Abstract
The expanding realm of exploratory proteomics has added a unique dimension to the study of the complex pathophysiology involved in sickle cell disease. A review of proteomic studies published on sickle cell erythrocytes and plasma shows trends of upregulation of antioxidant proteins, an increase in cytoskeletal defects, an increase in protein repair and turnover components, a decrease in lipid raft proteins and apolipoprotein dysregulation. Many of these findings are consistent with the pathophysiology of sickle cell disease, including high oxidant burden, resulting in damage to cytoskeletal and other proteins, and erythrocyte rigidity. More unexpected findings, such as a decrease in lipid raft components and apolipoprotein dysregulation, offer previously unexplored targets for future investigation and potential therapeutic intervention. Exploratory proteomic profiling is a valuable source of hypothesis generation for the cellular and molecular pathophysiology of sickle cell disease.
Keywords: 2D-DIGE, apolipoprotein, cytoskeleton, exploratory proteomics, lipid rafts, MALDI-TOF, oxidative stress, pulmonary hypertension, SELDI-TOF, sickle cell disease
Sickle cell disease (SCD) is an autosomal recessive genetic disorder that involves a single adenine-to-thymine point mutation in the gene encoding the β-subunit of hemoglobin. This translates to a single amino acid substitution of valine to glutamic acid. Patients with SCD demonstrate marked phenotypic heterogeneity in disease expression, particularly in the prevalence and severity of its complications and its mortality rate. The fundamental pathophysiological basis of SCD is the polymerization of sickle hemoglobin in the cytoplasm of the red blood cell (RBC) [1–4]. The degree of sickle hemoglobin polymerization varies with microenvironmental changes, including oxygen saturation and anatomic location in the body, and varies over time. Most prominent of its consequences is diminished RBC flexibility, which impairs blood flow through the microvasculature. Unique to SCD are episodes of acute vascular occlusion that promote tissue ischemia and pain, sometimes progressing to organ infarction. Vascular occlusion may also cause a pneumonia-like condition, known as ‘acute chest syndrome’, and bone necrosis, especially in the femoral head. Vascular occlusion due to polymerization of sickle hemoglobin remains the hallmark and virtually unique feature of SCD.
Sickle hemoglobin polymerization leads to a remarkable spectrum of biochemical, cellular and physiological pathology, which is associated with a wide array of clinical complications. Sickling appears to promote adhesion of various blood cells to the activated, adhesive vascular endothelium, which may contribute to vascular occlusion [5,6]. Sickle hemoglobin polymerization robustly induces RBC oxidant stress, with linked damage to RBC metabolism, the cytoskeleton and membrane, leading to loss of membrane integrity and hemolysis. Recently, proposed models of SCD pathophysiology have focused on understanding the variable expression of pain, infarction and a host of additional complications. Strong evidence has accumulated from one recent model, suggesting that the hemolysis induced by sickle hemoglobin polymerization promotes deficiency of nitric oxide (NO), increasing the risk of pulmonary hypertension, cutaneous leg ulceration and priapism, a painful, persistent erection of the penis [7]. These complications are reported in other hemolytic diseases as well [8]. Cerebrovascular disease and stroke are also common in SCD, with some suggestion that hemolysis may play a role in this risk [9]. However, the contribution of hemolysis to vascular dysfunction has also been disputed [10].
The field of proteomics has grown exponentially in the last decade with significant technological advances that allow high-throughput proteomic profiling of multiple patient samples, database-driven identification of proteins on a molecular level with highly sensitive and precise mass spectrometry (MS), and improved resolution of a wider range of molecular mass:charge ratios, as well as techniques that are able to complement and validate each other for improved accuracy of findings. The intent of this article is to examine what has been discovered to date using these proteomic tools, to study the RBC and plasma proteome involved in SCD and to suggest future directions of study.
Normal erythrocyte proteome
To summarize a recent comprehensive review of the RBC membrane proteome [11], the major categories of known proteins are those involved in membrane repair, maintaining RBC shape and deformability, regulation of cell volume, the transport of nutrients and intercell signaling and interaction.
The RBC membrane consists of the phospholipid bilayer and supporting cytoskeletal network, which are attached to each other by protein 4.1, protein 3 and ankyrin [12–14]. The RBC is unable to synthesize phospholipids itself, so any loss of integrity of the phospholipid membrane depends on exchange with the environment for repair – the outer layer, by incorporating free cholesterol obtained from plasma lipoproteins, and the inner layer by ATP-mediated acylation of membrane lysophospholipids [15–17].
Red blood cell shape and deformability is largely determined by the cytoskeletal network, which consists of highly coiled helical rods of spectrin tetramers, conferring flexibility and spring-like properties [18,19], and actin, which polymerizes to increase cell rigidity and vice versa. Dematin (protein 4.9) and α- and β-adducin heterodimers bundle the actin filaments. Adducin also acts as an actin polymer cap and, together with tropomyosin and tropomodulin, tightly controls the ratio of polymerized:depolymerized actin to affect cell malleability [20–23]. ATP-driven phosphorylation of the cytoskeletal network [24,25], calcium extrusion and various membrane-associated enzymes [26] have also been noted to mediate RBC plasticity.
Deformability of the RBC is a crucial property to facilitate its passage through capillaries, and for successful oxygen delivery to the target tissues. Two factors that have been identified as major contributors to the deformability of the cell are the reduced surface area:volume ratio [27,28] and cytoplasmic viscosity, which depends on the concentration of hemoglobin [29]. Viscoelastic properties of the membrane itself have actually only been a minor factor [30]. Owing to cell volume being an important determinant, the red cell has a number of transporters believed to have a role in volume regulation, including several different cation ATPase transporters [31–33], passive K:Cl co-transporters and calcium-dependent potassium transporters [34], aquaporins that respond to changes in osmolarity [35–37] and amino acid transporters that are also suspected to be involved in osmoregulation [38,39].
Other transporters found in the RBC membrane are responsible for RBC function (the band 3 anion exchanger facilitating proton-mediated oxygen release from hemoglobin to tissues [40]) and nutrient influx (facilitated glucose transporters providing RBCs with their primary energy source [41] and nucleoside transporters [42]).
RBC membrane proteomics in SCD
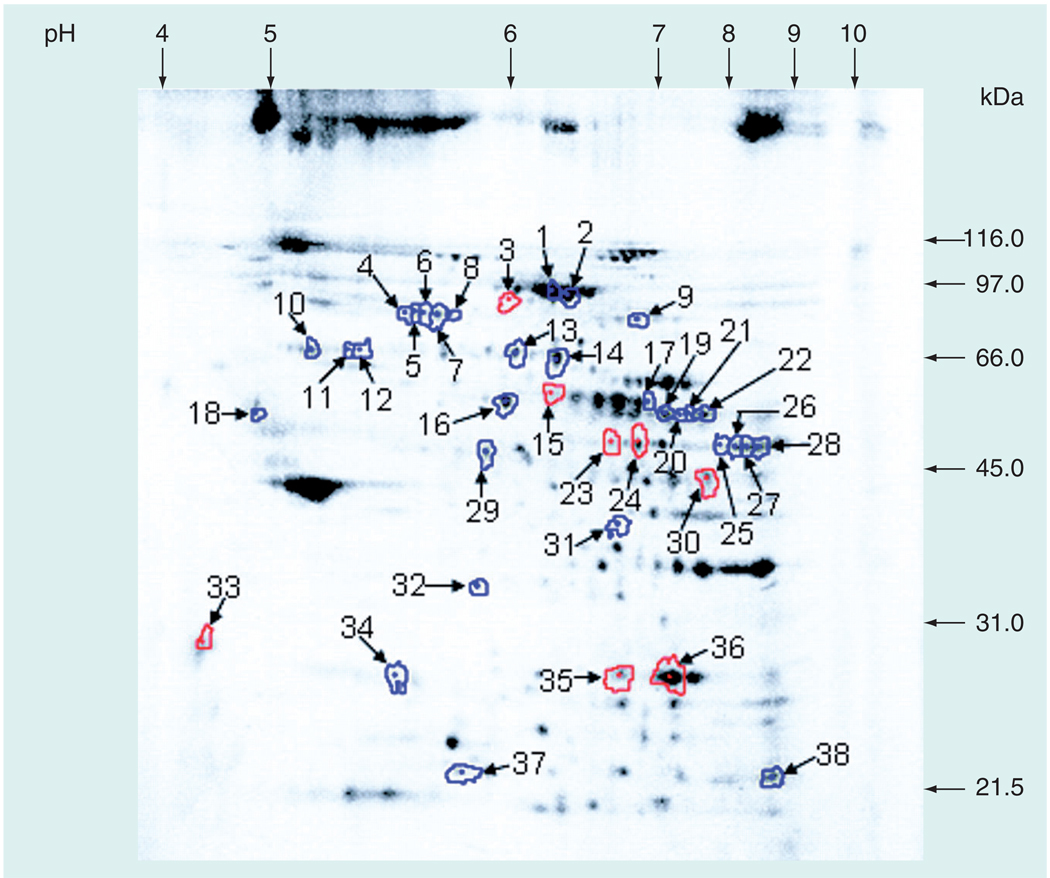
In 2005, Kakhniashvili et al. evaluated the sickle cell erythrocyte membrane proteome using 2D fluorescence difference gel electrophoresis (2D-DIGE; Figure 1), trypsin digest, HPLC and tandem MS (LC-MS/MS). A total of 22 proteins were identified (44 protein forms, including post-translational modifications) from sickle cell RBC membranes that had a 2.5-fold or greater difference from control RBC membranes, according to the threshold deemed statistically significant, based on normal variation of protein levels in normal healthy controls [43]. Proteins with the most pronounced increases in sickle cell RBCs included the 70-kDa heat-shock protein 8 isoform 1 and chaperonin-containing T-complex protein 1 (TCP1) – both involved in protein repair; protein 4.1; and ankyrin – both involved in cytoskeleton attachment to the phospholipid bilayer, and the α-subunit of ATP-synthase. Interestingly protein 4.1 was both increased and decreased in differing spots on the 2D gel. The two isoforms diverging in quantity had a notable difference in isoelectric point (pI) and molecular weight (MW). The isoform with a pI of 5.98 and MW of 89.2 kDa was decreased 3.2-fold in sickle cell RBCs, in contrast to the isoform with a pI that increased from 5.6 to 5.78 and a MW of 82.7 kDa, which was increased 3.3– to 6.6-fold. Ankyrin’s MW was increased 3.9– to 4.9-fold in sickle erythrocytes (Table 1).
Figure 1. Representative 2D fluorescence difference gel electrophoresis image from sickle cell membrane proteomics study.
