Abstract
The combination of diphtheria, tetanus, and pertussis vaccines into a single product has been central to the protection of the pediatric population over the past 50 years. The addition of inactivated polio, Haemophilus influenzae, and hepatitis B vaccines into the combination has facilitated the introduction of these vaccines into recommended immunization schedules by reducing the number of injections required and has therefore increased immunization compliance. However, the development of these combinations encountered numerous challenges, including the reduced response to Haemophilus influenzae vaccine when given in combination; the need to consolidate the differences in the immunization schedule (hepatitis B); and the need to improve the safety profile of the diphtheria, tetanus, and pertussis combination. Here, we review these challenges and also discuss future prospects for combination vaccines.
Keywords: Adjuvant, Combination vaccine, Diphtheria, Haemophilus influenza, Hepatitis B, Neisseria meningitidis, Pertussis, Poliovirus, Streptococcus pneumoniae, Tetanus
INTRODUCTION
The development of combination vaccines for protection against multiple diseases began with the combination of individual diphtheria, tetanus, and pertussis (DTP) vaccines into a single product; this combined vaccine was first used to vaccinate infants and children in 1948.[1] It has become the cornerstone of pediatric and adult immunization programs, and over the years we have seen the addition of other vaccines to the combination and the replacement of components to improve its reactogenicity profile. An important advancement was the replacement of whole-cell pertussis antigens (wP) with less reactogenic acellular antigens (aP) in the early 1990s. This paved the way for the combination of diphtheria, tetanus, and acellular pertussis antigens (DTaP) with other routine vaccines such as inactivated polio vaccine (IPV), Haemophilus influenzae vaccine (Hib), and hepatitis B vaccine (HepB). In this article, we will describe in detail the composition of DTaP-based vaccines and provide an overview of the different variations of this combination that are licensed and in use. Furthermore, we will extensively review the technical challenges that have been faced as additional vaccines have been added to these combinations and speculate on the future developments for this group of vaccines.
Another significant combination vaccine that protects against more than one disease is the measles, mumps, and rubella vaccine (MMR). However, this vaccine will not be discussed extensively here as, unlike the DTaP combination, MMR has not been built upon with the inclusion of additional vaccines. For almost 40 years, the MMR vaccine has remained a trivalent vaccine that is given as a single product.
THE NEED FOR COMBINATION VACCINES
The number of immunizations recommended for children in the first 2 years of life has dramatically increased over time. In the United States the recommended immunization schedules for 2010 indicate that in the first 2 years children are expected to receive vaccines against 14 diseases [Figure 1]. Even with the use of the available pentavalent combination vaccine DTaP-IPV/Hib we have calculated that this recommendation can be achieved through a minimum of 17 injections.[2] In a single visit to the pediatrician, infants may need to receive as many as six injections to comply with these recommendations.[2] With such a complex immunization schedule, it becomes increasingly more challenging to incorporate new vaccines into the schedule.
Figure 1.
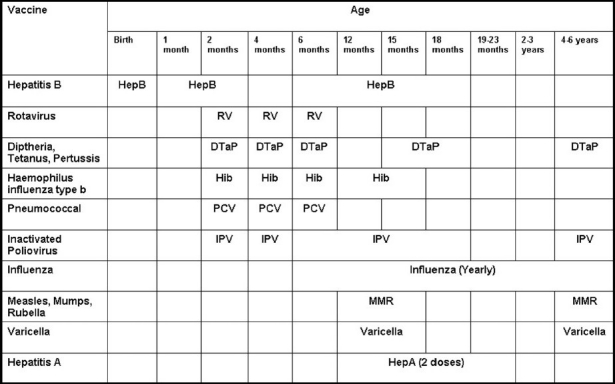
Recommended immunization schedule for children aged 0 through 6 years. This schedule has been adapted from the recommended immunization schedule from the Centers for Disease Control and Prevention.[2] Recommendations are for all children except certain high-risk age-groups. For more information please consult original source.[2]
Simplifying immunization schedules by combining multiple vaccines into a single syringe has been reported to have numerous positive effects [Table 1]. Reducing injections by combining vaccines reduces trauma to the infant and has been found to lead to higher rates of compliance with complex vaccination schedules.[3,4] A number of studies have also reported increased vaccine coverage with the use of combination vaccines. An example of this is a US study reporting increased coverage rates with a pentavalent DTaP-HepB-IPV (Pediarix™) vaccine than with multiple lower-valent vaccines containing the same antigens.[3]
Table 1.
Potential advantages of combination vaccines

COMPOSITION OF DTAP VACCINES
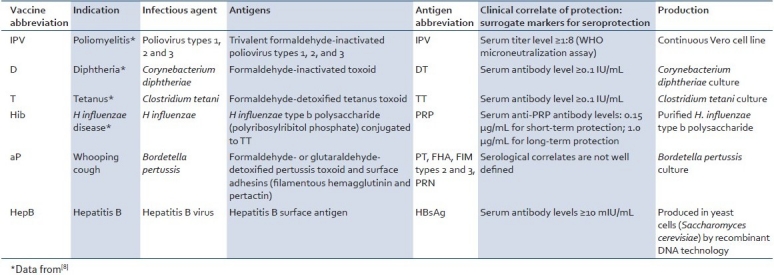
DTaP vaccines from different manufactures are very similar in their composition, with the main differences being related to the number, amount, and detoxification method of the pertussis components.[5] Furthermore, some combinations contain additional vaccines such as HepB, Hib, and IPV in addition to the DTaP base. Here, we will use GlaxoSmithKline's (GSK) hexavalent vaccine Infanrix™-hexa as a model to describe the different components of DTaP-based combination vaccines, as this represents the latest advance in licensed combination vaccine development[Table 2]. There is no other licensed combination vaccine on the market that protects against as many indications as Infanrix™-hexa, which combines diphtheria, tetanus, acellular pertussis, hepatitis B, Haemophilus influenzae, and inactivated poliovirus vaccines (DTaP-HepB-Hib/IPV).[6] Thus, this is composed of diphtheria, tetanus, and acellular pertussis antigens; hepatitis B surface antigen; inactivated poliovirus; and Haemophilus influenzae polyribosylribitol phosphate antigen conjugated to tetanus toxoid. Detailed descriptions of these antigens together with common abbreviations are summarized in Table 2. Infanrix™-hexa is adjuvanted with both aluminum hydroxide and aluminum phosphate adjuvants, which is due to the combination of DTaP vaccine adsorbed onto aluminum hydroxide and HepB vaccine adsorbed onto aluminum phosphate.
