Abstract
Introduction:
Candida species are normal inhabitants of the skin and mucosa. The importance of epidemiological monitoring of yeasts involved in pathogenic processes is unquestionable due to the increase of these infections over the last decade;
Materials and Methods:
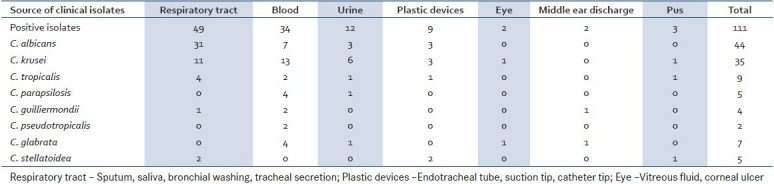
The clinical samples from the respiratory tract (sputum, bronchial wash, tracheal secretions), saliva, blood, urine, middle ear discharge, vitreous fluid, corneal ulcer, and plastic devices (endotracheal tube, catheter tip, suction tip) were collected and cultured. The species of Candida isolated were identified.
Results:
A total of 111 isolates of Candida species were recovered from 250 diverse clinical sources. C. albicans (39.64%) was the most isolated species, although the Candida non albicans species with 60.36% showed the major prevalence. In blood cultures, C. krusei (38.23%) and C. albicans (20.58%) were isolated frequently. C. albicans (63.27%) was the predominant species in mucosal surface. Urinary tract infections caused by yeasts were more frequent in hospitalized patients, C. krusei (50.0%) being commonly isolated, followed by C. albicans (25.0%).
Discussion:
Several virulence factors like, biofilm, proteinase, phospholipase, etc. contribute to the pathogenecity. Early detection of virulence factors by Candida is useful in clinical decision making. We therefore have aimed at demonstrating the formation of biofilm using the method proposed by Branchini et al, (1994). The proteinase produced by Candida was estimated as per the method of Staib et al, (1965). Phospholipase assay was carried out as per the method of Samaranayake et al, (2005).
Conclusions:
The data suggests that the capacity of Candida species to produce biofilm may be a reflection of the pathogenic potential of the isolates. C. krusei and C. tropicalis showed strong slime production. The non-Candida albicans produced more proteinase than C. albicans. C. albicans produced higher levels of phospholipase than non Candida albicans in this study.
Keywords: Biofilm, Candidiasis, Clinical samples, Phospholipase, Proteinase, Slime
INTRODUCTION
Candida species are normal inhabitants of the skin and mucosa. The importance of epidemiological monitoring of yeasts involved in pathogenic processes is unquestionable due to the increase of these infections over the last decade; so are the changes observed in species causing candidiasis and empirical antifungal treatment.[1]
Although C. albicans is the organism most often associated with serious fungal infection, other Candida species also have emerged as clinically important opportunistic pathogens.
Most pathogens, including Candida species, havedeveloped an effective battery of putative virulence factorsand specific strategies to assist in colonization, invasion, and pathogenesis. Thevirulence factors expressed by Candida species, to cause infections may vary depending on the type of infection,the site and stage of infection, and the nature of the host response. The main virulence factors are biofilm formation, production of acid proteinase, phospholipase, etc. Once the contact is made, enzymes facilitate adherence by damaging or degrading cell membranes and extracellular proteins thus permitting the yeast to enter the host, whereas phenotypic switching or coating with platelets may be used to evade the immune system.
Biofilms are the structured microbial communities that are attached and encased in a matrix of exopolymeric material,[2] and are important for the development of clinical infection. The most external layers of Candida cells are essential for the adherence to host surface, thereby playing a pivotal role in the pathophysiology of candidiasis.[3] The advantages of forming a biofilm include protection from the environment, nutrient availability, metabolic cooperation, and acquisition of new genetic traits.