Two different fluorescent dyes were used – Cy3 for normal healthy controls and Cy5 for sickle cell patients. The above image is the Cy3 image of the gel, showing spots from normal healthy control samples. Spots outlined in red represent spots that were decreased in sickle cell disease; those that were increased in sickle cell disease are outlined in blue.
Reproduced with permission from [43].
Table 1.
Most notable erythrocyte proteins quantitatively or qualitatively altered in sickle cell disease as compiled from exploratory proteomic studies
| Category | Function | Protein (MW) | GI no. | Change in SCD | Ref. |
|---|---|---|---|---|---|
| Cytoskeletal | Actin accessory | Ankyrin 1 (92 kDa) | 105337 | Increased 3.9–4.9-fold; increased threefold with HU | [43, 49] |
| Protein 4.1 (82 kDa); pI: 5.72 | 14916944 | Increased 2.7–6.6-fold; increased two–threefold without HU† | [43, 49] | ||
| Protein 4.1 (89.2 kDa); pI: 5.98, 6-kDa larger | 14916944 | Decreased 3.2-fold; increased two–threefold without HU† | [43] | ||
| Dematin protein 4.9 (44.1 kDa) | 22654240 | Decreased 2.6-fold | [43] | ||
| Tropomyosin 3 (30 kDa) | 24119203 | Decreased 2.6-fold | [43] | ||
| Anion exchanger band 3 (52 kDa) | 4507021 | Increased 2.5-fold with HU | [49] | ||
| Tropomodulin (40 kDa) | 135922 | Increased two–threefold with HU | [49] | ||
| Actin, β-actin (41 kDa) | 1703156, 1419444 | Increased 2- to 3.5-fold with HU | [49] | ||
| Palmitoylated membrane protein p55 (55 kDa) | 62898353 | Increased two–threefold with HU | [49] | ||
| Membrane | Active transport | ATP synthase α-subunit (48.9 kDa) | 4757810 | Increased 2.6–5.1-fold | [43] |
| Lipid raft | Flotillin-1 (49 kDa) | 5031699 | Decreased 2.8–3.3-fold | [43] | |
| Stomatin isoform a (27.4 kDa); pI: 6.5 | 38016911 | Decreased 3.8-fold | [43] | ||
| Stomatin isoform a (27.4 kDa); pI: 5.56 | 38016911 | Increased 2.7-fold | [43] | ||
| Vesicle transport | RAB-8b GTPase (22 kDa) | 7706563 | Increased 2.8-fold | [43] | |
| Cytoplasmic | Protein turnover | Proteasome-β 1 subunit (22 kDa) | 4506193 | Increased 2.8-fold | [43] |
| Proteasome 26S ATPase subunit 6 (39 kDa) | 24430160 | Increased 2.6-fold | [43] | ||
| Proteasome-α 1 subunit, isoform 1 (34 kDa) | 23110935 | Increased 2.5-fold | [43] | ||
| Protein folding | Chaperonin containing TCP1 subunit 7 (54 kDa) | 5453607‡, 1729870‡ | Increased 2.9–4.2-fold | [43] | |
| Protein repair | Heat-shock protein 8 (68 kDa) | 5729877 | Increased 7.3-fold | [43] | |
| Antioxidant | Peroxiredoxin 3 isoform b (22.2 kDa) | 32483377 | Increased 2.9-fold | [43] | |
| Peroxiredoxin 1 (22 kDa) | 4505591 | Increased 2.8-fold | [43] | ||
| Antioxidant | Catalase (56 kDa) | 4557014 | Increased 3.1-fold | [43] | |
| Glycolysis | Glyceraldehyde-3-phosphate dehydrogenase (36 kDa) | 7669492 | Increased two–fourfold with HU | [49] | |
| Fructose bisphosphate aldolase (39 kDa) | 113606 | Increased twofold with HU | [49] | ||
Experimental MW, corresponding GI number (National Center for Biotechnology Information [NCBI] database accession number), relevant alterations in pI or MW and quantitative change in SCD or SCD-related complication are noted.
Unclear which isoform represents the increase.
Representative NCBI database accession number.
GI: GenInfo identifier; HU: Hydroxyurea; MW: Molecular weight; pI: Isoelectric point; SCD: Sickle cell disease; TCP: T-complex protein.
Decreases in sickle cell membranes tended to be among components of the lipid raft (flotillin-1 and stomatin) and actin accessory proteins (dematin and tropomyosin 3). The functions of flotillin-1 and stomatin have not been completely elucidated, but they are transmembrane moieties thought to be involved in signal transduction. Stomatin is known to regulate transmembrane monovalent cation flux [44].
More recently, the sickle cell membrane proteome was studied in RBCs exposed to hydroxyurea, the only drug approved by the US FDA expressly for SCD [45]. This agent is known to promote elevation of fetal hemoglobin, increasing the mean corpuscular volume suggestive of improved RBC hydration and decreasing endothelial adhesion. In this exploratory proteomic approach, the investigators sought to identify changes to the sickle cell erythrocyte membrane induced by exposure of the RBCs to hydroxyurea in vitro. To assess quantitative changes in protein forms and post-translational modifications, 2D-DIGE and MS/MS were applied to erythrocyte membranes from samples of sickle cell whole blood, incubated at physiologic temperatures for 15 h with and without a physiologic concentration of hydroxyurea (50 µM). This approach yielded ten proteins of interest: a trend involving antioxidants (catalase, thioredoxin peroxidase, flavin reductase and peroxiredoxin-2 isoform a), an oxidoreductase (aldehyde dehydrogenase), proteins involved in protein repair (chaperonin-containing TCP1 subunits δ and ε) and protein degradation (proteasomal α2 subunit). In addition, key players included a structural membrane component (palmitoylated membrane protein p55), carbonic anhydrase and β-globin.
The rise in antioxidants was attributed to a compensatory response to the oxidative stress effect of hydroxyurea from its mechanism for induction of altered erythroid differentiation. The increase in protein repair and turnover constituents was suggested as a response to oxidative protein damage. Further investigation to assess the post-translational modification of catalase using 2D quantitative western blotting showed a twofold increase in tyrosine phosphorylation with hydroxyurea exposure. This observation is consistent with prior studies that showed phosphorylation of catalase by tyrosine kinases in response to oxidative stress, increasing catalase activity at a post-translational level [46–48]. It is remarkable that this set of in vitro hydroxyurea experiments yielded alterations in protein abundance, since the principal effects of hydroxyurea have previously been attributed to alterations in transcriptional regulation that are not possible in enucleated mature RBCs. In patients with SCD, there are large numbers of circulating reticulocytes – enucleated immature RBCs that contain abundant RNA and are translationally active – so it is possible that these ex vivo hydroxyurea experiments might have reflected both its translational and post-translational effects.
A follow-up study with a similar design examined the RBC membrane proteome following in vivo administration of therapeutic doses of hydroxyurea (400 µm) in five patients with excellent clinical responses to treatment, compared with five untreated SCD patients [49]. The most significant increases were observed in cytoskeletal components (anion exchanger band 3, ankyrin, protein 4.1, p55, actin, tropomodulin and stomatin) and glycolytic enzymes (glyceraldehydes-3-phosphate dehydrogenase and fructose bisphosphate aldolase). The increase in glycolytic enzymes was attributed to an increased demand for ATP synthesis as a result of hydroxyurea-induced oxidative stress; however, an alternate explanation might involve activation of the hypoxia-inducible factor (HIF)-1, which is known to regulate anaerobic glycolysis [50].
Decreases were observed in chaperonin-containing TCP1 subunit 2 (2.45-fold) and proteasome subunit-α type 4 (4.13-fold). Although the authors again attributed these decreases to increased oxidative stress in response to hydroxyurea, these changes may actually reflect a clinical improvement in response to treatment, considering that both of these proteins are elevated in SCD at baseline [43]. The only hydroxyurea-induced change common to both the in vivo and in vitro studies was the increase in p55. Identity was confirmed by immunoblot, which demonstrated a five–tenfold increase of p55 in two representative hydroxyurea-treated patients, as compared with untreated patients. Conclusions are limited by the lack of paired control specimens from each subject prior to hydroxyurea, providing potential confounding by variability of inherent characteristics of each individual sickle cell patient. The authors also acknowledged the limitation of the small sample size of this study. A larger confirmatory study, although possible with 2D-DIGE, would likely require incorporation of more high-throughput proteomic techniques.
As a precursor to formal sickle cell proteomic studies, Chou et al. published a pilot study with a primary intent to establish the utility of cleavable isotope affinity tag (cICAT) methodology as a proteomic technique [51]. Its secondary aim was to preliminarily identify structural proteins with statistically significant differences in sickle cell RBC membranes. Core RBC membrane skeletons of patients with SCD and normal healthy African–American controls were analyzed by ICAT and nano-LC-MS/MS. Samples were analyzed in three combinations: a control sample to itself (AA1/AA1), a control sample to a different control sample (AA1/AA2) and a control sample to a sickle cell sample (SS/AA), looking at mean ratios of each of the four cytoskeletal core proteins (α-spectrin, β-spectrin, protein 4.1 and actin). Mean ratios were not significantly different from 1.0, with similar variation among ratios for each protein. Variation contributed by the cICAT method in quantifying protein amounts was calculated to be 14.1%. The authors concluded that cICAT has improved precision in the quantification of proteins over 2D-DIGE, but is limited by missing many post-translational modifications, and, thus, recommended it as a complementary technique to 2D-DIGE in proteomic analysis. The secondary analysis showed no statistically significant differences between sickle cell and normal healthy subject RBC membranes, but with only nine subjects, the experiment probably lacked sufficient statistical power to definitively rule out a difference.
Monocyte proteomics in sickle cell disease
Elevated PlGF levels correlate with the severity of vaso-occlusive manifestations in SCD [52]. PlGF also activates endothelial cell adhesion molecule expression by increasing monocyte production of IL-1β and TNF-α [53]. This premise prompted examination of the proteome of monocytes from ten well-phenotyped adolescents with SCD by 2D-DIGE, followed by trypsin digest and LC-MS/MS [54]. Quantities of identified proteins were correlated with the vaso-occlusive crisis rate, a surrogate measure of clinical severity.
Monocyte membrane proteins most strongly correlated with disease severity were glycolytic enzyme transketolase, coronin and moesin – actin binding proteins involved in motility – and cell signaling mediator guanine nucleotide-binding protein. These proteins were all negatively correlated with vaso-occlusive manifestations.
