Abstract
Pyogenic liver abscess secondary to dissemination from Sigmoid Diverticulitis is rare. Streptococcus Anginosus has been linked to abscesses but has been rarely reported from a Sigmoid Diverticulitis source. We report a case of liver abscess in which the source was confounding but eventually was traced to Sigmoid Diverticulitis on laparotomy.
Keywords: Diverticulitis, Liver abscess, Streptococcus Anginosus
INTRODUCTION
Pyogenic liver abscess is a life threatening disease. Early recognition and timely intervention is key to the successful treatment and better prognosis of the patients. In recent years, its management has been enhanced by imaging techniques, and the use of percutaneous drainage and improvement in microbiological characterization have allowed the use of most appropriate antibiotic therapy. Major underlying conditions include biliary tract disease, malignancy, and disorders of the colon. Sigmoid Diverticulitis as the source of hepatic abscess is uncommon.[1–3]
The “Streptococcus milleri Group” (SMG) of bacteria is frequently linked to abscess.[4–7] Members of this group are now actually classified into three distinct species; Streptococcus intermedius, Streptococcus constellatus, and Streptococcus Anginosus.[4] The first case of Streptococcus milleri hepatic abscess was reported in 1975. Since then they have been identified as one of the important pathogens of liver abscess.[8–11] SMG bacteremia from a Sigmoid diverticular source leading to hepatic and intra-abdominal abscess is rarely reported.
We present a case report in which our patient had Streptococcus anginosus bacteremia with hepatic and intraabdominal abscesses. Further work-up revealed sigmoid diverticulitis as the source of infection.
CASE REPORT
A 71-year-old Caucasian male with a past medical history of hypertension, arthritis, benign prostatic hypertrophy, and osteoarthritis presented to the emergency room with complaints of intermittent fever, chills, generalized weakness, myalagia, and malaise of two weeks duration. He also complained of dysuria and intermittent right-sided subcostal pleuritic pain. He denied diarrhea, melena, any recent travels, or any sick contacts. On physical examination, patient had a temperature of 36.7ºC, was hypotensive initially with systolic blood pressure in the 70s-80s; however, with aggressive fluid resuscitation, his blood pressure came up and was recorded as 98/52 on admission. His pulse was 90 and respiratory rate was 18 saturating 98% on room air. Remainder of the examination was unremarkable. Pt was anicteric. Lungs were clear on auscultation. Cardiac examination findings were normal. Abdomen was soft, with active bowel sounds. No significant tenderness, guarding or rigidity was elicited in the right upper quadrant of abdomen. There was no costorenal tenderness. Initial laboratory testing revealed a WBC count of 36.6*1000 with bands of 7%; creatinine was 2.1, BUN 54, AST 84, ALT 51, alkaline phosphatase 182, total bilirubin 2.5, direct bilirubin 1.3, albumin 1.7, total protein 5.5, PT 15.1, PTT 38, and INR 1.5. Urinalysis was positive for large leukocyte esterase with marked pyuria of more than 60/HPF. The chest X-ray was unremarkable.
Patient was started on empiric intravenous antibiotics with Ampicillin-Sulbactam, and blood and urine cultures were sent. In view of his complaint of intermittent right-sided sub costal pain and a negative chest X-ray, abdominal ultrasound was obtained. Interestingly, the ultrasound revealed heterogenous liver masses and associated partial main portal vein thrombosis. To further characterize these findings, a CT scan of abdomen and pelvis was performed [Figure 1]. The CT scan showed multiple low-density liver lesions with enhancing rims as well as perihepatic fluid collections suggestive of multiple hepatic abscesses. There was also associated partial main portal vein thrombosis and right portal vein thrombosis. The presence of diverticulosis was noted as well. The finding of diverticulosis raised the possibility of diverticulitis as the source of infection; however, this could not be determined convincingly. After consultation with the surgeon and radiologist, CT guided drain was placed in the largest hepatic abscess with a view aimed at interval follow up of the lesions. In the meantime patient's blood culture revealed Streptococcus Anginosus (2/2), susceptible to penicillins. Patient continued to do well with mild discomfort intermittently in the right subcostal/ right upper abdominal quadrant. His leukocytosis overall improved with some fluctuations, but did not resolve. His creatinine levels normalised. His abdomen remained benign on examination. The hemodynamic parameters were stable. Repeat blood cultures did not show any growth and the cultures from hepatic abscess drain showed no growth as well. Surprisingly, a repeat CT scan done 6 days after the drain was placed showed that there was overall increased size of the intraparenchymal and extrahepatic abscesses. New interloop abscess were present in the right mid abdomen and in the pelvis. Right portal vein thrombosis was redemonstrated. In view of progression of the disease, decision was made for exploratory laparotomy. Intraoperative findings were consistent with multiple intra-abdominal abscesses and adhesions. The sigmoid colon was noted to be completely adherent to the bladder with extensive fibrolytic area. This was consistent with Sigmoid Diverticulitis with the formation of a colovesical fistula. The patient underwent sigmoid resection with primary anastomosis, repair of the colovesical fistula, enterolysis, and drainage of multiple intraabdominal abscesses.