Aspartyl proteinases are secreted by pathogenic species of Candida in vivo during infection.[4] Theenzymes are secreted in vitro when the organism is cultured inthe presence of exogenous protein (usually bovine serum albumin)as the nitrogen source. Proteinase production is believed to enhance the ability of the organism to colonize and penetrate host tissues and to evadethe host immune system.[5]
Phospholipase enzymes are associated with membrane damage of the host cells, adherence, and penetration. Invasion of host cells by microbes entails penetration and damage of the outer cell envelope. Early data suggestthat direct host cell damage and lysis are the main mechanismscontributing to fungal virulence.
MATERIALS AND METHODS
A total of 250 different clinical samples were collected from patients being treated in hospitals and nursing homes in and around Bangalore. The patients had no history of antifungal drug exposure prior to collection. The samples collected include 102 from respiratory tract (sputum, bronchial wash, tracheal secretion) and saliva, 120 from blood, 12 from urine, 2 from middle ear discharge, 1 from vitreous fluid, 1 from corneal ulcer, and 9 samples from plastic devices (endotracheal tube, catheter tip, suction tip).
All the respiratory specimens and exudates were examined in 10% KOH. In addition, the smears were gram stained and examined. The samples were inoculated on to Sabouraud's dextrose agar as the main isolation medium. For blood samples, SDA biphasic medium with chloramphenicol and gentamicin was used. The culture medium was incubated at 37°C for a week or longer if required.
The identification of the species was conducted by assessing the germ tube formation, pellicle formation, assimilation, fermentation of sugars. They were cultured on cornmeal agar for demonstration of chlamydospores. Culture on candid chrom agar was used for identification of the species.
Biofilm formation
Biofilm formation was determined for all the isolates and the standard strains by using a method proposed by Branchini et al.[6] A loopful of organisms from the SDA plate was inoculated into a tube containing 10 ml sabouraud's liquid medium supplemented with glucose (final concentration of 8%). The tubes were incubated at 37°C for 24 h after which the broth was aspirated out and the walls of the tubes were stained with safranin. Biofilm formation was scored as negative (0+), weak positive (1+), moderate positive (2+), or strong positive (3+).
Proteinase detection
The Candida proteinase was detected by the slightly modified Staib et al, method[7] using Bovine serum albumin medium (dextrose 2%, KH2 PO4 0.1%, MgSO4 0.05%, agar 2% mixed after cooling to 50°C with 1% bovine serum albumin solution). Proteinase activity was detected by inoculating 10 μl aliquots of the yeast suspension (approximately 108 yeast cells /ml) into the wells punched onto the surface ofthe medium. The plates were incubated at 37°C for 2 days. After incubation, the plates were fixed with 20% trichloracetic acid and stained with 1.25% amidoblack. Decolourisation was performed with 15% acetic acid. Opaqueness of the agar, corresponding to a zone of proteolysis around the wells that could not be stained with amidoblack indicated degradation of the protein. The diameter of unstained zones around the well was considered as a measure of proteinase production. The proteinase activity (Pz) was determined in terms of the ratio of the diameter of the well to the diameter of the proteolytic unstained zone. When Pz = 1, no proteinase activity was detected in the strain. Thus, low Pz means high production of the enzyme.
Phospholipase estimation
Slightly modified method of Samaranayake et al,[8] was used to estimate phospholipase.The egg yolk medium used consisted of 13.0 g sabouraud dextrose agar (SDA), 11.7 g NaCl, 0.111 g CaCl2, and 10% sterile egg yolk.The egg yolk was centrifuged at 500 g for 10 min at room temperature,and 20 ml of the supernatant was added to the sterilized medium.Extracellular phospholipase activity was detected by inoculating 10 μl aliquots of the yeast suspension (approximately 108 yeast cells /ml) into the wells punched onto the surface ofthe egg yolk medium. The diameter of the precipitation zone around the well was measured after incubation at 37°C for 48 h. Phospholipase activity(Pz value) was determined. When Pz = 1, no phospholipase activity was detected in the strain. Thus, Low Pz means high production of the enzyme.