Cytosolic monocyte proteins with the greatest positive correlation with disease severity were the far upstream element-binding protein, α-actinin, the myristic acid/tri-iodobenzoic acid/albumin complex, filamin A, integrin, the apo form of human mitochondrial aldehyde dehydrogenase chain A and leukotriene A-4 hydrolase. Negatively correlated cytosolic monocyte proteins largely consisted of those involved in protein folding, repair and turnover – heat-shock proteins (70 kDa and mitochondrial 60 kDa), TCP and chaperonin-containing TCP1 (Table 2). A mitochondrial apoptotic mediator, adenylate kinase 2 (variant AK2D), was also negatively correlated with recurrent vaso-occlusive events, as was phosphoglycerate kinase 1, an enzyme involved in glycolysis and angiogenesis. These findings are potentially useful as hypothesis-generating information, but are limited by unclear statistical significance. Validation of each candidate marker by independent assay is also necessary for definitive identification.
Table 2.
Most significant monocyte proteins altered in sickle cell disease, as compiled from exploratory proteomic studies.
| Category | Function | Protein (MW) | GI no. | Change in SCD | Ref. |
|---|---|---|---|---|---|
| Membrane | Glycolysis | Transketolase (75 kDa) | 4507521 | Negatively correlated with VOC rate | [54] |
| Actin-binding; cell motility | Coronin (62 kDa) | 5902134 | Negatively correlated with VOC rate | [54] | |
| Actin-binding; cell motility | Moesin (81 kDa) | 4505257 | Negatively correlated with VOC rate | [54] | |
| Cell signaling | Guanine nucleotide-binding protein (32 kDa) | 5174447 | Negatively correlated with VOC rate | [54] | |
| Cytosolic | ATP-dependent DNA helicase | Far upstream element-binding protein (96 kDa) | 37078468 | Positively correlated with VOC rate | [54] |
| Actin accessory | α-actinin (103 kDa) | 4501891, 2804273 | Positively correlated with VOC rate | [54] | |
| Unclear | Myristic acid/tri-iodobenzoic acid/albumin complex | 4389275 | Positively correlated with VOC rate | [54] | |
| Actin accessory | Filamin A (272 kDa) | 116241365 | Positively correlated with VOC rate | [54] | |
| Intra-/extra-cellular signal transduction | Integrin (168 kDa) | 64654539 | Positively correlated with VOC rate | [54] | |
| Aldehyde oxidation | Mitochondrial aldehyde dehydrogenase (63 kDa) | 28949044 | Positively correlated with VOC rate | [54] | |
| Arachidonic acid metabolism | Leukotriene A-4 hydrolase (82 kDa) | 4505029 | Positively correlated with VOC rate | [54] | |
| Protein repair | Heat-shock protein (70 kDa) | 12585261 | Negatively correlated with VOC rate | [54] | |
| Protein repair | Mitochondrial heat-shock protein (60 kDa) | 129379 | Negatively correlated with VOC rate | [54] | |
| Protein folding | TCP1, subunit-α (73 kDa) | 57863257 | Negatively correlated with VOC rate | [54] | |
| Protein folding | Chaperonin-containing TCP1, subunit 8 (69 kDa) | 48762932 | Negatively correlated with VOC rate | [54] | |
| Mitochondial apoptotic mediator | Adenylate kinase 2 (64 kDa) | 5453595 | Negatively correlated with VOC rate | [54] | |
| Glycolysis/angiogenesis | Phosphoglycerate kinase 1 (46 kDa) | 48145549 | Negatively correlated with VOC rate | [54] | |
Experimental MW, corresponding GI number (National Center for Biotechnology Information [NCBI] database accession number) and alteration in SCD is noted. VOC rate was used as a surrogate marker of SCD severity.
GI: GenInfo identifier; MW: Molecular weight; SCD: Sickle cell disease; VOC: Vaso-occlusive crisis.
Sickle cell plasma proteomics
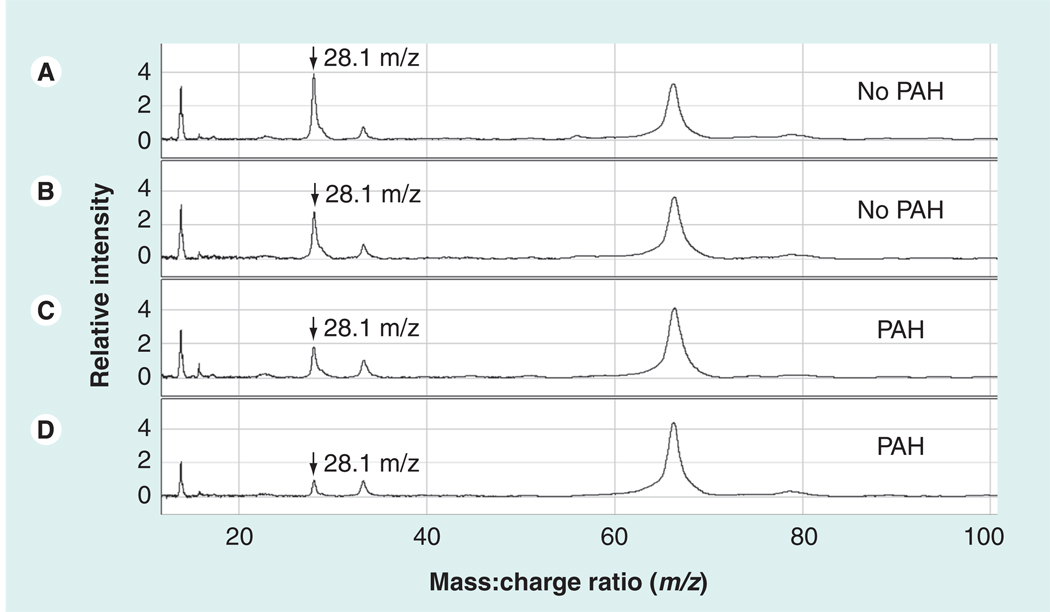
Pulmonary hypertension (PH) is an independent risk factor for mortality in SCD [55]. Our group carried out a high-throughput exploratory proteomic study searching for markers of pulmonary arterial hypertension in the plasma of sickle cell patients utilizing a variety of proteomic techniques [56]. Proteomic profiling by SELDI-TOF MS was performed on plasma samples from a cohort of 56 age- and gender-matched patients with homozygous SCD, with and without PH as determined by high versus normal tricuspid regurgitant jet velocity on a Doppler echocardiogram. The most significant mass to charge (m/z) species (Figure 2) were identified through statistical analysis using the random forest computational algorithm and stepwise logistical regression. Species of interest were then identified using protein electrophoresis, in-gel trypsin digest and LC-MS/MS with or without confirmation by MALDI-TOF MS. These results were validated by correlation with available standard clinical immunoassays. This approach suggested decreased plasma levels of apolipoprotein A-I (apoA-I) as a potential marker of PH risk in the setting of SCD. Other preliminary markers found were increased apolipoprotein A-II (apoA-II) and serum amyloid A (SAA)-4 levels, and decreased levels of plasminogen dimer and haptoglobin dimer (Table 3). ApoA-I levels were validated by correlation with clinical assays for both apoA-I and high-density lipoprotein cholesterol (HDL-C; p = 0.0001; Spearman’s rank correlation: 0.59). Apolipoprotein B (apoB) levels, though not detected through SELDI-TOF MS, were also measured by a clinical assay, with high levels correlating with PH (p = 0.05). This led to calculation of the apoB:apoA-I ratio, which was significantly elevated in PH (p = 0.006). In a supporting blood flow physiology study of vasomotor reactivity in SCD subjects, lower levels of apoA-I were associated with an impaired vasodilatory response to the endothelial-dependent agonist acetylcholine (p = 0.001), indicating association of endothelial dysfunction with lower apoA-I levels in SCD. These results taken together suggest that apolipoprotein dysregulation, manifested by low apoA-I levels, contributed to endothelial dysfunction and PH associated with SCD.
Figure 2. Representative SELDI-TOF MS spectra from plasma from patients with sickle cell disease with and without pulmonary hypertension.
The vertical axes represent intensity of peaks in arbitrary units, and the horizontal axes represent mass:charge ratio (m/z). These four spectra were obtained from plasma eluted from anion exchange resin, bound to IMAC30–Cu2+ matrix, and ionized by SELDI-TOF MS. Two specimens are from sickle cell disease (SCD) patients without pulmonary arterial hypertension (A & B) and two are from SCD patients with PAH (C & D). A peak at m/z 28.1 kDa was observed at lower average intensity in SCD patients with PAH (arrows). IMAC: Immobilized metal-affinity capture; PAH: Pulmonary arterial hypertension. Reproduced with permission from [56].
Table 3.
Most significant plasma proteins altered in sickle cell disease, as compiled from exploratory proteomic studies.
| Function | Protein (MW) | GI no. | Change in SCD | Ref. |
|---|---|---|---|---|
| Antioxidant/anti-inflammatory, HDL component | ApoA-I (28.1 kDa) | 4960066† | Decrease in SCD vs control | [56] |
| Decrease in SCD + PH vs SCD - PH | [58] | |||
| Decreased during acute pain episodes | ||||
| LDL/VLDL component | ApoB | 178790† | Decrease in SCD vs control | [56] |
| Ratio | ApoB:ApoA-I ratio | – | Decrease in SCD vs control | [56] |
| HDL component | ApoA-II (8.9 kDa) | 296633†, 178424† | Increase in SCD + PH vs SCD - PH | [56] |
| HDL component, constitutive SAA | SAA-4 (13.4 kDa) | 13937846†, 119588821† | Increase in SCD + PH vs SCD - PH | [56] |
| Glycoprotein precursor of plasmin | 89.4 kDa‡ | 387031† | Decrease in SCD + PH vs SCD - PH | [56] |
| Scavenges cell-free hemoglobin | Haptoglobin dimer (75.2 kDa) | 386783† | Decrease in SCD + PH vs SCD - PH | [56] |
| Uncertain identity | 18.4 | – | Decrease in SCD + PH vs SCD - PH | [56] |
| Acute phase reactant, antagonizes apoA-I | SAA | 40316910† | Increase in acute pain episodes | [58] |
| Ratio | SAA:apoA-I ratio | – | Decrease in acute pain episodes | [58] |
| Oxidative post-translational modification | Malondialdehyde–albumin adduct | – | Qualitative increase in SCD + PH vs SCD - PH | [57] |
All proteins mentioned belong to the ‘plasma’ category. Experimental MW, corresponding GI number (National Center for Biotechnology Information [NCBI] database accession number) and change in SCD or SCD-related complication are noted.
Representative NCBI database accession number.
Protein of uncertain identity.
Apo: Apolipoprotein; GI: GenInfo identifier; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MW: Molecular weight; PH: Pulmonary hypertension; SAA: Serum amyloid A; SCD: Sickle cell disease; VLDL: Very low-density lipoprotein.