Table 2.
Antigen components of Infanrix™-hexa
Overview of licensed DTP-based pediatric combinations in the US and Europe
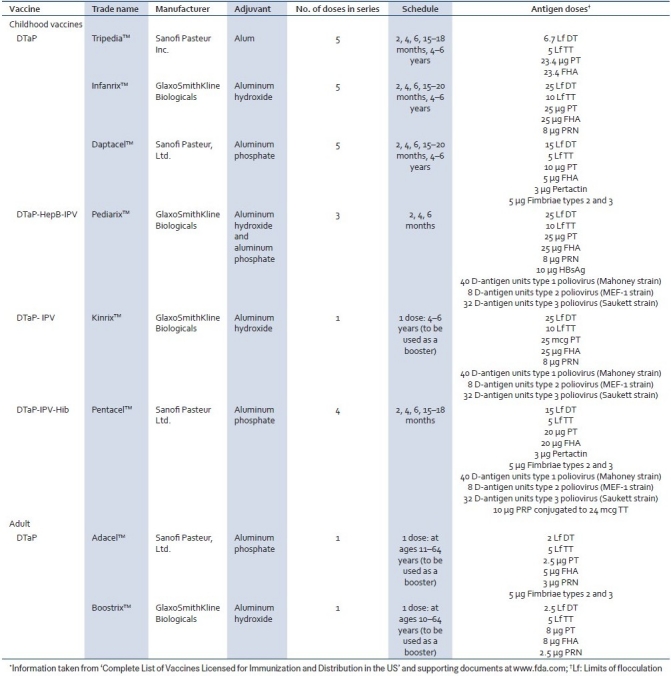
In the US, three diphtheria, tetanus, and acellular pertussis (DTaP) combinations are licensed for use in the pediatric population: Tripedia™ and Daptacel™ from Sanofi Pasteur and Infanrix™ from GSK. Two larger licensed combinations currently in use are pentavalent Pediarix™ , a DTaP-HepB-IPV vaccine from GSK and pentavalent Pentacel™, a DTaP-IPV/Hib combination from Sanofi Pasteur. In addition, there is the tetravalent booster DTaP-IPV vaccine Kinrix™ for use in 4- to 6-year-old children and adult booster DTaP vaccines Adacel™ and Boostrix™. Further details of these vaccines are outlined in Table 3. DTaP-based combinations currently in use outside the US include several other vaccines built upon Sanofi's five-pertussis antigen DTaP vaccine Daptacel™ and GSK's three-pertussis antigen DTaP vaccine Infanrix™ . Infanrix™ is the leading DTaP vaccine used worldwide[7] and provides the backbone of the hexavalent vaccine Infanrix™ -hexa which is licensed in Europe and protects against six different diseases and, as mentioned previously, defines the latest advance in combination vaccine development. Hexavalent DTaP combination vaccines are yet to gain licensure in the US, the authorities’ reluctance stemming from reported reductions in antibody titers to Hib.[8]
Table 3.
Avaliable DTaP-based vaccines in the US*
TECHNICAL CHALLENGES FACED WHEN COMBINING VACCINES
Although there are clear benefits with combination vaccines, the main challenge in their development is the risk that the efficacy or safety of the combination would be less than that seen with the administration of the vaccines separately. New combinations cannot be less immunogenic, less efficacious, or more reactogenic than the previously licensed uncombined vaccines. Immunological, physical, and/or chemical interactions between the combined components have the potential to alter the immune response to specific components. Furthermore, if the vaccines to be combined have differing immunization schedules, consolidation of these should also not negatively affect immunogenicity, efficacy, or safety. Finally, and ideally, the many advantages of combination vaccines should not be achieved at the cost of reduced product stability. From a practical standpoint, uncommon transport and storage conditions and complicated bedside mixing could hamper the development of a combination vaccine.
In this section, we discuss in depth some of the key technical challenges that have faced the development and implementation of DTaP-based combination vaccines. With the use of specific examples, we highlight cases where putting together vaccines in DTaP-based combinations has affected the immunogenicity or safety of the final product.
Reduced Hib response when combined with DTaP
The most commonly reported example of immune interference in DTaP-based combination vaccines is the reduction in antibody titers to the Hib component of the vaccine polyribosylribitol phosphate antigen.[9–11] This has been reported for many DTaP-based vaccines, including the hexavalent vaccine DTaP-HBV-IPV/Hib.[12,13] The interference has not been reported to the same extent for DTwP-based combination vaccines, mainly, it has been suggested, due to the adjuvant effect of the whole-cell pertussis (wP) component.[10,14,15] However, the adjuvant effect simply masks the underlying interference, and the mechanism by which the Hib response is reduced after combination with DTaP is complex and is still not completely understood.