RESULTS
The species spectrum of the isolate was as follows, of the 111 isolates 49 were C. albicans, 7 C. glabrata, 4 C. guilliermondi, 2 C. kefyr, 35 C. krusei, 5 C. parapsilosis, and 9 C. tropicalis. Candida species distributions in different clinical samples are shown in Table 1.
Table 1.
Candida species isolated from different clinical samples
A total of 81 (73%) out of 111 Candida species isolates obtainedfrom the clinical isolates produced biofilm. Only 51% (25 of 49) of C. albicans isolates produced biofilm, which was significantly lower than the percentageof all non albicans Candida species isolates producing slime (90.32%, 56 of 62; P<0.0001). Strong biofilm production was seen in C. krusei and C. tropicalis. Weak biofilm production was seen in C. albicans.
Proteinase activity was detected in 89 (80.18%) isolates. Highest proteinase producers were C. kefyr (Pz 0.16), C. guilliermondii (Pz 0.17), followed by C. albicans (Pz 0.18), whereas the least producer in the group was C. glabrata (Pz 0.29).
Phospholipase activity was detected in 49 (44.14%) isolates. Highest phospholipase producer is C. guilliermondii (Pz 0.07), followed by C. parapsilosis (Pz 0.08). Least producer is C. tropicalis (Pz 0.27).
Biofilm, proteinase, and phospholipase production by Candida species isolated from clinical specimen are shown in the Figure 1.
Figure 1.
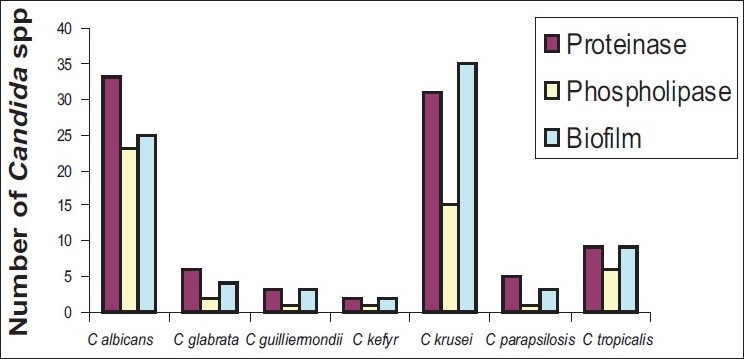
Number of Candida species producing proteinase, phospholipase, and biofilm
DISCUSSION
Candida is an asexual, diploid, dimorphic fungus that is present on humans and in their environment . A relatively small number of Candida species are pathogenic for humans. These organisms are capable of causing a variety of superficial and deep-seated mycoses such as cutaneous, mucocutaneous, subcutaneous, or systemic candidiasis. Candida organisms are commensals; and to act as pathogens, interruptionof normal host defenses is necessary. Therefore, general riskfactors for Candida infections include immunocompromised states,diabetes mellitus, and iatrogenic factors like antibiotic use,indwelling devices, intravenous drug use, and hyperalimentationfluids. Candidiasis has emerged as an alarming opportunistic disease as there is an increase in number of patients who are immunocompromised, aged, receiving prolonged antibacterial and aggressive cancer chemotherapy or undergoing invasive surgical procedures and organ transplantation.
The present study showed the distribution of Candida species in different clinical samples and the predominance of non-Candida albicans, as was also shown by Mujika et al.[1] The most common isolate from all samples was C. krusei. C. albicans (41.37%) was the predominant species recovered from respiratory tract samples. The patients aged above 60 years of age and had productive cough; they probably had secondary infection due to Candida. A total of 120 blood samples were collected from the ICUs and dialysis units. The predominant species isolated from the blood samples were non-Candida albicans. The most common isolate was C. krusei. Most catheter-related septicemias are caused by microorganisms that invade the intracutaneous wound during catheter insertion or thereafter.[9–11] The proportion of such infection due to non-Candida albicans species is persistently rising.[12–14] The saliva samples were collected from 84 diabetic individuals aged above 60 years. Saliva samples from 31 patients yielded C. albicans 23 (74.19%) and non-Candida albicans 8 (25.8%). Urine samples yielded C. krusei 6 (50.0%); followed by C. albicans 3 (25.0%) and these patients had symptoms of urinary tract infection. The isolates from pus, middle ear discharge, and eye were predominantly non Candida albicans species. Plastic devices like endotracheal tube, suction tip, and catheter tip were collected from patients. These cultures yielded C. albicans and C. krusei predominantly.
