Abstract
Background:
Soil transmitted helminths (STH) remain a global public health concern in spite of occasional dosing campaigns.
Aims:
To determine baseline prevalence and intensity of STH infection in east Guatemalan school children, and describe the associated epidemiology of anemia, stunting, and wasting in this population. Setting and design: Ten schools in Izabal province (eastern Guatemala) were identified, and 1,001 school children were selected for this study. Half of the schools were used as clinical testing sites (blood and stool).
Materials and Methods:
Anthropometric measures were collected from all children. Over 300 children were tested for anemia and 229 for helminth infection. Ova and parasite specimens were examined via Direct, Kato Katz, and McMaster techniques. Hemoglobin was measured from venipuncture following the hemacue system. Statistical analysis: Correlation between infection intensities and growth indicators were examined. Chi Square or t tests were used for bivariate analysis. Multiple logistic regression was performed on significant variables from bivariate techniques.
Results:
Over two-thirds of school children were positive for infection by any STH. Prevalence of Hookworm was 30%; Ascaris, 52%; and Trichuris, 39%, most as low-intensity infection. Over half of the children were co-infected. In bivariate analysis, anemia was significantly associated with polyparasitism.
Conclusions:
For a Guatemalan child who experiences a unit decrease in hemoglobin, one expects to see a 24% increase in the odds of being infected with STH, controlling for age, sex, lake proximity, and growth characteristics. Infection with more than one STH, despite low intensity, led to a significant decrease in hemoglobin.
Keywords: Anemia, Guatemala, Helminth, Polyparasitism, School children
INTRODUCTION
Soil transmitted helminths (STH) remain a widespread and neglected public health concern, particularly in tropical rural areas throughout the world.[1–3] Some consequences of STH infection in children include stunting, wasting, cognitive deficits, apathy, reduced levels of physical activity and social interaction, and a diminished perception of security.[4]
STH infections account for nearly 40% of all tropical diseases in children, excluding malaria.[5] Two recent reports estimated Hookworm prevalence in Latin America at 8%-10%, ascariasis at 16%-18%, and trichuriasis at 16%-19%.[2,6] In Guatemala alone, 8 million individuals are estimated to be infected with Hookworm, 8.6 million with Ascaris, and 7.9 million with Trichuris, a remarkably high number since Guatemala is a relatively small country of less than 14 million individuals.[2,7] Guatemala has the highest case load of ascariasis and trichuriasis in all of Latin America. Hookworm is particularly insidious and more devastating in disability-adjusted life years than dengue fever, Chagas disease, schistosomiasis, or leprosy together.[1] A major effect of Hookworm infection in children is iron-deficiency anemia. This leads to an increased risk for learning disabilities, and infected children are nearly four times more likely to be stunted.[3,5]
Less dramatic and often overlooked are low-intensity infections from various STH species. Ezeamama and colleagues demonstrated that low-intensity polyparasitism can be just as adverse to health outcomes as high-intensity infection with a single helminth.[8] In endemic areas, low-intensity infections are more common than moderate or high-intensity infections.[8]
The aim of this study was to establish prevalence and intensity of STH in East Guatemalan school children, to report their baseline levels of hemoglobin, height for age Z scores (HAZ), weight for height Z scores (WHZ), and to describe the epidemiology of anemia, stunting, and wasting in this population.
