. Metabolism of cAMP to adenosine: role in vasodilation of rat pial artery in response to hypotension. American Journal of Physiology. 1999;276:H376–82. doi: 10.1152/ajpheart.1999.276.2.H376. [DOI] [PubMed] [Google Scholar]
- 5.Chiavegatti T, Costa VL, Jr., Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway.[see comment]. British Journal of Pharmacology. 2008;153:1331–40. doi: 10.1038/sj.bjp.0707648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giron MC, Bin A, Brun P, et al. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology. 2008;134:1116–26. doi: 10.1053/j.gastro.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001;37:1095–100. doi: 10.1161/01.hyp.37.4.1095. [DOI] [PubMed] [Google Scholar]
- 8.Resta R, Thompson LF. T cell signalling through CD73. Cellular Signalling. 1997;9:131-. doi: 10.1016/s0898-6568(96)00132-5. [DOI] [PubMed] [Google Scholar]
- 9.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. Journal of Pharmacology & Experimental Therapeutics. 1995;273:728–33. [PubMed] [Google Scholar]
- 10.Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:765–71. doi: 10.1161/01.hyp.28.5.765. [DOI] [PubMed] [Google Scholar]
- 11.Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. Journal of Pharmacology & Experimental Therapeutics. 1998;287:926–30. [PubMed] [Google Scholar]
- 12.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension. 2000;36:337–42. doi: 10.1161/01.hyp.36.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. Journal of Pharmacology & Experimental Therapeutics. 2006;317:1219–29. doi: 10.1124/jpet.106.101360. [DOI] [PubMed] [Google Scholar]
- 14.Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. Journal of Pharmacology & Experimental Therapeutics. 2003;307:888–96. doi: 10.1124/jpet.103.057166. [DOI] [PubMed] [Google Scholar]
- 15.Jackson EK, Mi Z, Zacharia LC, Tofovic SP, Dubey RK. The pancreatohepatorenal cAMP-adenosine mechanism. Journal of Pharmacology & Experimental Therapeutics. 2007;321:799–809. doi: 10.1124/jpet.106.119164. [DOI] [PubMed] [Google Scholar]
- 16.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Research. 2001;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 17.Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiological Reviews. 2006;86:849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 18.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Archiv - European Journal of Physiology. 2007;453:661–73. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 19.Kruh GD, Zeng H, Rea PA, et al. MRP subfamily transporters and resistance to anticancer agents. Journal of Bioenergetics & Biomembranes. 2001;33:493–501. doi: 10.1023/a:1012827221844. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Krishnamurthy PC, Penmatsa H, et al. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–51. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. Journal of Pharmacology & Experimental Therapeutics. 2000;295:23–8. [PubMed] [Google Scholar]
- 22.Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. Journal of Pharmacology & Experimental Therapeutics. 1997;283:177–82. [PubMed] [Google Scholar]
- 23.Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. Journal of Pharmacology and Experimental Therapeutics. 2008;325:920–6. doi: 10.1124/jpet.108.137752. [DOI] [PubMed] [Google Scholar]
- 24.Jackson EK, Gillespie DG, Jackson TC. Phospholipase C and Src modulate angiotensin II-induced cyclic AMP production in preglomerular microvascular smooth-muscle cells from spontaneously hypertensive rats. Journal of Cardiovascular Pharmacology. 2007;49:106–10. doi: 10.1097/FJC.0b013e31802ee3d5. [DOI] [PubMed] [Google Scholar]
- 25.Jackson TC, Mi Z, Jackson EK. Modulation of cyclic AMP production by signal transduction pathways in preglomerular microvessels and microvascular smooth muscle cells. Journal of Pharmacology & Experimental Therapeutics. 2004;310:349–58. doi: 10.1124/jpet.103.063081. [DOI] [PubMed] [Google Scholar]
- 26.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiological Reviews. 2006;86:901–40. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 27.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. American Journal of Cardiology. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 28.Boison D. Adenosine as a modulator of brain activity. Drug News & Perspectives. 2007;20:607–11. doi: 10.1358/dnp.2007.20.10.1181353. [DOI] [PubMed] [Google Scholar]
- 29.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacology & Therapeutics. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]