A biofilm is a community of microorganisms and their extra cellular polymers that are attached to a surface.[15] Biofilms are a collection of microorganisms surrounded by the slime they secrete. The ability to form biofilms is associated with the pathogenecity and as such should be considered as an important virulence determinant during candidiasis. Biofilms may help maintain the role of fungi as commensal and pathogen, by evading host immune mechanisms, resisting antifungal treatment, and withstanding the competitive pressure from other organisms. Consequently, biofilm related infections are difficult to treat.[16] The biofilm production is also associated with high level of antimicrobial resistance of the associated organisms.[17] Biofilm positivity occurred most frequently in isolates of C. krusei followed by C. tropicalis, C. kefyr, C. guilliermondii, C. parapsilosis, C. glabrata, and C. albicans. In contrast, Hawser and Douglas[18] reported thatisolates of C. parapsilosis and C. glabrata were significantlyless likely to produce biofilms than the more pathogenic C. albicans. Biofilm production in this study was more related to the species of Candida than to the site of infection. There were no significant differences in biofilm production when grouping the strains according to the patients′ age, and site of infection.
Aspartyl proteinases are secreted by pathogenic species of Candida in vivo during infection. Secreted aspartic proteinases (Saps) are responsible for the adhesion, tissue damage, and invasion of host immune responses. Proteinases fulfill a number of specialized functionsduring the infective process, they include digesting molecules for nutrient acquisition, digesting ordistorting host cell membranes to facilitate adhesion and tissueinvasion, and digesting cells and molecules of the host immunesystem to avoid or resist antimicrobial attack by the host. The proteinase-producing capacity of Candida non albicans 56 (50.45%) was less than that of C. albicans 33 (67.34%) in this study. The interspecies variation in the amount of proteinase produced varied significantly (P<0.05).
The term “phospholipases” refers to a heterogeneous group of enzymes that share the ability to hydrolyze one or more esterlinkage in glycerophospholipids. Since phospholipase targets membrane phospholipids and digests these components, leading to cell lysis;[19] direct hostcell damage and lysis has been proposed as a major mechanism contributingto microbial virulence. A total of 23 (46.93%) of C. albicans isolates and 26 (42%) of non-Candida albicans isolates produced phospholipase. The result in this study agrees with the reports of Ibrahim et al,[20] in proving that C. albicans isolated from the blood samples showed greater extracellular phospholipase activity.