MATERIALS AND METHODS
This study was conducted in the eastern tropics of Guatemala, within the northwest quadrant of the Department of Izabal. Recent reports place the proportion of the population living in poverty at 56%-75%.[7,9] Within Department of Izabal, the province (municipalidad) of El Estor lies on the northwestern edge of the largest freshwater lake in the country (Lago Izabal). The province has a population of 43,000, 85% of whom are identified as Q’eqchi of Mayan origin.[10] The local economy is primarily agriculture-based, and municipal leaders classify this region as isolated. There are 89 registered primary and secondary schools in the municipality of El Estor, serving 8,500 children.[10]
Study participants were selected using a nested approach by first selecting schools, and then students within schools. Schools were stratified according to semi-urban/rural status. Among the criteria for semi-urban school selection was suspected high parasite prevalence, whether the neighbourhood housed a public clinic (for primary healthcare needs), and low economic status. The local director of the Ministry Of Health (MOH), in consultation with local Department Of Education (DOE), generated this list of schools. Among the criteria for rural school section was ease of transportation to and from the province capital for clinical laboratory testing. Schools were categorised by proximity to the lake shore to investigate possible associations between STH infestation and soil type. Previous research indicated that certain STHs are more likely to thrive in sandier, moist soils than in other soil types.[1,11,12] Lake proximity was defined as 500 m or less from Lago Izabal. A total of 26 schools were originally identified. Finally, 10 schools were selected, half of which were used as clinical testing sites (for blood and stool analysis).
Students within selected schools were chosen by a systematic random strategy using complete student enrollment lists generated from each school by respective school directors. In five schools, approximately 70 students per school were selected for clinical testing and anthropomorphic measurement. Another 50-70 students per school, from the remaining five schools, were chosen for anthropometric measurement only.
The institutional review board of the lead academic institution approved the study. On-site consent and permission proceeded in the following order: DOE and MOH representatives granted access to the schools, and then approval for the research study was obtained by each school director. Afterwards, consent forms were distributed to parents via school administrators along with household surveys. Consent was collected and witnessed by school administrators at subsequent times. After obtaining parental consent, student assent was requested and noted. After completing data collection, all students, teachers, and administrators were given albendazole (400 mg tablet). Baseline data were collected during the spring of 2006.
A survey was given to parents before clinical testing began. The survey included questions about household characteristics including water source, latrine/bathroom and floor conditions, number of household children, etc. within each household. These responses were matched to children enrolled in the study. A total of 196 surveys were returned and processed (estimated response rate = 20%).
Weight and height measures were collected using a portable stadiometer and a digital weight scale accurate to 0.2 kg (QuickMedical, Snoqualmie, WA). Stunting was defined as low height for age of less than 2 Z scores on a stature reference population. Wasting was defined as weight for height, also less than 2 Z scores, on a reference population.[3,13] Hemoglobin was measured from 2-ml sample of whole blood obtained by venipuncture, and collected in EDTA lavender-top vials. Hemoglobin was measured using the hemacue system (with portable battery-operated photometer and disposable microcuvetts; Hemocue Inc, Mission Viejo, CA). Anemia was defined as hemoglobin level ≤ 11 g/dL.
Ova and Parasite stool kits were supplied by a Nongovernmental Organisation (NGO) that operates in adjacent Guatemalan provinces. Both the NGO and an academic institution contributed to supplying laboratory technicians for this research team. All stool samples were analysed at least twice using the Direct, Kato Katz,[3,14] and McMaster techniques. The Kato Katz and McMaster methods furthermore provided a measure of infection intensity in eggs per gram of faeces (epa). Only Kato Katz intensities are reported here. Intensity thresholds were defined by the World Health Organization (WHO).[15]
This study utilised a standard sample size calculation for controlled trials,[16] whereby alpha = 0.05 and beta = 0.80, based on a median Hookworm prevalence of 30%[6] for the control group. Furthermore, we assumed a conservative 34% attrition rate due to absenteeism.[17] Therefore, 355 students were originally sought for clinical analysis. Inclusion criteria included female and male students in the age group 5-17 years. A total of 1,001 students were finally enrolled in this study. The mean age was 9.2 years and 52.8% were female. Other sample characteristics are shown in Table 1.
Table 1.
Characteristics of sample school children (El Estor, n=1,001)

Bivariate Chi Square or t test statistics were accomplished using baseline data to assess associations between demographic, community, household, or maternal health indicators and infection status. Pearson correlations between infection intensities and growth indicators were examined. Multiple logistic regression identified significant factors, controlling for demographic or growth variables, from the bivariate tests. Analysis was performed with the SPSS software (v.15; SPSS Inc., Chicago, IL).