Protein function and structure alterations due to oxidative stress are thought to potentially be a contributor to the pathogenesis of PH. This premise generated a hypothesis of whether oxidative post-translational modifications of abundant plasma proteins could potentially serve as markers of disease severity, which was proteomically explored [57]. Albumin isolated from the plasma of sickle cell patients, with and without PH, revealed an increased presence of a malondialdehyde (MDA) adduct in PH, by MALDI-TOF MS and immunoblot. LC-MS/MS and x-ray crystallography further localized the adduct to the K159 residue on albumin. A similar study of albumin from patients with non-SCD-related idiopathic pulmonary arterial hypertension also showed increased post-translational modification with MDA at the same site. It was concluded that this may represent an oxidative lesion specific to the pathogenesis of pulmonary hypertension, and it could be a candidate to serve as a biomarker. This study also suggested that such post-translational modifications may be present on other abundant, as well as potentially nonabundant, plasma proteins.
A second study from our group also used SELDI-TOF MS and clinical immunoassays to profile plasma from 26 adult sickle cell patients at steady state versus during acute pain episodes (APEs) [58]. Significant differences in intensity during APEs included an elevated m/z species of 11.7 kDa and a decreased m/z species of 28.1 kDa, identified as SAA and apoA-I, respectively. The relative intensities of these m/z species correlated strongly with their respective immunoassays. Four patients with clinically severe extraosseous complications during APE were distinguished by extreme elevations of SAA (median: 413.5 vs 6.5 mg/ml). Further analysis of immunoassay data also demonstrated an inverse correlation between the two markers during APE, such that an increased SAA:apoA-I ratio (p < 0.004) may be an even more robust marker of APE (Table 3).
The two SELDI-TOF studies confirmed and extended previous observations regarding apoA-I and SAA in SCD [59–62]. Validation of results generated by this technique with clinical assays, high-resolution MS and immunoassays supports the validity of SELDI-TOF MS when used as a screening tool for further confirmatory immunoassays or high-resolution MS. SELDI-TOF alone lacks the resolution for independent identification of protein markers.
Sickle cell transcriptomics versus proteomics
A few studies have examined reticulocyte, erythrocyte, platelet and monocyte gene-expression profiles in the setting of SCD, as well as hydroxyurea treatment, and offer an additional perspective. Several findings from a high-throughput microarray mRNA genomic-profiling study of sickle cell whole blood coincide with recurring proteins in exploratory proteomic studies, in particular, significant upregulation of ankyrin, erythrocyte membrane protein bands 3 and 4.1, peroxiredoxin and stomatin. Increased levels of enzyme 2,3-bisphosphoglycerate kinase mRNA supports the theory of stimulation of glycolytic pathways in SCD, suggested by proteomic data. Other differentially expressed transcripts included selenium-binding protein, exportin, spermine oxidase, chemokines, interleukin receptor and peptidyl arginine deaminase [63].
A similar study design assessing platelet mRNA expression identified 100 differentially expressed genes in SCD, major categories being arginine and nitrogen metabolism, redox homeostasis, cell growth, adhesion and signaling pathways. Most notably, there was a significant increase in mRNA of arginase II and ornithine decarboxylase, both enzymes being involved in converting arginine to polyamines. The absence of SCD platelet proteomic studies limits direct comparisons between this study and proteomic data, but the finding of increased redox proteins glutathione peroxidase 4, thioredoxin reductase and superoxide dismutase continues to support the recurrent SCD theme of a response to oxidative stress [64].
Mononuclear gene-expression profiles were investigated in 27 patients with steady state SCD (ten hydroxyurea-treated, 17 untreated) and 13 healthy controls, identifying a highly specific leukocyte transcriptional response in SCD. A total of 112 genes were identified as being differentially expressed in SCD by global transcriptional microarray analysis, major categories involving heme metabolism, cell cycle regulation, antioxidants, inflammatory mediators and angiogenesis. Most notable were the upregulations of hemeoxygenase 1, biliverdin reductase, cyclin-dependent kinase p21, IL-15, ECGF-1 and antioxidants glutathione peroxidase, thioredoxin and thioredoxin peroxidase. Of note, hydroxyurea did not appear to have a direct effect on leukocyte gene expression, but the study appears underpowered to definitively comment on this effect [65].
A reverse transcriptase PCR-based transcriptomic study of hydroxyurea-induced gene expression in reticulocytes of eight SCD patients on hydroxyurea for at least 3 months, compared with eight SCD patients unexposed to hydroxyurea, found upregulation of a host of metal-ion-binding proteins and transporters, RNA binding proteins, ubiquitin-protein ligases and glycolytic enzymes, the latter of which also appeared in the in vivo hydroxyurea proteomic study by Ghatpande et al. [49,66].
A second similar study of patients with homozygous SCD at steady state, 14 of whom were on hydroxyurea therapy for at least 3 months, and 28 of whom were not treated with hydroxyurea, focused specifically on the hydroxyurea-induced expression of several low-affinity reticulocyte adhesion molecules, and suggested that hydroxyurea reduces the adhesive properties of sickle cell erythrocytes and does so at the transcriptional level [67].
As demonstrated by these gene-expression studies, SCD transcriptome results overlap with exploratory proteomic results. However, each sphere additionally provides a distinct set of nonoverlapping information. This is probably due to differences in disease-associated alterations occurring at the transcriptional versus post-translational levels, as well as biases inherent to specific methodologies. Global mRNA expression studies will not detect post-translational alterations of protein levels or products, which a proteomic approach is more likely to detect. Conversely, unstable proteins with short half-lives might not yield proteomics signals as strong as the corresponding mRNA abundance.
Another important distinction between proteomic and transcriptomic analysis, as it concerns SCD, is the tissue of interest to which either is applied. It is difficult to study platelets by exploratory proteomics due to the difficulty in isolating them in abundance. Gene-expression profiling can employ PCR amplification to permit the study of minute quantities of cells.
Thus, these two methodologies are complementary and both useful in examining a global expression profile of a disease of interest. By utilizing both, the pool of candidate biomarkers to be further studied can be maximized and functionally compartmentalized to an extent, allowing for more comprehensive hypothesis generation. The study of SCD in particular, stemming from a simple point mutation at the nucleic acid level but manifesting with an enormous range of phenotypic variation, conceptually emphasizes the importance of involving broad-based approaches.
Multiple globin gene-expression studies have identified polymorphic alterations within the SCD population, which is an important direction in understanding the phenotypic variation among sickle cell patients. However, since our focus is on proteins identified by the exploratory proteomic approach, we refer readers to a recent extensive review of this subject [68].
SCD proteomic interactome
In a step beyond exploratory proteomic profiling, interactome mapping attempts to define disease-specific interactions among proteomically identified candidate proteins. This additional analytic method was performed on the sickle cell erythrocyte membrane proteome [43] in the context of a preliminary erythrocyte interactome network constructed from a compilation of 751 erythrocyte membrane proteins identified by proteomic methods [69]. An extension of this analysis also attempted to quantify the impact of the SCD proteome on the erythrocyte protein interactome using a small number of different statistical clustering algorithms [70].
In these analyses, the relationships between the proteins were derived mathematically from Spearman correlations and database information, not from experimentally demonstrated interactions between the proteins of interest. Several of the SCD-altered proteins coincided with the repair or destroy box, a categorization of closely correlated proteins that share functions responsible for protein folding, repair and degradation. Several others (ankyrin 1, dematin, protein 4.9, heat-shock protein 8, proteasome subunit-α type 1 and -β type 1, chaperonin-containing TCP1, proteasome subunit-α and 26S subunit, and ATPase 6) appeared as key articulation points on the interactome map, with unclear significance.
This approach was admittedly incomplete and carried a certain degree of false discovery risk, although an attempt was made to minimize this with the use of Spearman correlation coefficients of at least 0.3 [69]. Interactome analysis may prove useful as a tool for the functional categorization of proteomically identified proteins. It is unclear at this point whether one of the interactome mapping methods is better than another, and this certainly deserves further study.