CONCLUSION
The present study showed predominance of non-Candida albicans in different clinical samples. The number of non Candida albicans producing proteinase, phospholipase and biofilm are more than the number of C. albicans producing these virulence factors. This result suggests that the biofilm production is more important for non-Candida albicans strains and Candida albicans possess mechanisms other than biofilm production to establish infections. Our study showed that the percentage of non-Candida albicans producing proteinase is higher than C. albicans, whereas C. albicans are higher producers of phospholipase than non- Candida albicans.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mujika MT, Finquelievich JL, Jewtuchowicz V, Iovannitti CA. Prevalence of Candida albicans and Candida non-albicans in clinical samples during 1999-2001. Rev Argent Microbiol. 2004;36:107–12. [PubMed] [Google Scholar]
- 2.Ramage G, Saville SP, Thomas DP, López-Ribot JL. Candida biofilms; an update. Eukaryot Cell. 2005;4:633–8. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senet JM. Candida adherence phenomena, from commensalism to pathogenicity. Int Microbiol. 1998;1:117–22. [PubMed] [Google Scholar]
- 4.De Bernardis F, Agatensi L, Ross IK, Emerson GW, Lorenzini R, Sullivan PA, et al. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis. 1990;161:1276–83. doi: 10.1093/infdis/161.6.1276. [DOI] [PubMed] [Google Scholar]
- 5.Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 6.Branchini ML, Pfaller MA, Rhine- chalk berg J, Frempong T, Isenberg HD. Genotype variation and slime production among blood and catheter isolates of C Parapsilosis. J Clin Microbiol. 1994;32:452–6. doi: 10.1128/jcm.32.2.452-456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staib F. Serum-proteins as nitrogen source for yeast like fungi. Sabouraudia. 1965;4:187–93. doi: 10.1080/00362176685190421. [DOI] [PubMed] [Google Scholar]
- 8.Samaranayake YH, Dassanayake RS, Jayatilake JA, Cheung BP, Yau JY, Yeung KW, et al. Phospholipase B enzyme expression is not associated with other virulence attributes in Candida albicans isolates from patients with human immunodeficiency virus infection. J Med Microbiol. 2005;54:583–93. doi: 10.1099/jmm.0.45762-0. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti A, Singh K, Das S. Changing face of nosocomial candidaemia. Indian J Med Microbiol. 1999;17:160–6. [Google Scholar]
- 10.Matsumato FE, Gandra RF, Ruiz LS, Auler ME, Marques SA, Pires FC, et al. Yeast isolated from blood and catheter in children from a public hospital of Sao Paulo, Brazil. Mycopathologia. 2002;154:63–9. doi: 10.1023/a:1015540224658. [DOI] [PubMed] [Google Scholar]
- 11.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, et al. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of blood stream isolates from other sources. J Clin Microbiol. 2002;40:1244–8. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Antonio D, Romani F, Pontieri E, Carruba G. Catheter related candidaemia caused by Candida lipolytica in a patient receiving allogenic bone marrow transplantation. J Clin Microbiol. 2002;40:1381–6. doi: 10.1128/JCM.40.4.1381-1386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. Biofilm formation by Candida dubiliensis. J Clin Microbiol. 2001;39:3234–40. doi: 10.1128/JCM.39.9.3234-3240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinitha M, Ballal M. Proteinase and phospholipase as virulence factors in Candida isolated from blood. Rev Iberoam Micol. 2008;25:208–10. doi: 10.1016/s1130-1406(08)70050-0. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller MA. Nosocomial candidiasis: Emerging species, reservoirs and modes of transmission. Clin Infect Dis. 1996;22:S89–94. doi: 10.1093/clinids/22.supplement_2.s89. [DOI] [PubMed] [Google Scholar]
- 16.Baillie GS, Douglas LJ. Candida biofilm and their susceptibility to antifungal agents. Methods Enzymol. 1999;310:644–56. doi: 10.1016/s0076-6879(99)10050-8. [DOI] [PubMed] [Google Scholar]
- 17.Ozkan S, Kaynak F, Kalkanci A, Abbasoglu U, Kustimur S. Slime production and proteinase activity of Candida species isolated from blood samples and comparison of these activities with minimum inhibitory concentration values of antifungal agents. Mem Inst Oswaldo Cruz. 2005;100:319–24. doi: 10.1590/s0074-02762005000300019. [DOI] [PubMed] [Google Scholar]
- 18.Hawser SP, Douglas LJ. Biofilm formation of Candida species on the surface of catheter materials in vitro. Infect Immun. 1994;62:915–21. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salyers A, Witt D. Virulence factors that damage the host. In: Salyers A, Witt D, editors. Bacterial pathogenesis: A molecular approach. Washington: D.C.: ASM Press; 1994. pp. 47–62. [Google Scholar]
- 20.Ibrahim AS, Mirbod F, Filler SG, Banno Y, Cole GT, Kitajima Y, et al. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–8. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