RESULTS
Sex and age group proportions were evenly distributed. Stunting was identified in 16% of female children and 18% of male children, according to CDC standards.[18] About half of the children were severely underweight [Table 1]. Table 2 shows the breakdown of household and maternal characteristics, highlighting socio-economic conditions in the region. Of the parents who reported an ill child within the last week, 61.6% mentioned that the child had febrile-like symptoms, 45.9% reported nausea/vomiting, and 17.0% mentioned diarrhoea. Only 2.5% of the parents mentioned languid or anemia-like symptoms in their ill children.
Table 2.
Household characteristics (El Estor, n = 196)
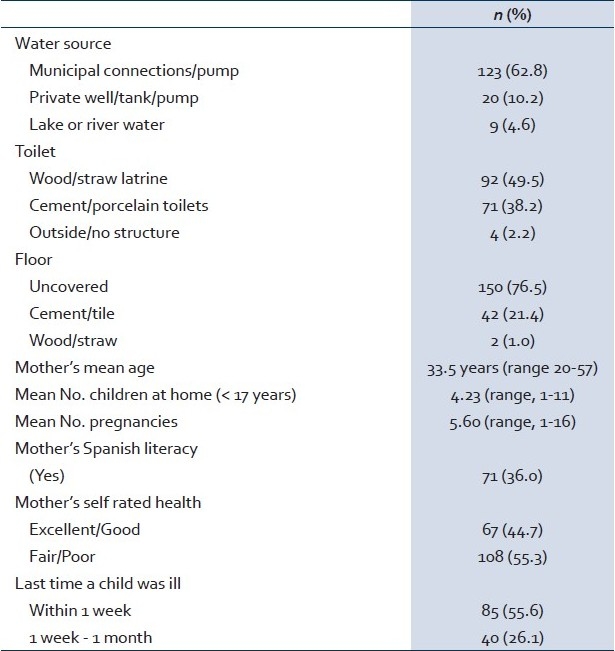
A total of 229 stool samples (one per child) were examined for the presence of helminth eggs. The mean age of children producing stool samples was 9.2 years, which is the same mean age as of the entire sample. However, although 47.2% of the originally selected children were male, 52.7% of the stool specimens came from males. Of the children examined for STH infection, over two-thirds (68.6%) were STH-positive detected by any method: 30.1% were positive for Hookworm (mean intensity = 657.5 ± 1,075 epg); 51.5% were positive for Ascaris (mean intensity = 8,228.9 ± 15,478.3 epg); and 38.9% were positive for Trichuris (mean intensity = 624 ± 3,209.2 epg). About 2% of Hookworm infections were heavy, as were 4% of the Ascaris infections and 1% of the Trichuris infections [Figure 1]. Of the children who were infected, the majority (56.1%) were polyparasite infected.
Figure 1.
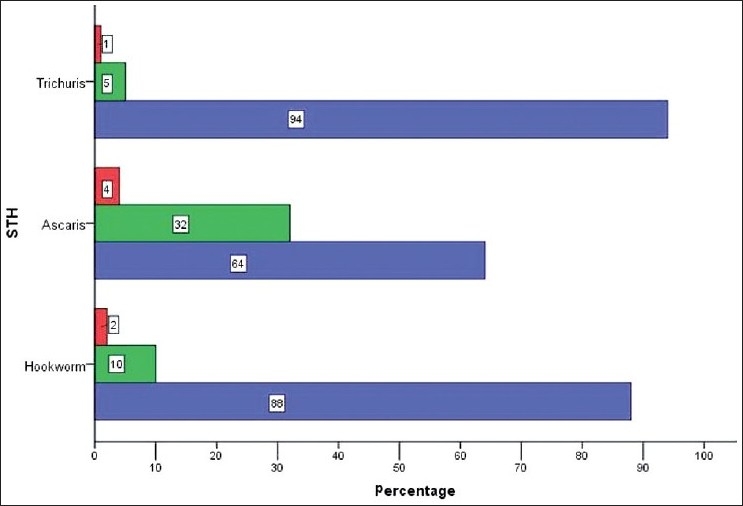
Intensities of STH by Kato Katz Analysis; Legend; Red - heavy; Green - moderate; Blue - light
Of the children testing positive for intestinal helminths, more males than females were infected (54.1% vs. 45.9%). The proportion of younger children was higher than older ones (54.1% at <10 years vs. 45.9% at 10+ years), and more lake-proximate children were infected than those attending school further away from the lake (70.9% vs. 64.0%). However, none of these differences were significant. The overall mean Hemoglobin level was 13.1 g/dL (range, 7.0-16.6), with males having statistically marginally higher levels (P=0.10; t test). Infection status demonstrated a significant difference in hemoglobin measures (13.0 g/dL infected vs. 13.4 g/dL not infected; Table 3). Anemia (blood hemoglobin level ≤11 g/dL) was present in 5.1% of the children and was significantly associated with polyparasitism in bivariate analysis.