Discussion
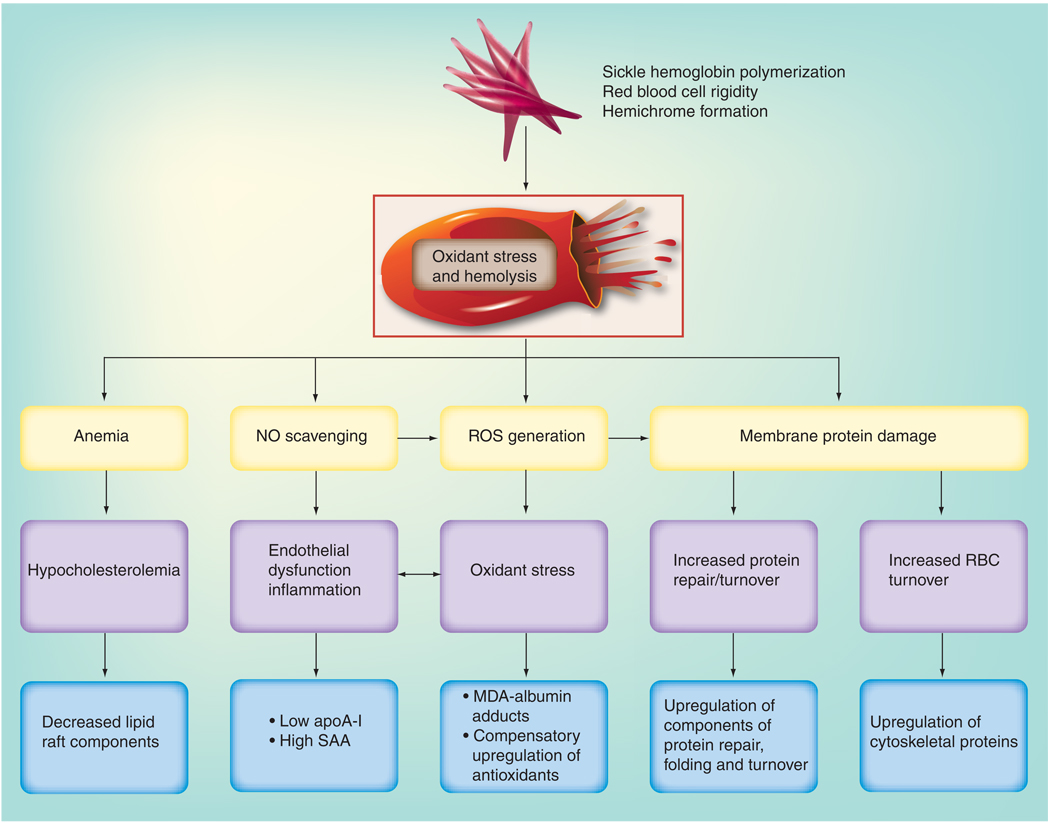
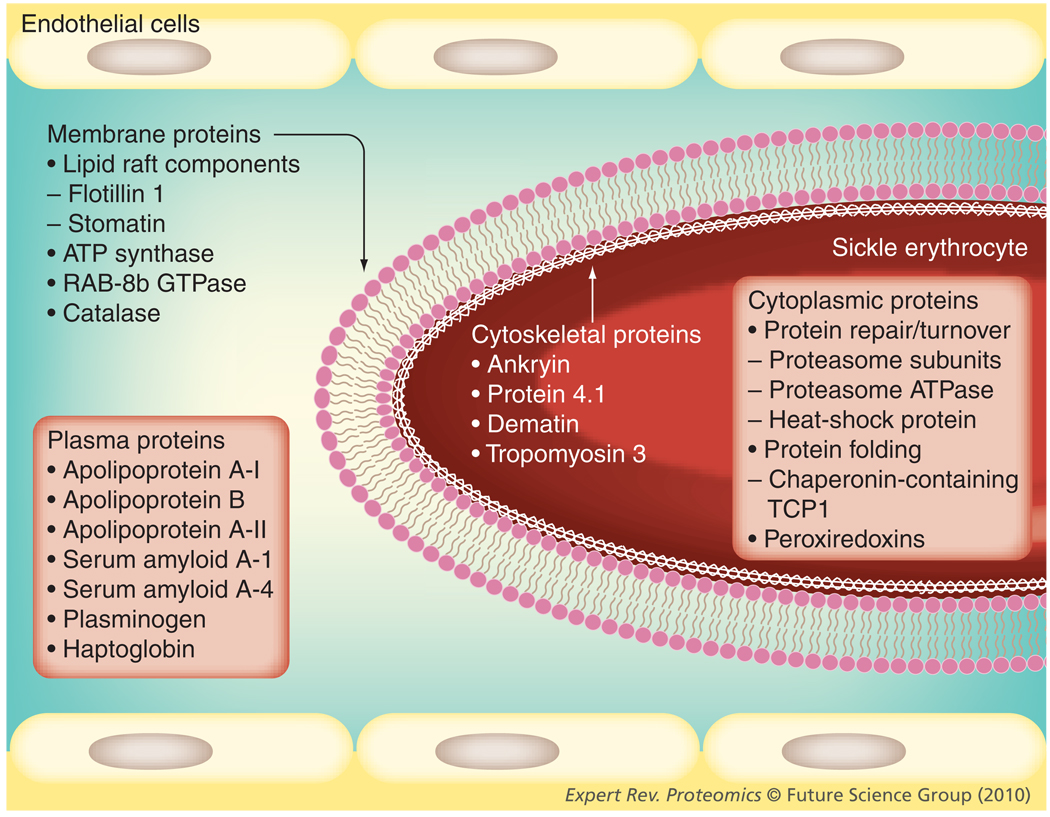
Only a limited number of studies exist that characterize the proteome of SCD. Erythrocyte membranes have been studied most comprehensively. In these studies, some cytoplasmic proteins have surfaced as potentially significant players in SCD, although the cytoplasmic proteome of RBCs or other tissues has not yet been specifically evaluated. Proteomic profiling of sickle cell plasma has thus far been directed at finding biomarkers of known complications of SCD, namely pulmonary hypertension and APEs. A variety of methods have been used in these exploratory proteomic studies, including 2D-DIGE and ICAT in the erythrocyte proteomic studies, SELDI-TOF and MALDI-TOF MS in the plasma proteomic studies, and trypsin digest and MS/MS in all studies, for identification of m/z species of interest. Differentially expressed proteins have been quantitatively validated using immunoassays. Their complementary nature is advantageous as this allows one to examine results trends across methods, as well as promoting nonredundant discoveries. These studies have allowed conclusions to be drawn, largely of quantitative protein alterations in SCD. Some hypotheses can be generated regarding suspected post-translational modification related to SCD, based on quantitative changes of the same identified protein bearing different pI and MW characteristics, but as yet, these putative modifications have not been rigorously validated. In examining the trends seen among the exploratory sickle cell proteomic studies to date, in the context of sickle erythrocyte proteins being described through protein biochemistry, several general pathophysiological themes are found to recur (Figures 3 & 4).
Figure 3. Summary of major categories of erythrocyte and plasma proteins altered in sickle cell disease as identified by exploratory proteomic profiling.
ApoA-I: Apolipoprotein A-I; MDA: Malondialdehyde; NO: Nitric oxide; RBC: Red blood cell; ROS: Reactive oxygen species; SAA: Serum amyloid A.
Figure 4. Physiologic and biochemical consequences of sickle hemoglobin polymerization-associated oxidant stress and hemolysis as a common pathway leading to alterations within the major protein biomarker categories.
TCP: T-complex protein.
Antioxidants in SCD
Upregulation of antioxidant proteins seems to be common to most of the studies discussed in this article, most notably, increases in catalase and peroxiredoxins. A compensatory increase in antioxidants is expected in the setting of the high oxidative burden of SCD. Oxidative stress is believed to be a consequence of several mechanisms, including: hemolysis, causing NO depletion [71] and generation of oxidant species [72–78]; generation of reactive oxidative species by upregulation of NADPH oxidase and xanthine oxidase [72]; chronic ischemic reperfusion in the setting of intermittent microvascular vasoocclusion [79]; and loss of catalytic antioxidants [80–86]. Oxidative stress is thought to promote the formation of irreversibly sickled cells, which, in a vicious cycle, fuels further oxidative stress. Oxidative damage is manifested on RBC membranes via the lower presence of sulphydryl groups, increased lipid peroxidation and deformation of the cytoskeleton [87].
Cytoskeletal components in SCD RBCs
Cytoskeletal proteins are consistently found to be altered in proteomic profiling of sickle cell erythrocytes. Cytoskeletal attachment proteins 4.1 and ankyrin were both increased significantly in SCD. In addition, levels of an isoform of protein 4.1 with an altered pI and MW were decreased, suggestive of an SCD-related alteration in a post-translational modification. Such protein alterations may be consistent with previously reported oxidative forms of protein 4.1 [88,89], β-actin and spectrin in sickle erythrocytes. No significant quantitative changes have been found in β-actin or spectrin in the exploratory sickle cell proteomic studies, but other studies have reported disulfide bridge formation by β-actin when oxidized [90], as well as glutathiolation of the cysteine residues of α-spectrin, which impedes dissociation from a ternary complex with protein 4.1 and β-actin [90–93]. These changes reduce cytoskeletal flexibility, which may contribute to sickled RBC rigidity [93]. The increase of the cytoskeletal attachment proteins ankyrin and protein 4.1 may reflect a compensatory cellular response to impaired anchoring of the phospholipid bilayer to the damaged cytoskeleton. Potentiation of this response may be reflected in the relative increase in these proteins seen in patients undergoing hydroxyurea treatment versus untreated SCD patients [49]. The function of the p55 protein is to link the plasma membrane with the cytoskeleton in a ternary complex with protein 4.1, thereby maintaining the stability of the erythrocyte membrane. Its increased expression, induced by both in vivo and in vitro hydroxyurea treatment, may hypothetically contribute to decreased RBC damage and hemolysis.
Besides protein 4.1, other actin accessory proteins show quantitative changes in the setting of SCD. Dematin and tropomyosin were both decreased by proteomic profiling, and both play a central role in RBC malleability by controlling actin bundling and the actin polymerization:depolymerization ratio. A decrease in these proteins may thus also reflect contribution to increased RBC rigidity. Proteomic analysis of RBC membranes, obtained from blood stored in blood banks by 2D-DIGE [94], revealed shifts in isoelectric points in cytoskeletal proteins after 7 days, which is thought to represent progressive degradation of actin, dematin, and ankyrin, due to oxidation by reactive oxygen species. Why no compensatory increase in dematin expression, similar to that of ankyrin, has been observed is not clear, but may be related to the nature of dematin’s dynamic function, as opposed to the more static one of ankyrin. Tropomyosin, also with a dynamic function, has a similar response to dematin.
Protein repair, protein turnover & protein folding components in SCD
A major consequence of oxidative stress is protein damage. It is therefore not surprising that several proteins involved in protein repair, folding and turnover emerged as being increased in the sickle cell erythrocyte and monocyte proteomic studies; namely components of proteasomes, chaperonin-containing TCP1 and several heat-shock proteins. Decrease of the former two proteins with in vivo hydroxyurea treatment could hypothetically reflect a lower net rate of protein damage, and thus a reduced demand for them. Proteasomes, heat-shock protein and the TCP1 ring complex are ATP dependent in their functions, which coincides with an increase in ATP synthase detected in sickle cell exploratory proteomic profiling [95]. Alterations in enzymes involved in glycolysis may be speculatively related to such an increase in demand for ATP synthesis, or alternatively, to a shift from oxidative phosphorylation to anaerobic glycolysis as an adaptation to hypoxia (see later). The ATP dependence of these protein-folding, turnover and repair processes may explain the upregulation of ATP synthase and, possibly, the increased metabolic rate seen in sickle cell patients. However, as stated previously, until many of these findings are validated by independent clinical assays, firmer mechanistic conclusions cannot be drawn.
Alterations in glycolytic enzymes in SCD
A recurrent pattern in the studies reviewed here is the association of upregulation of glycolytic enzymes with a favorable clinical course in SCD. In the monocyte, higher levels of phosphoglycerate kinase-1 and transketolase correlated with a lower vaso-occlusive crisis rate [54]. In vivo treatment with hydroxyurea, a medication known to promote clinical improvement in SCD patients, revealed upregulation of GADPH and fructose bisphosphate aldolase, compared with untreated patients.
Glycolytic enzyme expression is now known to be under the control of HIF-1, the master switch of the gene-expression response to hypoxia [50,96]. HIF-1 coordinates adaption to hypoxia, shifting ATP production away from oxygen-dependent mitochondrial oxidative phosphorylation, and shunting pyruvate away from the mitochondria via activation of pyruvate dehydrogenase kinase and toward anaerobic ATP production via HIF-1-induced upregulation of glucose uptake and glycolytic enzyme genes [50]. Proteomics results indicate a three- to five-fold increase in the level of an ATP synthase subunit in SCD patients at baseline that may reflect a compensation for reduced oxidative phosphorylation synthesis of ATP. HIF-1 target genes within these pathways with proteomically identified alterations include phosphoglycerate kinase-1 and GADPH [96–98]. Fructose bisphosphate aldolase, which favors gluconeogenesis, was identified as being possibly induced by hydroxyurea, which potentially benefits meeting the increased requirement for glucose under less-efficient anaerobic pathways.