Consistent with clinical data, this reduction in Hib immunogenicity has also been demonstrated in preclinical animal models. Studies in a rat model looking at the interference of TT and different aP antigens with Hib reported reduced anti-PRP response with combined administration of Hib and TT, or Hib and FHA (a component of aP vaccine).[16] The level of this reduction was reported to be comparable to that observed for combined administration of DTaP and Hib.[16] The interference of TT with Hib is of particular interest, as TT is also present in the Hib vaccine as a carrier protein conjugated to the capsular polysaccharide PRP. Possible mechanisms for the effect of free unconjugated TT on the Hib PRP-TT conjugate includes competition between TT-specific and PRP-specific B cells for the Hib conjugate antigen, suppression of PRP response by clonal expansion of TT-specific B cells, and physical prevention of binding of the conjugate antigen to PRP-specific B cells by the TT carrier protein.[17] The reduced anti-PRP response with FHA is in line with the finding that it is a potent suppressor of IL-12 and IFN-γ production in vivo and in vitro, suppressing immune responses to co-injected antigens.[16,18] Another explanation for the reduced Hib response when combined with DTaP vaccines is incompatibility with the alum adjuvant. Experiments in the rat model with Hib alone have reported 5- to 11-fold lower levels of anti-PRP antibodies when adsorbed to aluminum hydroxide adjuvant.[16] Taken together, the reduced Hib response in DTaP/Hib combinations is at least in part a result of the interaction of Hib with TT, FHA, and aluminum hydroxide adjuvant.
The reduced immunogenicity of Hib in DTaP-based combination vaccines vs DTwP-based combination vaccines has been suggested to be a contributory factor in the increase in Hib disease incidence observed in the UK between 1999 and 2002 and a similar increase observed in Ireland.[19–21] It must be stated however that other factors have been suggested to explain the increased incidence, including decreasing herd immunity, the short UK immunization schedule, and the lack of a booster immunization in the second year of life.[21] Despite multiple reports of lowered Hib antibody responses with DTaP combination vaccines containing Hib, the effect of changing from whole-cell to acellular pertussis was actively monitored in Canada and this shows that among children 1–4 years of age incidence rates of pertussis disease has been reduced by 85% in the acellular-pertussis vaccine era.[22] Furthermore, extensive data generated by Eskola and coworkers[10] indicate that the current serological correlate of efficacy for Hib vaccines, which is set at anti-PRP levels of 1.0 μg/mL, is too high and that antibody responses below this threshold are reported which are not associated with impaired antibody function and loss of immune memory. These findings led to the approval of many DTaP-based Hib combinations in Europe.[7]
Combining the differing HepB and DTaP immunization schedules
Another significant technical issue in the implementation of combination vaccines is consolidation of the different immunization schedules of the vaccines to be combined. For example, hepatitis B is often transmitted from mothers to their newborns at the time of birth.[23] Therefore, in countries where a high percentage of HBV infections are acquired, the WHO recommends that the first HepB vaccine dose be administered <24 hours after birth. Furthermore, the Advisory Committee on Immunization Practices (ACIP) recommends a birth dose of hepatitis B vaccine for all US infants. This is to be followed by second and third doses of the HepB vaccine at 1 and 6 months. The primary immunization series of DTaP, on the other hand, requires doses at 2, 4, and 6 months. Therefore, combination of HepB and DTaP still requires administration of monovalent HepB at birth followed by doses in combination with DTaP at 2, 4, and 6 months, resulting in an unnecessary fourth dose of HepB at the 6th month. A study comparing DTaP-HepB combination administered a 2, 4, and 6 months against separate administration of HepB at birth, 1 and 6 months and DTaP at 2, 4, and 6 months showed significantly lower HepB antibody titers with the combined vaccine.[24] However, antibody levels were still above serologically recognized levels of protection in 99% of the subjects.[25] Furthermore, administration of a DTaP-HBV-IPV/Hib vaccine at 2, 4, and 6 months after a dose of HepB vaccine shortly after birth did not impact protective anti-HBs titers and was not more reactogenic than the same combination given without the birth dose of HepB.[26] Nevertheless, reduced efficacy for hepatitis B antigen was the reason for the suspension of another hexavalent combination vaccine, Hexavac™, by the EMEA in 2005.[27] Of particular concern was the reduction in long-term protection, which is important in hepatitis B, as individuals immunized as infants need to have protective levels of antibody when they reach adolescence and adulthood.[28,29]
Improving the safety and tolerability profile of DTP-based vaccines
Safety and tolerability is another important consideration and a potential technical hurdle to overcome when combining vaccines. An improvement for DTP-based vaccines in this respect has been the substitution of wP with aP antigens. Acellular pertussis combinations were first licensed in 1992 and eventually replaced wP vaccines in the USA, primarily due to their improved reactogenicity profile.[30] Significantly higher incidence of localized redness and swelling was reported with wP in studies comparing DTwP-IPV/Hib and DTaP-HBV-IPV/Hib vaccines.[31] Also, increased frequency of adverse events with whole-cell pertussis vaccine was observed in a study comparing DTwP-IPV/Hib and DTaP-IPV/Hib.[8]
When compared to DTwP vaccines, DTaP vaccines are considered to have a good safety profile with low incidence of systemic adverse events such as fever, fatigue, or appetite loss.[32,33] However, reactions are still seen with all DTaP vaccines, notably increased frequency and severity of swelling with the booster immunizations given as the fourth or fifth dose.[34] These were first reported in 1997[35] and despite several hypotheses regarding the cause of these reactions[34,36–39] the immunological mechanisms responsible remain unexplained. For example, incidences of local symptoms were higher after administration of a booster dose of hexavalent DTaP-HBV-IPV/Hib in the second year of life than following the primary doses.[40] Other examples include redness (erythema) at the site of injection (reported in up to 37% of subjects) and swelling (seen in up to 27% of individuals) following a fifth dose of DTaP.[34,36,41] Also, more local responses were observed with a fourth dose of DTaP-IPV/Hib vaccine than following the primary series given in the first 6 months of age.[8,42] Although most countries continue to use full-dose DTaP vaccines for booster doses, there is a growing body of reactogenicity and immunogenicity data to support the use of reduced antigen content in the 4-6 year age-group.[43–45] This has already been implemented in the UK and Germany, where reduced-dose DTaP or DTaP-IPV booster vaccines are administered in the 3–6 year age-group.[46,47]
FUTURE PERSPECTIVES ON DTAP-BASED VACCINES
Until recently there has been reluctance in the US to license pentavalent DTaP combination vaccines and, at the time of writing, hexavalent vaccines still have not gained licensure despite approval for use in Europe. This reluctance stems from reported decreases in antibody titers to Hib when combined with other components in a single syringe.[8] However, clinical data generated with hexavalent vaccines combining DTaP-HepB-Hib/IPV is consistent, with efficacy and safety profiles comparable to that with separate administration of these antigens.[6]
The ACIP recommends that combination vaccines be used whenever possible. As new vaccines that target previously unaddressed diseases are added to the vaccination calendar, the use and improvement of currently available combination vaccines will be paramount if high vaccine coverage is to be maintained.