Table 3.
Bivariate factors and P values associated with STH (infection status Y/N)
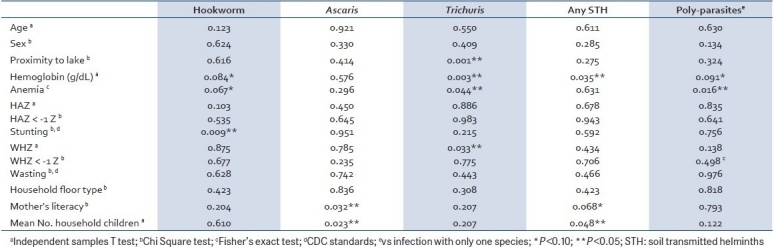
Correlations [Table 4] between STH intensities, hemoglobin, and growth indicators showed four significant relationships. First, a negative correlation was seen between Hookworm intensity and hemoglobin. On further inspection, this relationship persisted only with female and not with male children (data not shown). Next, hemoglobin held a positive relationship with body bulk (weight for height), but a negative relationship with height for age. On controlling for sex, the inverse relationship between hemoglobin and height for age was significant only in males (data not shown). Finally, there was a negative relationship between the growth indices, ie, body bulk decreased with increasing height in both sexes. Interestingly, STH intensities did not correlate with each other.
Table 4.
Correlation among STH intensities, age/growth indicators, and hemoglobin
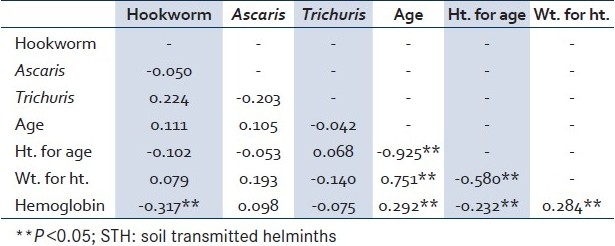
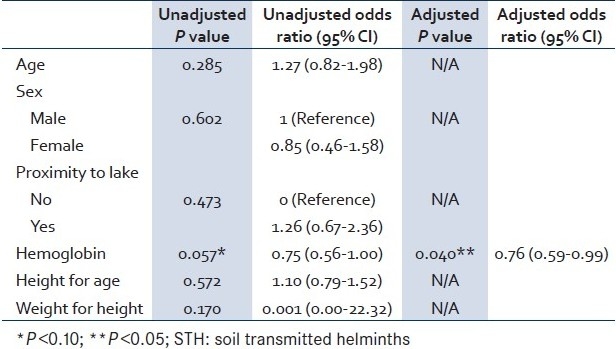
In logistic regression (with any STH infection), household characteristics from Table 3 were dropped from the model due to the low number of responses, and the remaining significant, bivariate factors were used [Table 5]. Only the hemoglobin level emerged significant after controlling for all other factors. For every one unit decrease in hemoglobin in Guatemalan children from this region, we would expect to see a 24% increase in the odds of being infected with any STH, controlling for age, sex, lake proximity, and growth characteristics.