Figure 1.
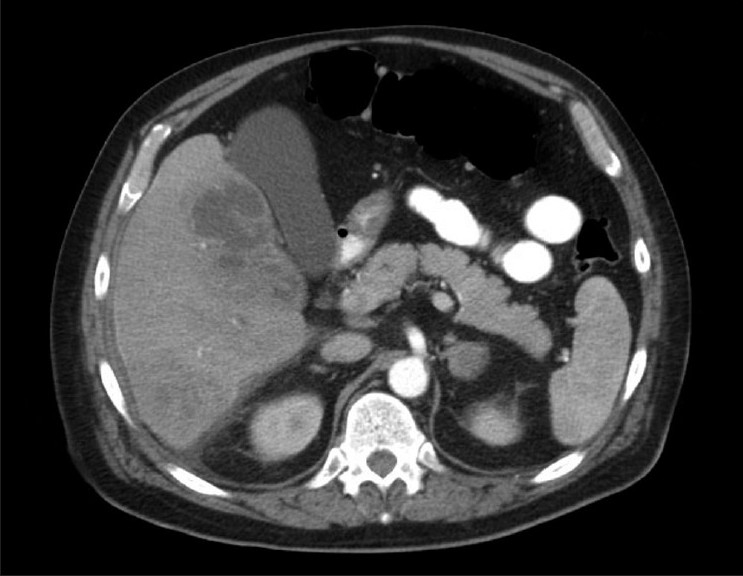
CT scan of the abdomen showing multiple liver abscesses
Patient's postoperative course was unremarkable. He showed gradual recovery. A repeat CT scan of the abdomen and pelvis done 6 days post surgery showed improvement in liver lesions. Patient was discharged home on hospital day 24 to complete a total 4-week course of Amoxicillin-Clavulanate. A follow-up CT scan done about 3 months later did not show any intra-abdominal or hepatic abscess.
DISCUSSION
Streptococcus Anginosus is a member of the SMG of organisms. SMG has been known by a variety of names, and in the past has been regarded as a single species that is loosely synonymous with Streptococcus Anginosus.[5] SMG, however, is actually composed of 3 species – S. intermedius, S.constellatus, and S.anginosus.[4]
The organisms are regularly encountered in clinical specimens and typically produce small colonies on culture with variable hemolytic properties and variable carriage of Lancefield group antigens including F, C, G, and A.[9] Because many phenotypic tests for the characterization of these species yield similar result, identification of isolates can be difficult. S. anginosus and S. constellatus both belong to Lancefiled group F, but S. constellatus is β hemolytic while S. anginosus is non-hemolytic.[7]S. intermedius is serological ungroupable and non-hemolytic.[7] Whitley et al.[7] was the first group to differentiate the SMG species through a biochemical scheme. They found that S intermedius produce α-glucosidase, β-glucodidase, β-D fucosidase, β-N-acetylgalactosaminadase, β-N-acetyglucosaminadase, and sialadase with 4 methylumbelliferl linked fluorogenic subsilates. S. constellatus was distinguished by the presence of a glucosidase and hyaluronidase. S. anginosus produces beta glucosidase. Although biochemical evaluation first enabled SMG isolates into 3 species, SMG can now be distinguished through PCR amplified 16 S r RNA gene sequences followed by species-specific hybridization probes.