In the final section below we will discuss future developments in this area, speculating on the possibility of addition of more vaccines to DTaP-based combinations as well as improvements to current combinations by increasing the compatibility of antigens, the addition of more potent adjuvants, or development of new methods for monitoring vaccine production.
Addition of pneumococcal or meningococcal vaccines to DTaP-based vaccines
Although described as hexavalent on the basis of the number of diseases the vaccine targets, the currently available vaccine in this class, Infanrix™ -hexa, carries nine antigens plus inactivated polio virus. Other vaccines which protect solely against pneumococcal infections carry more antigens: for example, the 23-valent pneumococcal polysaccharide vaccine Pneumovax 23™; the updated conjugate vaccine Prevenar™ will contain 13 valences. It is likely that new combination vaccines will reach the market in the coming years, targeting additional diseases such as pneumococcal or meningococcal infections. Although there is no clinical data for DTaP-based combinations containing pneumococcal or meningococcal vaccine, there is considerable experience with the co-administeration of these vaccines.
Multiple clinical studies evaluating concurrent administration of DTaP-based combination vaccines with a seven-valent pneumococcal conjugate vaccine found no significant immunological interference between the vaccines.[8,48,49]
For larger combinations, a study looking at co-administration of DTaP-HepB-Hib/IPV with a seven-valent pneumococcal conjugate vaccine found that after three primary immunizations and a fourth booster dose, titers for several of the antigens were reduced compared to administration of DTaP-HepB-Hib/IPV or pneumococcal vaccine separately according to their respective immunization schedules.[40] However, there was minimal effect on seroprotection/vaccine response rates, with these ranging from 96.8%–100%.[40]
As seen with DTaP vaccines, increased local reactions are also observed with pneumococcal polysaccharide vaccines, which may pose another hurdle to combining these vaccines.[50] A couple of studies with seven-valent pneumococcal conjugate vaccine co-administered with hexavalent DTaP-HepB-Hib/IPV reported increased incidence of systemic reactions, specifically fever, compared to administration of the two vaccines separately.[40,51] Encouragingly, a review of the data available on this topic by Tozzi and coworkers[52] identified no negative effects of particular note in the few published studies that have examined the co-administration of pneumococcal or meningococcal conjugate vaccines with hexavalent DTaP-HepB-Hib/IPV.
Studies looking at co-administration of DTaP-HepB-Hib/IPV with meningococcal serogroup C vaccines have found that the combination is well tolerated and remains immunogenic with both CRM197-conjugated vaccines (Meningitec™ and Menjugate™ ) and the tetanus toxoid conjugate vaccine (NeisVac-C™ ).[53–56] A significant increase in antibody titers to the meningococcal antigens was observed in one study when the vaccines were administered on separate schedules. This was attributed to the ‘priming’ effect of the diphtheria toxoid in the DTaP-HepB-Hib/IPV vaccine, enhancing the response to subsequent immunizations with the CRM197-conjugated meningococcal vaccine.[53] Another study, monitoring co-administration of DTaP-IPV/Hib vaccines with meningococcal conjugate (MCC) vaccine showed no negative effects on antibody responses.[57] A study in adolescents and young adults receiving Novartis's MenACWY polysaccharide conjugate vaccine (Menveo™) found the response to be the same whether administered alone or in combination with DTaP.[58]
New carrier protein strategies for conjugate vaccines
It is generally the case that each of the vaccines contained in DTP-based combination vaccines have usually been previously licensed either as a stand-alone vaccine or as part of a simpler combination. As well as the addition of more vaccines to existing combinations, the future could also see reformulation of existing combinations by changing either the antigens or the carrier proteins used for conjugates. The problems associated with combining conjugate vaccines with carrier proteins which are also present as antigens in the combination have been discussed above. To address this, the future could see new approaches to carrier protein technology, with novel carrier proteins replacing TT. One strategy described for a candidate 11-valent pneumococcal conjugate vaccine has been to use two carrier proteins, TT and DT, and to further reduce the amount of TT by conjugating the polysaccharides that required the largest carrier amounts to DT.[59]
Another approach has been to replace full-length protein carriers such as DT and TT with peptides containing T helper cell epitopes and lacking B cell epitopes. Universal CD4+ T cell epitopes could enable a strong helper effect to the conjugated antigen without inducing an antibody response to the carrier itself. One example of this approach is the N19 recombinant polyepitope made up of CD4+ T cell epitopes from Clostridium tetani, Plasmodium falciparum, HBV, and influenza virus.[60] When evaluated in the mouse model as a carrier protein for a combination of capsular polysaccharides from Neisseria meningitides serogroups A, C, W, and Y, good antibody responses to all four polysaccharides were obtained after a single immunization. Importantly N19-specific antibodies do not cross-react with TT or influenza virus hemagglutinin, the parent proteins from which N19 is obtained.[61]
Novel approaches to adjuvants
Another future challenge will be to extend the period of protection from pediatric vaccine combinations administered in infancy, beyond childhood into adolescence and adulthood. Improvements in this area may involve optimization of vaccine formulation and schedule and the use of adjuvants more potent than alum. Many studies have reported that antibody responses to Hib are reduced when delivered in combination with DTaP. One explanation for this is incompatibility with alum adjuvant, and experiments with Hib alone have reported 5- to 11-fold lower levels of anti-PRP antibodies when adsorbed to aluminum hydroxide adjuvant.[16] Furthermore, reduction in Hib response has come to the fore since the substitution of wP antigens with aP antigens and, with it, the removal of the adjuvant properties of the endotoxin-containing wP antigens. Use of alternative adjuvants may be able to improve immunogenicity and overcome the reduced responses observed with Hib and HepB in DTaP combinations. Novel adjuvants can potentially bring a range of other advantages to combination vaccines, such as antigen dose reduction and reduction in the number of immunizations in the schedule.[62]
Singh and coworkers[63] explored the use of alternative adjuvant formulations, including the oil-in-water emulsion MF59 and polylactide co-glycolide (PLG) microparticles, with established vaccine antigens such as DT, TT, HepB (HBsAg), N meningitides serotype C conjugate (MenC), and a N meningitides serotype B recombinant antigen (MenB). MF59 emulsion stood out as a good alternative to alum for TT, HBsAg, MenC, and MenB vaccines, with the indication that it may be possible to replace alum with MF59 and improve immune responses to these antigens. PLG microparticles also showed promise, with responses comparable to or better than alum with both MenC and MenB vaccines.