Table 5.
Multiple logistic regression factors associated with any STH infection
DISCUSSION
We examined anthropometric, clinical, and household measures of children, age group 5-17 years, in the eastern district of Izabal, Guatemala. Our results confirm other studies showing a moderate prevalence of helminths in this region. We found the prevalence of Hookworm to be 30% in children, as well as a 52% prevalence of Ascaris and 39% prevalence of Trichuris. Over half of the children tested were polyparasite infected. In bivariate analysis, polyparasitism (infection with more than one STH vs. infection with only one STH) was significantly associated with anemia. However, stunting, wasting, or anemia did not persist in multivariate techniques even though our analysis revealed a significant drop in hemoglobin levels. As such, low to moderate intensity co-infection can be just as precarious as high-intensity infection with only one STH.[8]
Here, we report similar STH prevalence regarding comparisons with other studies done in Central America. Proximal with our results, a prevalence of 45% for Ascaris and 38% for Trichuris was found in neighbouring Honduras.[19] One recent Guatemalan study reported a 33% Ascaris prevalence in school children.[9] With regard to intensity, another Guatemalan study tested children aged 7-12 and reported that 25% of the children had heavy Ascaris infestations and more than half had moderate infestations.[20] Our study, on the contrary, showed relatively light STH infestation with all three helminths. The lighter intensities in El Estor children may be explained by any one of three factors, or a combination thereof. First, the semi-urban nature of El Estor may permit harsher environments in which STH struggle to thrive. Second, The Guatemalan MOH maintains a series of public clinics in the District, which occasionally dispense anti-helminth medication. This governmental intervention may have steadily eroded the severity of infections in this area. Third, general improvements in socio-economic status in El Estor induce better health outcomes in general that obfuscate STH morbidity.
In regards to stunting and wasting, this study runs contrary to other reports from Guatemala, which reveal a high prevalence of stunting in children in the range 26%-56%.[2,21,22] We show an 18% stunting prevalence in boys and 16% in girls. On the other hand, our data confirm with those by Groeneveld et al, in that, stunting increased with increasing age. Our study demonstrates that Guatemalan children are more likely to be thinner rather than shorter. This observation highlights the notion that these children have received, or are receiving, beneficial breaks from the chronic malnutrition cycle during their childhood. If STH are causal agents in malnutrition, the breaks may be short-lived because of STH re-infection. Watkins et al, report that STH re-infestation in Guatemala is high and, though treatment shows an immediate effect in egg depletion in the hosts of Ascaris and Trichuris, after six months their programme had no significant effect on STH prevalence or in growth gain in children.[20]
We confirm the findings in previous reports that Hookworm is associated with anemia and stunting.[3,5,23] Hookworm intensity negatively correlated with hemoglobin measures as well. An accepted physiological model is that a decrease in hemoglobin is due to iron depletion,[24,25] which mediates between infection and poor growth. Furthermore, our study confirms[19] as well as contradicts[24] the findings of previous research that Trichuris associates with deleterious health outcomes (decreased hemoglobin and anemia), though we did not observe an association with stunting. Interestingly, there was no correlation between Hookworm and Trichuris intensities (nor with Ascaris). Moreover, except for Hookworm, helminth intensity did not correlate with body growth measures or with age. A second physiological model advances the role of inflammation in anemia, rather than of iron depletion.[26,27] If this is true then co-infection should amplify hemoglobin loss, as was also evident from our bivariate analysis.
Our data suggest that household conditions are important factors in the epidemiology of STH infection. We confirm two studies demonstrating that mothers′ education had an effect on their children's infection status, albeit the effect only emerged with Ascaris infection here. Both reports allude to hygiene as the mediator.[19,28] Likewise, we demonstrate that the number of children in the household was positively associated with Ascaris infection in bivariate analysis. Similarly, other researchers found significant characteristics with the number of household children.[19,23] Finally, the association between Trichuris infection and lake proximity in our study is interesting. Some research delineates changes in Hookworm prevalence according to soil type.[11,23] Another research team, in Venezuela, linked Trichuris prevalence to soil type.[12] We assumed that inhabitants closer to Lago Isabal are in frequent contact with wet and sandy soil, which could partially explain the higher risk for Trichuris infection. Future studies should be carried out to confirm this.