Lipid raft proteins in SCD
A less obvious category of proteins altered in SCD by proteomic profiling are lipid raft components, namely flotillin-1 and stomatin. Both are known to be part of the stomatin prohibin flotillin Hflk/c (SPFH) domain-containing protein family, which is found in lipid raft microdomains in various cellular membranes and is, as yet, incompletely characterized. Lipid rafts are cholesterol-rich regions in the phospholipid bilayer. In a proposed ‘lipid shell’ hypothesis, SPFH domain-containing proteins form large multimeric complexes, which bind cholesterol and form cholesterol-rich microdomains. They are then thought to actively recruit other types of proteins to these domains and mediate various protein–protein interactions. Functions of SPFH proteins have not been completely elucidated, but processes attributed to them include ion channel regulation, membrane protein chaperoning, vesicle and protein trafficking, membrane–cytoskeletal communication and mechanosensation. Flotillin-1 has specifically been cited in roles handling neuronal regeneration, adipocyte GLUT4 trafficking, cell proliferation, endocytosis, phagocytosis and signaling from the cell surface to the cytoskeleton. Stomatin has been putatively implicated in ion channel regulation [99]. Of note, proteasome activation and inactivation has been characterized as a lipid raft-compartmentalized event in macrophages [100], which suggests a possible relationship between the decrease in lipid raft components and the increase in proteasome proteins observed by exploratory proteomics in SCD.
Furthermore, in the cell membrane, the actin cortex plays a central role in organizing lipid raft sphingolipid–cholesterol assemblage potential [101], so the sickle cell-associated decrease in flotillin-1 and stomatin may be a consequence of impaired actin functioning. Impaired mechanosensation, along with poor communication between the cytoskeleton and plasma membrane, could cause inappropriate responses to environments calling for increased cell malleability, and thus contribute to RBC microvascular clumping and increased cell fragility. Decreases in lipid raft components could also be related to the hypocholesterolemia generally associated with SCD [102–104]. Low serum cholesterol levels may be a consequence of increased cholesterol consumption by membrane synthesis during increased erythrocyte production [103], but are more likely to be due to equilibration with the reservoir of RBC membrane cholesterol, which is diminished owing to anemia [105]. It is possible that the depleted cholesterol levels lead to a lower presence of lipid rafts in the RBC membrane, reflected by the proteomically identified decrease in stomatin and flotillin-1. Interestingly, stomatin levels increased following in vivo hydroxyurea treatment, which hypothetically suggests reversal of one or more of these potential mechanisms. It would be interesting to investigate, especially in the context of the link we established between apolipoprotein dysregulation and endothelial dysfunction, the impact of a lipid raft deficiency on erythrocyte and endothelial functioning. Signaling and interactions between erythrocytes and their environment generally remains a poorly understood subject that, with further elucidation may shed more light on the contribution of lipid raft defects to SCD pathophysiology. This presents a limitation in fully appreciating the impact and involvement of lipid rafts in SCD, but is an interesting direction for hypothesis generation and further research.
Oxidative post-translational modifications
The presence of a MDA adduct at the K157 residue of albumin in both SCD-associated PH and idiopathic pulmonary hypertension is interesting, in that MDA adducts represent an oxidative lesion and that they are a candidate biomarker for PH that warrants further clinical validation. This discovery also beckons further exploration of the existence of post-translational modifications on other abundant, as well as nonabundant, plasma proteins that may lend clues to disease pathogenesis and/or serve as biomarkers for sequelae of SCD. These approaches are technically challenging.
Apolipoprotein dysregulation associated with complications of SCD
The study of complications of SCD through proteomic profiling of plasma brought to the foreground an unexpected involvement of apolipoproteins in the pathogenesis of sickle cell-associated pulmonary hypertension and APEs. Low apolipoprotein levels were associated with each, on a chronic basis in pulmonary hypertension and more acutely in APEs. ApoA-I is known to have both antioxidant and anti-inflammatory properties. The increase in acute-phase SAA along with the apoA-I depletion observed in both studies (by Yuditskaya et al. and Tumblin et al.) suggests involvement of a possible mechanism of apoA-I replacement by acute-phase SAA in HDL, either by direct displacement or, more likely, by competition during HDL synthesis [56,58]. This replacement would render HDL a proinflammatory particle, and inhibit its ability to prevent oxidation of apoB-containing particles, such as low-density lipoprotein, particularly in the subendothelial space. In other systemic inflammatory disorders, HDL has been observed to be less effective at preventing low-density lipoprotein oxidation as well, which is suggestive of a similar incapacitation of the normal functioning of HDL [106,107].
Apolipoprotein A-I is a key participant in the activation of endothelial NO synthase by HDL-mediated phosphorylation and thus plays a central role in increasing NO production in the presence of reactive oxygen species [108]. As such, beyond reverse cholesterol transport and atherogenesis inhibition, apoA-1 protects against direct endothelial injury. HDL, in its anti-inflammatory state, also inhibits adhesion molecule expression on endothelial cells. These phenomena are of particular interest in the setting of SCD, especially with our evidence that a deficiency in apoA-I predisposes to the development of PH, and a precipitous drop in apoA-I level from steady state levels occurs during a sickle cell APE. The model of altered apoA-I levels in sickle cell patients opens a translational target for therapeutically increasing apoA-I levels in sickle cell patients to prevent the long-term complication of PH, which might increase life expectancy, as well as diminish the severity of APEs, thereby improving quality of life.
Apolipoprotein A-II was identified as being elevated in the setting of PH by proteomic profiling of sickle cell plasma. ApoA-II, along with acute-phase SAA, impairs the antioxidant properties of HDL by displacing paraoxonase-1, a HDL-associated enzyme [109,110]. Paraoxonase-1 enables HDL to accept and inactivate oxidized phospholipids from cell membranes [111]. Impairment of this function might be especially problematic in a setting of severe oxidative stress such as SCD. As evidenced by the elevation of apoA-II, this mechanism might contribute to vasculopathy and the development of PH in SCD.
The significance of the elevated constitutive SAA-4 in the setting of sickle cell-associated pulmonary hypertension is less clear as its function has not been as well characterized, but it does suggest a disorder of lipoprotein metabolism.
Expert commentary & five-year view
Exploratory proteomic methodologies have, in recent years, revealed some proteins that were previously detected via standard protein biochemistry methods to be altered in SCD, essentially validating the exploratory proteomics scientific approach. Comparisons of the RBC and plasma proteome between healthy controls and subjects with SCD, as well as among SCD patients with different complications, using these exploratory proteomic approaches have presented some fresh mechanistic insights, including alterations of apolipoprotein function in vascular dysfunction, PH and vaso-occlusive pain in SCD. Such discoveries promote new directions of thought and research regarding the pathophysiology of SCD, pathogenesis of complications involved in SCD, potential treatments targeting these proteomically identified aberrations, and earlier, and more specific, screening and diagnostic modalities.
Future investigation in this area over the next 5 years holds promise for devising new biomarkers to predict prognosis of SCD, stratify risk and provide surrogate outcome markers for future clinical trials in SCD. Such biomarkers could help to guide risky curative therapies such as hematopoietic stem cell transplantation and gene therapy, and to accelerate drug development to reduce the burden of human suffering in this insidious disease.
Acknowledgments
The authors are supported by funds from the Division of Intramural Research of the National Heart, Lung and Blood Institute (MD, USA) and the Clinical Center of the NIH (MD, USA). Gregory J Kato has received funding from the NIH (grant number 1ZIAHL006014-01).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Vekilov PG. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia. Br. J. Haematol. 2007;139(2):173–184. doi: 10.1111/j.1365-2141.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrone FA. Polymerization and sickle cell disease: a molecular view. Microcirculation. 2004;11(2):115–128. doi: 10.1080/10739680490278312. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg MH. Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. Scientific World Journal. 2008;8:1295–1324. doi: 10.1100/tsw.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 5.Conran N, Costa FF. Hemoglobin disorders and endothelial cell interactions. Clin. Biochem. 2009;42(18):1824–1838. doi: 10.1016/j.clinbiochem.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Frenette PS. Sickle cell vasoocclusion: heterotypic, multicellular aggregations driven by leukocyte adhesion. Microcirculation. 2004;11(2):167–177. [PubMed] [Google Scholar]
- 7.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato GJ, Taylor JG. Pleiotropic effects of intravascular haemolysis on vascular homeostasis. Br. J. Haematol. 2010;148(5):690–701. doi: 10.1111/j.1365-2141.2009.08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernaudin F, Verlhac S, Chevret S, et al. G6PD deficiency, absence of α thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood. 2008;112(10):4314–4317. doi: 10.1182/blood-2008-03-143891. [DOI] [PubMed] [Google Scholar]
- 10.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 11.Pasini EM, Lutz HU, Mann M, Thomas AW. Red blood cell (RBC) membrane proteomics – part I: proteomics and RBC physiology. J. Proteomics. 2009;73(3):403–420. doi: 10.1016/j.jprot.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Hemming NJ, Anstee DJ, Staricoff MA, Tanner MJ, Mohandas N. Identification of the membrane attachment sites for protein 4.1 in the human erythrocyte. J. Biol. Chem. 1995;270:5360–5366. doi: 10.1074/jbc.270.10.5360. [DOI] [PubMed] [Google Scholar]
- 13.An XL, Takakuwa Y, Nunomura W, Manno S, Mohandas N. Modulation of band 3-ankyrin interaction by protein 4.1. Functional implications in regulation of erythrocyte membrane mechanical properties. J. Biol. Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- 14.Gimm JA, An X, Nunomura W, Mohandas N. Functional characterization of spectrin-actin-binding domains in 4.1 family of proteins. Biochemistry. 2002;41:7275–7282. doi: 10.1021/bi0256330. [DOI] [PubMed] [Google Scholar]
- 15.Yawata Y. Cell Membrane: The Red Blood Cell as a Model. Germany: Wiley-VCH, Weinheim; 2003. [Google Scholar]
- 16.Hunt AN, Fenn HC, Clark GT, Wright MM, Postle AD, McMaster CR. Lipidomic analysis of the molecular specificity of a cholinephosphotransferase in situ. Biochem. Soc. Trans. 2004;32:1060–1062. doi: 10.1042/BST0321060. [DOI] [PubMed] [Google Scholar]
- 17.Hunt AN, Alb JG, Koster G, Postle AD, Bankaitis VA. Use of mass spectrometry-based lipidomics to probe PITPα (phosphatidylinositol transfer protein α) function inside the nuclei of PITPα+/+ and PITPα−/− cells. Biochem. Soc. Trans. 2004;32:1063–1065. doi: 10.1042/BST0321063. [DOI] [PubMed] [Google Scholar]
- 18.Vertessy BG, Steck TL. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys. J. 1989;55:255–262. doi: 10.1016/S0006-3495(89)82800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altmann SM, Grunberg RG, Lenne PF, Ylanne J, Raae A, Herbert K. Pathways and intermediates in forced unfolding of spectrin repeats. Structure. 2002;10:1085–1096. doi: 10.1016/s0969-2126(02)00808-0. [DOI] [PubMed] [Google Scholar]
- 20.Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V. Primary structure and domain organization of human α and β adducin. J. Cell Biol. 1991;115:665–675. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri T, Ozaki K, Fujiwara T, Shimizu F, Kawai A, Okuno S. Cloning, expression and chromosome mapping of adducin-like 70 (ADDL), a human cDNA highly homologous to human erythrocyte adducin. Cytogenet. Cell Genet. 1996;74:90–95. doi: 10.1159/000134389. [DOI] [PubMed] [Google Scholar]
- 22.Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP. Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J. Cell Biol. 1993;120:411–420. doi: 10.1083/jcb.120.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J. Biol. Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- 24.Devaux PF, Zachowski A, Favre E, Fellmann P, Cribier S, Geldwerth D. Energy-dependent translocation of amino-phospholipids in the erythrocyte membrane. Biochimie. 1986;68:383–393. doi: 10.1016/s0300-9084(86)80005-0. [DOI] [PubMed] [Google Scholar]
- 25.Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC. Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J. Biol. Chem. 1998;273:13681–13692. doi: 10.1074/jbc.273.22.13681. [DOI] [PubMed] [Google Scholar]
- 26.Mohandas N, Shohet SB. The role of membrane-associated enzymes in regulation of erythrocyte shape and deformability. Clin. Haematol. 1981;10:223–237. [PubMed] [Google Scholar]
- 27.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 28.Mohandas N, Morrow JA. In: Hematology: Basic Principles and Practice. Hoffman R, Benz EJ, Shattil SJ, Furie B, editors. NY, USA: Churchill Livingstone; 2000. pp. 40–48. [Google Scholar]
- 29.Gallagher PG, Forget BG, Lux SE. In: Hematology of Infancy and Childhood. Nathan DG, Orkin SH, editors. PA, USA: WB Saunders; 1998. pp. 544–664. [Google Scholar]
- 30.Mohandas N, Winardi R, Knowles D, Leung A, Parra M, George E. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J. Clin. Invest. 1992;89:686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. • Comprehensive recent review on general erythrocyte proteomics.