Improved in vitro assays for measuring batch-to-batch consistency
Another future trend, whilst not limited to combination vaccines, will be the development of improved in vitro assays for measuring batch-to-batch consistency of vaccine production. For combination vaccines in particular, where one or more components may affect immunogenicity, in vivo potency testing remains the gold standard for evaluation of the final vaccine product. However, efforts are on to reduce the number of animals used in in vivo potency testing (e.g., many laboratories rely on a single-point potency test) and this may lead to increased difficulty in monitoring trends.[64] in vitro approaches as a correlate to in vivo protection have already been applied for some components of combination vaccines, e.g., HepB.[65,66] For other components of DTP-based vaccines, a recent example is the development of an enzyme-linked immunosorbent assay (ELISA) to quantify diphtheria toxoid antigen in DTP-based combination vaccines.[64] Similar assays for tetanus toxoid have also been described.[67,68] Specific antibody-based assays such as these have multiple advantages over other biochemical and biophysical tests applied to these toxins, such as gel electrophoresis, size-exclusion chromatography, and circular dichroism.[69–72] These tests are often limited to characterization of unformulated bulk antigens due to interference from other components in the final vaccine product. This can be overcome in antibody-based assays that are specific for the target antigen. Furthermore antibody-based assays are very sensitive and can detect very low concentrations of target antigen as may be found in booster vaccines.[64] Finally, a significant advantage of antibody-based assays is that they have the potential to measure antigen that is adsorbed to alum, avoiding potentially destructive methods otherwise required for the elution of antigens prior to quantification. For monovalent vaccines, this has recently been reported by a direct alum formulation immunoassay (DAFIA), which was able to quantify alum-bound malaria antigen AMA1-C1 over a linear detection range of 0.16-10 μg/mL.[73]
CLOSING REMARKS
Disease prevention and eradication are the ultimate and immediate goals of immunization, and central to reaching these goals is achieving vaccine coverage. Combination vaccines can help overcome some of the key challenges to maintaining coverage through simplification of vaccine schedules and reduction of injections required. However, there are multiple technical challenges in maintaining immunogenicity and safety when combining vaccines. Continuing vaccine development will only increase the need for the use of combination vaccines, and the future development of larger combinations appears inevitable.
Footnotes
Source of Support: Nil.
Conflict of Interest: The authors David A G Skibinski, Barbara C Baudner, Manmohan Singh and Derek T O’Hagan are all employees of Novartis Vaccines and Diagnotics.
REFERENCES
- 1.Edwards KM, Decker MD. Vaccines. In: Plotkin SA, Orenstein WA, Offitt P, editors. Pertussis vaccine. 5th ed. USA: Saunders PA; 2008. pp. 471–528. [Google Scholar]
- 2.Recommended Immunization Schedule for Persons Aged 0 Through 6 Years. Centers for Disease Control and Prevention - United States. 2010 [Google Scholar]
- 3.Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26:496–500. doi: 10.1097/INF.0b013e31805d7f17. [DOI] [PubMed] [Google Scholar]
- 4.Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006;25:507–12. doi: 10.1097/01.inf.0000222413.47344.23. [DOI] [PubMed] [Google Scholar]
- 5.Weston WM, Klein NP. Kinrix: A new combination DTaPIPV vaccine for children aged 46 years. Expert Rev Vaccines. 2008;7:1309–20. doi: 10.1586/14760584.7.9.1309. [DOI] [PubMed] [Google Scholar]
- 6.Zepp F, Schmitt HJ, Cleerbout J, Verstraeten T, Schuerman L, Jacquet JM. Review of 8 years of experience with Infanrix hexa (DTPaHBVIPV/Hib hexavalent vaccine) Expert Rev Vaccines. 2009;8:663–78. doi: 10.1586/erv.09.32. [DOI] [PubMed] [Google Scholar]
- 7.Bogaerts H. The future of childhood immunizations: Examining the European experience. Am J Manag Care. 2003;9:S30–6. [PubMed] [Google Scholar]
- 8.White C, Halperin SA, Scheifele DW. Pediatric combined formulation DTaPIPV/Hib vaccine. Expert Rev Vaccines. 2009;8:831–40. doi: 10.1586/erv.09.59. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt HJ. USA: San Francisco, CA; 1995. Immunogenicity and reactogenicity of two Haemophilus influenzae type b tetanus conjugate vaccines administered by reconstituting with diphtheria-tetanus-acellular pertussis vaccine or given as separate injections.In 35th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Immunogenicity and reactogenicity of two Haemophilus influenzae type b tetanus conjugate vaccines administered by reconstituting with diphtheria-tetanus-acellular pertussis vaccine or given as separate injections In 35 th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) San Francisco, CA, USA; 1995. [Google Scholar]