This study presents possible threats to internal validity since schools were not randomly selected. However, students within schools were randomised. The response rate from the household survey was low, contributing to lack of matches with children's clinical data. The low response rate from our household survey may contribute to Type I error in bivariate analysis. Consequently, household variables were removed from the multiple regression model. Therefore, it is likely that we under-report the impact of the social environment on STH infection; moreover, household measures should be considered in future studies dealing with STH.[23,26] With regard to laboratory techniques, under-reporting of ova counts is possible,[29] but our investigation validated counts by using different testing techniques.
Selection bias may be present due to selection of only participants enrolled in school. With one-third of the population in the age group 5-19 years in El Estor, it is estimated that 4,000 children in this age group do not attend school at all. Our study did not find significant differences in infection status by sex. More females than males miss school;[10] however, indicating a challenge in reaching girls through a school-based approach. Still, a school-based program is recommended as an easy delivery platform and should be considered as a cornerstone to launch programs to non-enrolled children as well as infants.[3,17]
CONCLUSION
Our study confirms a moderate prevalence of helminth infection in school-aged children in eastern Guatemala. A majority of children are infected with at least two species of intestinal parasite. These data support both iron depletion and inflammation-derived anemia models. In general, though our sample revealed low infection intensities, the co-morbidity of two or more infections may be detrimental to health outcomes much like high-intensity infection with one STH.[8] Long-term treatment programmes are needed to have any effect.[20] Albendazole is safe, effective, inexpensive, and the 400-mg dose is safe for children as young as 2 years. The WHO recommends dosing with albendazole three times a year in countries with STH prevalence of 50% or above.[3,30] In areas where STH prevalence is below 50%, dosage frequency may be decreased but treatment efforts should be sustained and monitored with periodic community surveys coupled with clinical feedback.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: A review of disease burden and distribution and roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:1–11. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva: WHO; 2005. Deworming for health and development: Report of the third global meeting of the partners for parasite control. [Google Scholar]
- 4.Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: Risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–57. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 5.Cappello M. Global health impact of soil-transmitted nematodes. Pediatr Infect Dis J. 2004;23:663–4. doi: 10.1097/01.inf.0000132228.00778.e4. [DOI] [PubMed] [Google Scholar]
- 6.de Silva N, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminthic infections: Updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Central Intelligence Agency. Field listing, population below poverty line. The World Factbook; TravLang. [Last cited on 2009 May 18]. Available from: http://www.travlang.com/factbook/fields/2046.html.
- 8.Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, et al. Functional significance of low-intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192:2160–70. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- 9.Cook DM, Swanson RC, Eggett DL, Booth GM. A retrospective analysis of prevalence of gastrointestinal parasites among school children in the Palajunoj Valley of Guatemala. J Health Popul Nutr. 2009;27:31–40. doi: 10.3329/jhpn.v27i1.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Municipalidad de El Estor. Municipal Diagnostics: El Estor, Izabal State, Guatemala. Diagnostico municipal. El Estor, Departamento Izabal. 2004. Guatemala, Oficina Municipal de Planificación, 2004.
- 11.Lilley B, Lammie P, Dickerson J, Eberhard M. An increase in Hookworm infection temporally associated with ecologic change. Emerg Infect Dis. 1997;3:391–3. doi: 10.3201/eid0303.970321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado AI, Rivero-Rodríguez Z, Chourio-Lozano G, Días IA, Calchi-La C, Marinella EA, et al. Prevalence of intestinal parasites and associated environmental factors in two indigenous communities in Zulia state.Prevalencia de enteroparásitos y factores ambientales asociados en dos comunidades indígenas del estado Zulia. Kasmera. 2008;36:53–66. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Growth indicators. [Last cited 2007 Jun 17]. Available from: http://www.cdc.gov/pednss/what_is/pednss_health_indicators.htm#growth .