- 32.Mercer RT, Schneider JW, Benz EJJ, Kaplan JH. In: Red Blood Cell Membranes: Structure, Function, Clinical Implications. Agre P, Parker JC, editors. NY, USA: Dekker; 1989. pp. 135–165.pp. 455–480. [Google Scholar]
- 33.Vincenzi FF. In: Red Blood Cell Membranes: Structure, Function, Clinical Implications. Agre P, Parker JC, editors. NY, USA: Dekker; 1989. pp. 481–505. [Google Scholar]
- 34.Parker JC, Dunham PB, Gunn RB, Froelich O. In: Red Blood Cell Membranes: Structure, Function, Clinical Implications. Agre P, Parker JC, editors. NY, USA: Dekker; 1989. pp. 507–561.pp. 563a–596a.pp. 663–705. [Google Scholar]
- 35.Abu-Hamdah R, Cho WJ, Cho SJ, Jeremic A, Kelly M, Ilie AE. Regulation of the water channel aquaporin-1: isolation and reconstitution of the regulatory complex. Cell Biol. Int. 2004;28:7–17. doi: 10.1016/j.cellbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Agre P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006;3:5–13. doi: 10.1513/pats.200510-109JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roudier N, Verbavatz JM, Maurel C, Ripoche P, Tacnet F. Evidence for the presence of aquaporin-3 in human red blood cells. J. Biol. Chem. 1998;273:8407–8412. doi: 10.1074/jbc.273.14.8407. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Whorton AR. Functional characterization of two S-nitroso-l-cystein transporters, which mediate movement of NO equivalents into vascular cells. Am. J. Physiol. Cell Physiol. 2007;292:C1263–C1271. doi: 10.1152/ajpcell.00382.2006. [DOI] [PubMed] [Google Scholar]
- 39.Fuijse H, Hamada Y, Mori M, Ochiai H. Na-dependent glutamate transport in high K and high glutathione (HK/HG) and high K and low glutathione (HK/LG) dog red blood cells. Biochim. Biophys. Acta. 1995;1239:22–26. doi: 10.1016/0005-2736(95)00140-x. [DOI] [PubMed] [Google Scholar]
- 40.Vince JW, Reithmeier RA. Carbonic anhyrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3-exchanger. J. Biol. Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 41.Mueckler MM, Low AG, Walmsley AR. In: Red Blood Cell Membranes: Structure, Function, Clinical Implications. Agre P, Parker JC, editors. NY, USA: Dekker; 1989. pp. 31–45.pp. 597–633. [Google Scholar]
- 42.Jarvis SM, Thorn JA, Glue P. Ribavirin uptake by human erythrocytes and the involvement of nitrobenzylthrioinosine-sensitive (es)-nucleoside transporters. Br. J. Pharmacol. 1998;123:1587–1592. doi: 10.1038/sj.bjp.0701775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kakhniashvili DG, Griko NB, Bulla LA, Jr, Goodman SR. The proteomics of sickle cell disease: profiling of erythrocyte membrane proteins by 2D-DIGE and tandem mass spectrometry. Exp. Biol. Med. 2005;230:787–792. doi: 10.1177/153537020523001102. •• Compilation of sickle cell disease-altered erythrocyte membrane proteins found by exploratory proteomics.
- 44.Green JB, Fricke B, Chetty MC, von During M, Preston GF, Stewart GW. Eukaryotic and prokaryotic stomatins: the proteolytic link. Blood Cells Mol. Dis. 2004;32:411–422. doi: 10.1016/j.bcmd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Ghatpande SS, Choudhary PK, Quinn CT, Goodman SR. Pharmaco-proteomic study of hydroxyurea-induced modifications in the sickle red blood cell membrane proteome. Exp. Biol. Med. 2008;233:1510–1517. doi: 10.3181/0805-S-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iyamu EW, Fasold H, Roa D, del Pilar Aguinaga M, Asakura T, Turner EA. Hydroxyurea-induced oxidative damage of normal and sickle cell hemoglobins in vitro: amelioration by radical scavengers. J. Clin. Lab. Anal. 2001;15:1–7. doi: 10.1002/1098-2825(2001)15:1<1::aid-jcla1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 47.Cao C, Leng Y, Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J. Biol. Chem. 2003;278:29667–29675. doi: 10.1074/jbc.M301292200. [DOI] [PubMed] [Google Scholar]
- 48.Eskenazi AE, Pinkas J, Whitin JC, Arguello F, Cohen HJ, Frantz CN. Role of antioxidant enzymes in the induction of increased experimental metastasis by hydroxyurea. J. Natl Cancer Inst. 1993;85:711–721. doi: 10.1093/jnci/85.9.711. [DOI] [PubMed] [Google Scholar]
- 49. Ghatpande SS, Choudhary PK, Quinn CT, Goodman SR. In vivo pharmaco-proteomic analysis of hydroxyurea induced changes in the sickle red blood cell membrane proteome. J. Proteomics. 2010;73:619–626. doi: 10.1016/j.jprot.2009.11.003. •• Proteomic profiling by 2D difference gel electrophoresis of red blood cell membranes from five sickle cell patients under hydroxyurea treatment versus five untreated sickle cell patients that most notably revealed an increase of p55 and glycolytic enzymes, GADPH and fructose bisphosphate aldolase with hydroxyurea treatment.
- 50.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou J, Choudhary PK, Goodman SR. Protein profiling of sickle cell versus control RBC core membrane skeletons by ICAT technology and tandem mass spectrometry. Cell. Mol. Biol. Lett. 2006;11:326–337. doi: 10.2478/s11658-006-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perelman N, Selvaraj SK, Batra S, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102:1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 53.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercolloti GM. Activated monocytes in sickle cell disease: potential role in activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- 54. Hryniewicz-Jankowska A, Choudhary PK, Ammann LP, Quinn CT, Goodman SR. Monocyte protein signatures of disease severity in sickle cell anemia. Exp. Biol. Med. 2009;234:210–221. doi: 10.3181/0807-RM-220. •• 2D difference gel electrophoresis proteomic profiling study of monocytes from ten sickle cell patients, in which proteins most strongly correlated with disease severity by surrogate marker of vaso-occlusive crisis rate were identified.
- 55.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl. J. Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 56. Yuditskaya S, Tumblin A, Hoehn GT, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113:1122–1128. doi: 10.1182/blood-2008-03-142604. •• High-throughput exploratory proteomic study of plasma from sickle cell patients with and without pulmonary hypertension (PH) revealed an association between low apolipoprotein (apo)A-I and endothelial dysfunction, as well as low apoA-I:high apoB/A-I ratio, high SAA-4, high plasminogen, high apoA-II and pulmonary hypertension by tricuspid regurgitant jet velocity measurement.
- 57. Odhiambo A, Perlman DH, Huang H, et al. Identification of oxidative post-translational modification of serum albumin in patients with idiopathic pulmonary arterial hypertension and pulmonary hypertension of sickle cell anemia. Rapid Comm. Mass Spect. 2007;21(14):2195–2203. doi: 10.1002/rcm.3074. •• Proteomic study of albumin isolated from plasma of sickle cell patients with and without PH that revealed an increased presence of a malondialdehyde adduct in PH.
- 58. Tumblin A, Tailor A, Hoehn GT, et al. Apolipoprotein A-I and serum amyloid A plasma levels are biomarkers of acute painful episodes in patients with sickle cell disease. Haematologica. 2010;95(9):1467–1472. doi: 10.3324/haematol.2009.018044. Proteomic study using SELDI-TOF MS and clinical immunoassays to profile plasma from 26 adult sickle cell patients at steady state versus acute pain episode that found an increased SAA:apoA-I ratio to be a robust marker of acute pain episode.