- 10.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999;354:2063–8. doi: 10.1016/S0140-6736(99)04377-9. [DOI] [PubMed] [Google Scholar]
- 11.Slack MH, Schapira D, Thwaites RJ, Burrage M, Southern J, Andrews N, et al. Immune response of premature infants to meningococcal serogroup C and combined diphtheria-tetanus toxoids-acellular pertussis-Haemophilus influenzae type b conjugate vaccines. J Infect Dis. 2001;184:1617–20. doi: 10.1086/324666. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt HJ, Knuf M, Ortiz E, Sänger R, Uwamwezi MC, Kaufhold A. Primary vaccination of infants with diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio virus and Haemophilus influenzae type b vaccines given as either separate or mixed injections. J Pediatr. 2000;137:304–12. doi: 10.1067/mpd.2000.107796. [DOI] [PubMed] [Google Scholar]
- 13.Decker MD. Principles of pediatric combination vaccines and practical issues related to use in clinical practice. Pediatr Infect Dis J. 2001;20:S10–8. doi: 10.1097/00006454-200111001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Dagan R, Poolman JT, Zepp F. Combination vaccines containing DTPa-Hib: Impact of IPV and coadministration of CRM197 conjugates. Expert Rev Vaccines. 2008;7:97–115. doi: 10.1586/14760584.7.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Decker MD, Edwards KM, Bogaerts HH. Combination vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. USA: Saunders PA; 2008. pp. 1069–101. [Google Scholar]
- 16.Mawas F, Dickinson R, Douglas-Bardsley A, Xing DK, Sesardic D, Corbel MJ. Immune interaction between components of acellular pertussis-diphtheria-tetanus (DTaP) vaccine and Haemophilus influenzae b (Hib) conjugate vaccine in a rat model. Vaccine. 2006;24:3505–12. doi: 10.1016/j.vaccine.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Schutze MP, Deriaud E, Przewlocki G, LeClerc C. Carrier-induced epitopic suppression is initiated through clonal dominance. J Immunol. 1989;142:2635–40. [PubMed] [Google Scholar]
- 18.McGuirk P, Johnson PA, Ryan EJ, Mills KH. Filamentous hemagglutinin and pertussis toxin from Bordetella pertussis modulate immune responses to unrelated antigens. J Infect Dis. 2000;182:1286–8. doi: 10.1086/315838. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay ME, McVernon J, Andrews NJ, Heath PT, Slack MP. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J Infect Dis. 2003;188:481–5. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald M, Canny M, O’Flanagan D. Vaccination catch-up campaign in response to recent increase in Hib infection in Ireland. Euro Surveill. 2005;10:E050929–2. doi: 10.2807/esw.10.39.02800-en. [DOI] [PubMed] [Google Scholar]
- 21.Steinhoff M, Goldblatt D. Conjugate HIB vaccines. Lancet. 2003;361:360–1. doi: 10.1016/S0140-6736(03)12441-5. [DOI] [PubMed] [Google Scholar]
- 22.Bettinger JA, Halperin SA, De Serres G, Scheifele DW, Tam T. The effect of changing from whole-cell to acellular pertussis vaccine on the epidemiology of hospitalized children with pertussis in Canada. Pediatr Infect Dis J. 2007;26:31–5. doi: 10.1097/01.inf.0000247055.81541.04. [DOI] [PubMed] [Google Scholar]
- 23.Mast E, Mahony F, Kane M, Margolis H. Hepatitis B vaccine. In: Plotskin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. USA: Saunders PA; 2008. pp. 205–241. [Google Scholar]
- 24.Greenberg DP, Wong VK, Partridge S, Chang SJ, Jing J, Howe BJ, et al. Immunogenicity of a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine when mixed with a diphtheria-tetanus-acellular pertussis-hepatitis B combination vaccine. Pediatr Infect Dis J. 2000;19:1135–40. doi: 10.1097/00006454-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg DP, Wong VK, Partridge S, Howe BJ, Ward JI. Safety and immunogenicity of a combination diphtheria-tetanus toxoids-acellular pertussis-hepatitis B vaccine administered at two, four and six months of age compared with monovalent hepatitis B vaccine administered at birth, one month and six months of age. Pediatr Infect Dis J. 2002;21:769–77. doi: 10.1097/00006454-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Pichichero ME, Blatter MM, Reisinger KS, Harrison CJ, Johnson CE, Steinhoff MC, et al. Impact of a birth dose of hepatitis B vaccine on the reactogenicity and immunogenicity of diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b combination vaccination. Pediatr Infect Dis J. 2002;21:854–9. doi: 10.1097/00006454-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 27.European Agency for the Evaluation of Medicinal Products (EMEA). European Medicines Agency recommends suspension of Hexavac. 2005. [last accessed on 2010 Jan]. Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/Hexavac/29736905en.pdf .