- 14.Idris MA, Al-Jabri AM. Usefulness of Kato-Katz and trichrome staining as diagnositc methods for parasitic infections in clinical laboratories. Squ J Scient Res Med Sci. 1999;3:65–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Montresor A, Crompton DW, Bundy DA, Hall A, Savioli L. Geneva: WHO; 1998. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. [Google Scholar]
- 16.Greenberg RS, Daniels SR, Fanders D, Eley JW, Boring JR. Medical Epidemiology Norwalk, CN: Appleton and Lange. 1993-2000 [Google Scholar]
- 17.Olsen A. Experience with school-based interventions against soil-transmitted helminthes and extension of coverage to non-enrolled children. Acta Trop. 2003;86:255–66. doi: 10.1016/s0001-706x(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 18.NCHS National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention. [Last cited 2007 Mar 20]. Available from: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/zscore/zscore.htm .
- 19.Smith HM, DeKaminsky RG, Niwas S, Soto RJ, Jolly PE. Prevalence and intensity of infections of Ascaris lumbricoides and Trichuris trichiura and associated socio-demographic variables in four rural Honduran communities. Mem Inst Oswaldo Cruz. 2001;96:303–14. doi: 10.1590/s0074-02762001000300004. [DOI] [PubMed] [Google Scholar]
- 20.Watkins WE, Cruz JR, Pollitt E. The effects of deworming on indicators of school performance in Guatemala. Trans R Soc Trop Med Hyg. 1996;90:156–61. doi: 10.1016/s0035-9203(96)90121-2. [DOI] [PubMed] [Google Scholar]
- 21.Martorell R, Kettel Khan L, Hughes ML, Grummer-Strawn LM. Obesity in Latin American women and children. J Nutr. 1998;128:1464–73. doi: 10.1093/jn/128.9.1464. [DOI] [PubMed] [Google Scholar]
- 22.Groeneveld IF, Solomons NW, Doak CM. Nutritional status of urban schoolchildren of high and low socioeconomic status in Quetzaltenango, Guatemala. Rev Panam Salud Publica. 2007;22:169–77. doi: 10.1590/s1020-49892007000800003. [DOI] [PubMed] [Google Scholar]
- 23.Olsen A, Samuelsen H, Onyango-Ouma W. A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches. J Biosoc Sci. 2001;33:569–84. doi: 10.1017/s0021932001005697. [DOI] [PubMed] [Google Scholar]
- 24.Stoltzfus RJ, Albonico M, Chwaya HM, Savioli L, Tielsch J, Schulze K, et al. Hemoquant determination of Hookworm-related blood loss and its role in iron deficiency in African children. Am J Trop Med Hyg. 1996;55:399–404. doi: 10.4269/ajtmh.1996.55.399. [DOI] [PubMed] [Google Scholar]
- 25.Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anemia in Zanzibari schoolschildren: The importance of Hookworms. Am J Clin Nutr. 1997;65:153–9. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JF, Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Wu H, et al. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. Am J Trop Med Hyg. 2005;72:527–33. [PubMed] [Google Scholar]
- 27.Olson CL, Acosta LP, Hochberg NS, Olveda RM, Jiz M, McGarvey ST, et al. Anemia of inflammation is related to cognitive impairment among children in Leyte, the Philippines. PLoS Negl Trop Dis. 2009;3:e533. doi: 10.1371/journal.pntd.0000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, Morales G, et al. Role of the employment status and education of Mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:225. doi: 10.1186/1471-2458-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raso G, Holmes E, Singer BH, N’Goran EK, Utzinger J. Polyparasitism, authors response.[Letter to the Editor] Int J Epidemiol. 2005;34:222–3. [Google Scholar]
- 30.The use and interpretation of anthropometry. World Health Organization. [Last cited on 2007 Jun 15]. Available from: http://www.who.int/childgrowth/faqs/beyond/en/index.html.