- 59.Monnet PD, Kané F, Konan-Waidhet D, Diafouka F, Sangare A, Yapo AE. Lipid, apolipoprotein AI and B levels in Ivorian patients with sickle cell anaemia. Ann. Biol. Clin. (Paris) 1996;54(7):285–288. [PubMed] [Google Scholar]
- 60.Sasaki J, Waterman MR, Cottam GL. Decreased apolipoprotein A-I and B content in plasma of individuals with sickle cell anemia. Clin. Chem. 1986;32:226–227. [PubMed] [Google Scholar]
- 61.Stuart J, Stone PC, Akinola NO, Gallimore JR, Pepys MB. Monitoring the acute phase response to vaso-occlusive crisis in sickle cell disease. J. Clin. Pathol. 1994;47(2):166–169. doi: 10.1136/jcp.47.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singhal A, Doherty JF, Raynes JG, et al. Is there an acute phase response in steady-state sickle cell disease? Lancet. 1993;341(8846):651–653. doi: 10.1016/0140-6736(93)90418-g. [DOI] [PubMed] [Google Scholar]
- 63.Raghavachari N, Xu X, Munson PJ, Gladwin MT. Characterization of whole blood gene expression profiles as a sequel to globin mRNA reduction in patients with sickle cell disease. PLoS ONE. 2009;4(8):E6484. doi: 10.1371/journal.pone.0006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raghavachari N, Xu X, Harris A, et al. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation. 2007;115(12):1551–1562. doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jison ML, Munson PJ, Barb JJ, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104(1):270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreira LS, de Andrade TG, Albuquerque DM, et al. Identification of differentially expressed genes induced by hydroxyurea in reticulocytes from sickle cell anaemia patients. Clin. Exp. Pharmacol. Physiol. 2008;35:651–655. doi: 10.1111/j.1440-1681.2007.04861.x. [DOI] [PubMed] [Google Scholar]
- 67.Gambero S, Canalli AA, Traina F, et al. Therapy with hydroxyurea is associated with reduced adhesion molecule gene and protein expression in sickle red cells with a concomitant reduction in adhesive properties. Eur. J. Haematol. 2006;78:144–151. doi: 10.1111/j.1600-0609.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 68.Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. Br. J. Haematol. 2009;145:455–467. doi: 10.1111/j.1365-2141.2009.07650.x. [DOI] [PubMed] [Google Scholar]
- 69. Goodman SR, Kurdia A, Ammann L, Kakhniashvili D, Daescu O. The human red blood cell proteome and interactome. Exp. Biol. Med. 2007;232:1391–1408. doi: 10.3181/0706-MR-156. • Comprehensive compilation and review of the general erythrocyte proteome as found by exploratory proteomics.
- 70.Ammann LP, Goodman SR. Cluster analysis for the impact of sickle cell disease on the human erythrocyte protein interactome. Exp. Biol. Med. 2009;234:703–711. doi: 10.3181/0806-RM-211. [DOI] [PubMed] [Google Scholar]
- 71.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 72.Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membranes: role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78(10):2753–2758. [PubMed] [Google Scholar]
- 73.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl Acad. Sci. USA. 1988;85(1):237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hebbel RP. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin. Hematol. 1990;27(1):51–69. [PubMed] [Google Scholar]
- 75.Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythocytes. J. Clin. Invest. 1982;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winterbourn CC, Carrell RW. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J. Clin. Invest. 1974;54:678–689. doi: 10.1172/JCI107806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuross SA, Rank BH, Hebbel RP. Heme in sickle erythrocyte inside-out membranes: possible role in thiol oxidation. Blood. 1988;71:876–882. [PubMed] [Google Scholar]
- 78.Rice-Evans C, Omorphos SC, Baysal E. Sickle cell membranes and oxidative damage. J. Biochem. 1986;237(1):265–269. doi: 10.1042/bj2370265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J. Clin. Invest. 2000;103(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho CS, Kato GJ, Yang SH, et al. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid. Redox Signal. 2010;13(1):1–11. doi: 10.1089/ars.2009.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schacter L, Warth JA, Gordon EM, Prasad A, Klein BL. Altered amount and activity of superoxide dismutase in sickle cell anemia. FASEB J. 1988;2(3):237–243. doi: 10.1096/fasebj.2.3.3350236. [DOI] [PubMed] [Google Scholar]
- 82.Natta CL, Chen LC, Chow CK. Selenium and glutathione peroxidase levels in sickle cell anemia. Acta Haematol. 1990;83(3):130–132. doi: 10.1159/000205188. [DOI] [PubMed] [Google Scholar]
- 83.Ren H, Ghebremeskel K, Okpala I, Lee A, Ibegbulam O, Crawford M. Patients with sickle cell disease have reduced blood antioxidant protection. Int. J. Vitam. Nutr. Res. 2008;78(3):139–147. doi: 10.1024/0300-9831.78.3.139. [DOI] [PubMed] [Google Scholar]
- 84.Manfredini V, Lazzaretti LL, Griebeler IH, et al. Blood antioxidant parameters in sickle cell anemia patients in steady state. J. Natl Med. Assoc. 2008;100(8):897–902. doi: 10.1016/s0027-9684(15)31402-4. [DOI] [PubMed] [Google Scholar]
- 85.Das SK, Nair RC. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br. J. Haematol. 1980;44(1):87–92. doi: 10.1111/j.1365-2141.1980.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 86.Chiu D, Lubin B. Abnormal vitamin E and glutathione peroxidase levels in sickle cell anemia: evidence for increased susceptibility to lipid peroxidation in vivo. J. Lab. Clin. Med. 1979;94(4):542–548. [PubMed] [Google Scholar]
- 87.Jain SK, Shokhet SB. A novel phospholipid in irrevesibly sickled cells: evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood. 1984;63(2):362–367. [PubMed] [Google Scholar]
- 88.Platt OS, Falcone JF, Lux SE. Molecular defect in the sickle erythrocyte skeleton. Abnormal spectrin binding to sickle inside-our vesicles. J. Clin. Invest. 1985;75(1):266–271. doi: 10.1172/JCI111684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwartz RS, Rybicki AC, Heath RH, Lubin BH. Protein 4.1 in sickle erythrocytes. Evidence for oxidative damage. J. Biol. Chem. 1987;262(32):15666–15672. [PubMed] [Google Scholar]
- 90.Goodman SR. The irreversibly sickled cell: a perspective. Cell. Mol. Biol. (Noisy-le-grand) 2004;50(1):53–58. [PubMed] [Google Scholar]
- 91.Chang TL, Kakhniashvili DG, Goodman SR. Spectrin’s E2/E3 ubiquitin conjugating/ligating activity is diminished in sickle cells. Am. J. Hematol. 2005;79(2):89–96. doi: 10.1002/ajh.20351. [DOI] [PubMed] [Google Scholar]
- 92.Hsu YJ, Zimmer WE, Goodman SR. Erythrocyte spectin’s chimeric E2/E3 ubiquitin conjugating/ligating activity. Cell. Mol. Biol. (Noisy-le-grand) 2005;51(2):187–193. [PubMed] [Google Scholar]
- 93.Ghatpande SS, Goodman SR. Ubiquitination of spectrin regulates the erythrocyte spectrin-protein-4.1-actin ternary complex dissociation: implications for the sickle cell membrane skeleton. Cell. Mol. Biol. (Noisy-le-grand) 2004;50(1):67–74. [PubMed] [Google Scholar]
- 94.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J. Proteome Res. 2007;6(8):3242–3255. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 95.Willison KR, Horwich AI. Structure and function of chaperonins in archaebacteria and eucaryotic cytosol. In: Ellis RJ, editor. The Chaperonins. Academic Press, CA: USA; 1996. p. 107. [Google Scholar]
- 96.Kaluz S, Kaluzova M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin. Chim. Acta. 2008;395:6–13. doi: 10.1016/j.cca.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell. Mol. Life Sci. 2003;60:1376–1393. doi: 10.1007/s00018-003-2370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr. Res. 2001;49(5):614–617. doi: 10.1203/00006450-200105000-00002. • Excellent review of hypoxia-inducible factor-1 effects.
- 99. Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17(8):394–402. doi: 10.1016/j.tcb.2007.06.005. • Good review on stomatin prohibitin flotillin Hflk/c proteins, including stomatin and flotillin, and their role in lipid rafts.
- 100.Dhungana S, Merrick BA, Tomer KB, Fessler MB. Quantitative proteomics analysis of macrophage rafts reveals compartmentalized activation of the proteasome and of proteasome-mediated ERK activation in response to lipopolysaccharide. Mol. Cell. Proteomics. 2009;8(1):201–213. doi: 10.1074/mcp.M800286-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 102.VanderJagt DJ, Shores J, Okorodudu A, Okolo SN, Glew RH. Hypocholesterolemia in Nigerian children with sickle cell disease. J. Trop. Pediatr. 2002;48:156–161. doi: 10.1093/tropej/48.3.156. [DOI] [PubMed] [Google Scholar]
- 103.Sasaki J, Waterman MR, Buchanan GR, Cottam GL. Plasma and erythrocyte lipids in sickle cell anaemia. Clin. Lab. Haematol. 1983;5:35–44. doi: 10.1111/j.1365-2257.1983.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 104.Rahimi Z, Merat A, Haghshenass M, Madai H, Rezaei M, Nagel RL. Plasma lipids in Iranians with sickle cell disease: hypocholesterolemia in sickle cell anemia and increase of HDL-cholesterol in sickle cell trait. Clin. Chim. Acta. 2006;365:217–220. doi: 10.1016/j.cca.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Westerman MP. Hypocholesterolaemia and anaemia. Br. J. Haematol. 1975;31(1):87–94. doi: 10.1111/j.1365-2141.1975.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 106. Tabet F, Rye K-A. High-density lipoproteins, inflammation and oxidative stress. Clin. Sci. 2009;116:87–98. doi: 10.1042/CS20080106. • Good review of high-density lipoprotein and the mechanisms behind its anti-inflammatory and antioxidant effects; covers how high-density lipoprotein can be rendered proinflammatory.
- 107.Lowenstein CJ, Cameron SJ. High-density lipoprotein metabolism and endothelial function. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17(2):166–170. doi: 10.1097/MED.0b013e32833727ee. [DOI] [PubMed] [Google Scholar]
- 108.Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein A-I increase endothelial NO synthase activity by protein association and multisite phsophorylation. PNAS. 2004;101(18):6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribas V, Sánchez-Quesada JL, Antón R, et al. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties. Circ. Res. 2004;95:789–797. doi: 10.1161/01.RES.0000146031.94850.5f. [DOI] [PubMed] [Google Scholar]
- 110.Cabana VG, Reardon CA, Feng N, Neath S, Lukens J, Getz GS. Serum paraoxonase: effect of the apolipoprotein composition of HDL and the acute phase response. J. Lipid Res. 2003;44:780–792. doi: 10.1194/jlr.M200432-JLR200. [DOI] [PubMed] [Google Scholar]
- 111.Klimov AN, Kozhevnikova KA, Kuzmin AA, Kuznetsov AS, Belova EV. On the ability of high density lipoproteins to remove phospholipid peroxidation products from erythrocyte membranes. Biochemistry (Mosc.) 2001;66(3):300–304. doi: 10.1023/a:1010203930470. [DOI] [PubMed] [Google Scholar]