- 28.Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: An Italian multicentre study. Lancet. 2005;366:1379–84. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 29.Da Villa G, Romanò L, Sepe A, Iorio R, Paribello N, Zappa A, et al. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133–6. doi: 10.1016/j.vaccine.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention - CDC. Pertussis vaccination: Acellular pertussis vaccine for reinforcing and booster use - supplementary ACIP statement. Recommendations of the Immunization Practices Advisory Committee (ACIP). 1992. Morbidity Mortality Weekly Report (MMWR) Recomm. Rep. 41(RR-1) :1–10. [PubMed] [Google Scholar]
- 31.Cohen R, Schuerman L. Reactogenicity of a new DTPa-HBV-IPV (+ and /Hib) vaccines after primary and booster doses.Presented at: 18th Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID) Noordwijk, The Netherlands, 2000 [Google Scholar]
- 32.Braun MM, Mootrey GT, Salive ME, Chen RT, Ellenberg SS. Infant immunization with acellular pertussis vaccines in the United States: Assessment of the first two years′ data from the Vaccine Adverse Event Reporting System (VAERS) Pediatrics. 2000;106:E51. doi: 10.1542/peds.106.4.e51. [DOI] [PubMed] [Google Scholar]
- 33.Deloria MA, Blackwelder WC, Decker MD, Englund JA, Steinhoff MC, Pichichero ME, et al. Association of reactions after consecutive acellular or whole-cell pertussis vaccine immunizations. Pediatrics. 1995;96:592. [PubMed] [Google Scholar]
- 34.Rennels MB, Deloria MA, Pichichero ME, Losonsky GA, Englund JA, Meade BD, et al. Extensive swelling after booster doses of acellular pertussis-tetanus-diphtheria vaccines. Pediatrics. 2000;105:e12. doi: 10.1542/peds.105.1.e12. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt HJ, Beutel K, Schuind A, Knuf M, Wagner S, Müschenborn S, et al. Reactogenicity and immunogenicity of a booster dose of a combined diphtheria, tetanus, and tricomponent acellular pertussis vaccine at fourteen to twenty-eight months of age. J Pediatr. 1997;130:616–23. doi: 10.1016/s0022-3476(97)70247-6. [DOI] [PubMed] [Google Scholar]
- 36.Scheifele DW, Halperin SA, Ferguson AC. Assessment of injection site reactions to an acellular pertussis-based combination vaccine, including novel use of skin tests with vaccine antigens. Vaccine. 2001;19:4720–6. doi: 10.1016/s0264-410x(01)00230-4. [DOI] [PubMed] [Google Scholar]
- 37.Rennels MB. Extensive swelling reactions occurring after booster doses of diphtheria-tetanus-acellular pertussis vaccines. Seminars in Pediatric Infectious Diseases. 2003;14:196–8. doi: 10.1016/s1045-1870(03)00033-5. [DOI] [PubMed] [Google Scholar]
- 38.Rowe J, Yerkovich ST, Richmond P, Suriyaarachchi D, Fisher E, Feddema L, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4-to 6-year-old children. Infect Immun. 2005;73:8130–5. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Southern J, Andrews N, Burrage M, Miller E. Immunogenicity and reactogenicity of combined acellular pertussis/tetanus/low dose diphtheria vaccines given as a booster to UK teenagers. Vaccine. 2005;23:3829–35. doi: 10.1016/j.vaccine.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Tichmann-Schumann I, Soemantri P, Behre U, Disselhoff J, Mahler H, Maechler G, et al. Immunogenicity and reactogenicity of four doses of diphtheria-tetanus-three-component acellular pertussis-hepatitis B-inactivated polio virus-haemophilus influenzae type b vaccine coadministered with 7-valent pneumococcal conjugate Vaccine. Pediatr Infect Dis J. 2005;24:70–7. doi: 10.1097/01.inf.0000148923.46453.48. [DOI] [PubMed] [Google Scholar]
- 41.Use of diphtheria toxoid-tetanus toxoid-acellular pertussis vaccine as a fivedose series. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–8. [PubMed] [Google Scholar]
- 42.Herz A, Black S, Shinefield H, Noriega F, Greenberg D. Pediatric Academic Societies Annual Meeting. USA: Washington, DC; 2005. May, Safety of Combined DTaP-IPV + PRP-T (Pentacel™) administered at 2, 4, 6, and 15 to 18 months of age; pp. 14–17. [Google Scholar]
- 43.Huang LM, Chang LY, Tang H, Bock HL, Lu CY, Huang FY, et al. Immunogenicity and reactogenicity of a reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine in healthy Taiwanese children and adolescents. J Adolesc Health. 2005;37:517. doi: 10.1016/j.jadohealth.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Kosuwon P, Warachit B, Hutagalung Y, Borkird T, Kosalaraksa P, Bock HL, et al. Reactogenicity and immunogenicity of reduced antigen content diphtheria-tetanus-acellular pertussis vaccine (dTpa) administered as a booster to 4-6 year-old children primed with four doses of whole-cell pertussis vaccine. Vaccine. 2003;21:4194–200. doi: 10.1016/s0264-410x(03)00496-1. [DOI] [PubMed] [Google Scholar]
- 45.Sänger R, Behre U, Krause KH, Loch HP, Soemantri P, Herrmann D, et al. Booster vaccination and 1-year follow-up of 4-8-year-old children with a reduced-antigen-content dTpa-IPV vaccine. Eur J Pediatr. 2007;166:1229–36. doi: 10.1007/s00431-006-0403-x. [DOI] [PubMed] [Google Scholar]
- 46.NHS Routine childhood immunization programme January 2008. [last accessed on 2010 Jan]. Available from: http://www.immunisation.nhs.uk/immunisation_schedule .
- 47.Robert Koch-Institut. Recommendation of the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute for pertussis vaccination. Epidemiologisches Bulletin. 2006;3:21–3. [Google Scholar]
- 48.Scheifele DW, Halperin SA, Smith B, Ochnio J, Meloff K, Duarte-Monteiro D. Assessment of the compatibility of co-administered 7-valent pneumococcal conjugate, DTaP.IPV/PRP-T Hib and hepatitis B vaccines in infants 2-7 months of age. Vaccine. 2006;24:2057–64. doi: 10.1016/j.vaccine.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Noriega F, Tsang P, Jemiolo D, Voloshen T, Greenberg DP. The 5th Pediatric Infectious Disease Conference. 5th ed. USA: Napa, CA; 2005. Oct, The Pentacel Investigators Study Group.Immunogenicity of a four dose series of DTaP-IPV//PRP-T (Pentacel™) [Google Scholar]
- 50.Prevention of pneumococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 51.Knuf M, Habermehl P, Cimino C, Petersen G, Schmitt HJ. Immunogenicity, reactogenicity and safety of a 7-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a DTPa-HBV-IPV/Hib combination vaccine in healthy infants. Vaccine. 2006;24:4727–36. doi: 10.1016/j.vaccine.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Tozzi AE, Azzari C, Bartolozzi G, Esposito S, Fara GM, Giudice ML. Can hexavalent vaccines be simultaneously administered with pneumococcal or meningococcal conjugate vaccines? Hum Vaccin. 2007;3:252–9. doi: 10.4161/hv.4626. [DOI] [PubMed] [Google Scholar]
- 53.Tejedor JC, Omeñaca F, García-Sicilia J, Verdaguer J, Van Esso D, Esporrín C, et al. Immunogenicity and reactogenicity of a three-dose primary vaccination course with a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-haemophilus influenzae type b vaccine coadministered with a meningococcal C conjugate vaccine. Pediatr Infect Dis J. 2004;23:1109–15. [PubMed] [Google Scholar]
- 54.Schmitt HJ, Habermehl P, Knuf M, Ypma E, Borkowski A. Spain: Valencia; 2005. May 18-20, Safety reactogenicity and mmunogenicity / priming following 2 vs. 3 doses of a meningococcal C conjugate vaccine (MCC) given concomitantly with DTaP-IPV-HBV/Hib vaccine to infants in 23rd Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID) [Google Scholar]
- 55.Schmitt HJ, Maechler G, Habermehl P, Knuf M, Saenger R, Begg N, et al. Immunogenicity, reactogenicity and immune memory after primary vaccination with a novel Haemophilus influenzae-Neisseria meningitidis serogroup C conjugate vaccine. Clin Vaccine Immunol. 2007;14:426–34. doi: 10.1128/CVI.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tejedor JC, Moro M, Ruiz-Contreras J, Castro J, Gómez-Campderá JA, Navarro ML, et al. Immunogenicity and reactogenicity of primary immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type B vaccine coadministered with two doses of a meningococcal C-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2006;25:713–20. doi: 10.1097/01.inf.0000227725.61495.c4. [DOI] [PubMed] [Google Scholar]
- 57.Halperin SA, McDonald J, Samson L, Danzig L, Santos G, Izu A, et al. Simultaneous administration of meningococcal C conjugate vaccine and diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine in children: A randomized double-blind study. Clin Invest Med. 2002;25:243–51. [PubMed] [Google Scholar]
- 58.Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull P, et al. Graz: Austria; 2008. Immunogenicity and safety of MenACWY-CRM, A novel quadrivalent meningococcal conjugate vaccine, administered concomitantly with Tdap in healthy subjects 11-25 years of age.Presented at: 26th Annual Meeting of the European Society for Paediatric Infectious Diseases. [Google Scholar]
- 59.Dagan R, Goldblatt D, Maleckar JR, Yaïch M, Eskola J. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect Immun. 2004;72:5383–91. doi: 10.1128/IAI.72.9.5383-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baraldo K, Mori E, Bartoloni A, Petracca R, Giannozzi A, Norelli F, et al. N19 polyepitope as a carrier for enhanced immunogenicity and protective efficacy of meningococcal conjugate vaccines. Infect Immun. 2004;72:4884–7. doi: 10.1128/IAI.72.8.4884-4887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baraldo K, Mori E, Bartoloni A, Norelli F, Grandi G, Rappuoli R, et al. Combined conjugate vaccines: Enhanced immunogenicity with the N19 polyepitope as a carrier protein. Infect Immun. 2005;73:5835–41. doi: 10.1128/IAI.73.9.5835-5841.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant-‘The long and winding road’. Drug Discov Today. 2009;14:541–51. doi: 10.1016/j.drudis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, et al. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine. 2006;24:1680–6. doi: 10.1016/j.vaccine.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 64.Coombes L, Stickings P, Tierney R, Rigsby P, Sesardic D. Development and use of a novel in vitro assay for testing of diphtheria toxoid in combination vaccines. J Immunol Methods. 2009;350:142–9. doi: 10.1016/j.jim.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Cuervo ML, Sterling AL, Nicot IA, Rodríguez MG, García OR. Validation of a new alternative for determining in vitro potency in vaccines containing Hepatitis B from two different manufacturers. Biologicals. 2008;36:375–82. doi: 10.1016/j.biologicals.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Giffroy D, Mazy C, Duchêne M. Validation of a new ELISA method for in vitro potency assay of hepatitis B-containing vaccines. Pharmeuropa Bio. 2006;2006:7–14. [PubMed] [Google Scholar]
- 67.Prieur S, Broc S, Gal M, Poirier B, Fuchs F. Advancing Science and Elimination of the Use of Laboratory Animals for Development and Control of Vaccines and Hormones. In: Brown F, Hendriksen C, Sesardic D, Cussler K, editors. Development of an in vitro potency test for tetanus vaccines. Basel: S. Karger AG Publiation; 2002. p. 37. [Google Scholar]
- 68.Xing DK, McLellan K, Corbel MJ, Sesardic D. Estimation of antigenic tetanus toxoid extracted from biodegradable microspheres. Biologicals. 1996;24:57–65. doi: 10.1006/biol.1996.0006. [DOI] [PubMed] [Google Scholar]
- 69.Bolgiano B, Fowler S, Turner K, Sesardic D, Xing DK, Crane DT, et al. Monitoring of diphtheria, pertussis and tetanus toxoid by circular dichroism, fluorescence spectroscopy and size-exclusion chromatography. In: Brown F, Corbel MJ, Griffiths E, editors. Physico-chemical Procedures for the Characterization of Vaccines. S. Karger AG Basel; 2000. p. 51. [PubMed] [Google Scholar]
- 70.Leenaars PP, Kersten GF, de Bruijn ML, Hendriksen CF. An in vitro approach in quality control of toxoid vaccines. Vaccine. 2001;19:2729–33. doi: 10.1016/s0264-410x(00)00510-7. [DOI] [PubMed] [Google Scholar]
- 71.Metz B, Jiskoot W, Hennink WE, Crommelin DJ, Kersten GF. Physicochemical and immunochemical techniques predict the quality of diphtheria toxoid vaccines. Vaccine. 2003;22:156–67. doi: 10.1016/j.vaccine.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Metz B, Jiskoot W, Mekkes D, Kingma R, Hennink WE, Crommelin DJ, et al. Quality control of routine, experimental and real-time aged diphtheria toxoids by in vitro analytical techniques. Vaccine. 2007;25:6863–71. doi: 10.1016/j.vaccine.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Zhu D, Huang S, Gebregeorgis E, McClellan H, Dai W, Miller L, et al. Development of a Direct Alhydrogel Formulation Immunoassay (DAFIA) J Immunol Methods. 2009;344:73–8. doi: 10.1016/j.jim.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