Members of the SMG group are part of the normal flora of human mucous membranes and are infrequent pathogens. However, these organisms are frequently isolated from purulent infections of the mouth and internal organs including brain, liver, lungs, and spleen and from cases of appendicitis, peritonitis, endocarditis, meningitis, and infections of the skin and soft tissue.[7] SMG bacteria are usually opportunistic in nature with some reports suggesting its association with patients having multiple co morbid illness, neoplasm, and diabetes. SMG as a causative organism in hepatic abscess have been reported in the literature.[8–11] In a study evaluating 29 cases of bactericidal with SMG in North Yorkshire[9] hepatic abscess was found to be the source in 14% of the cases. In a prospective study,[8] S. milleri was associated with 51% cases of hepatic abscess, the species isolated were S. intermedus and S. constellatus.
Liver abscess is most frequently associated with biliary tract disorder.[1] Biliary stone disease, obstructive malignancy affecting the biliary tree, stricture, and congenital diseases are common inciting conditions. Liver abscess may also develop from hematogenous dissemination of organisms in association with systemic bacteremia such as endocarditis and pyelonephritis. Local spread of bacteria can also occur from contiguous sites of infection within the peritoneal cavity. Infections in the portal bed can result in a localized septic thrombophlebitis, which can lead to liver abscess. Previously, rupture of appendicitis with subsequent spread via portal system was the most common pathway to progress to liver abscess. However, with advanced surgical management and antibiotics, this is less frequently seen nowadays. Although intra-abdominal infection is a potential source of hepatic abscess, there are only some cases listing Sigmoid Diverticulitis as the source for liver abscess.
In our case, the patient was an elderly male with hypertension, arthritis, benign prostatic hypertrophy, and osteoarthritis. There has been no particular link between any of these conditions to Streptococcus Anginosus or SMG, however, presence of multiple medical problems predisposes to infection with this group of organisms. The patient had bacteremia with S. anginosus; the primary source of the infection was Sigmoid Diverticulitis which was spread hematogenously as well as contiguously, resulting in multiple intra-abdominal abscess and hepatic abscess. The patient had a satisfactory recovery on oral antibiotics after undergoing sigmoid resection and removal of the source.
CONCLUSION
In conclusion, bacteremia with S. milleri (S. intermedius, S. constellatus, and S. anginosus) should alert the clinicians for abscess as a potential source of infection. Intraabdominal sources of infection should be looked for in the absence of any obvious source. If a hepatic abscess is identified, the source of infection should be traced. It is important to be aware of diverticulitis as a possible cause of liver abscess. Progression of intra-abdominal abscess despite drainage of hepatic abscess and institution of broad-spectrum antibiotics should raise the suspicion of diverticular source as the possible source of infection.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wang YJ, Wen SC, Chien ST, King J, Hsuea CW, Feng NH. Liver abscess secondary to sigmoid diverticulitis: A case report. J Intern Med Taiwan. 2005;16:289–94. [Google Scholar]
- 2.Wallack MK, Brown AS, Austrian R, Fitts WT. Pyogenic liver abscess secondary to asymptomatic sigmoid diverticultis. Ann Surg. 1976;184:241–3. doi: 10.1097/00000658-197608000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowles R, Rinaldo JA. Pyogenic liver abscess secondary to sigmoid diverticultis: Report of two cases. Gastroenetertology. 1960;38:262–6. [PubMed] [Google Scholar]
- 4.Clarridge JE, Attorri S, Musher DM, Herbert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus Anginosus (“Streptococcus milleri”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32:1511–5. doi: 10.1086/320163. [DOI] [PubMed] [Google Scholar]
- 5.Coykendall AL, Wesbecher PM, Gustafson KB. “Streptococcus milleri”, Streptococcus constellatus, and Streptococcus intermedius are later synonyms of Streptococcus Anginosus. Int J Syst Bacteriol. 1987;37:222–8. [Google Scholar]
- 6.Whiley RA, Beighton D, Winstanley TG, Fraser HY, Hardie JM. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus Anginosus (the Streptococcus milleri group): Association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–4. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiley RA, Fraser H, Hardie JM, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus Anginosus strains within the “Streptococcus milleri group. J Clin Microbiol. 1990;28:1497–501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corredoira J, Casariego E, Moreno C, Villanueva L, López Alvarez MJ, Varela J, et al. Prospective study of Streptococcus milleri hepatic abscess. Eur J Clin Microbiol Infect Dis. 1998;17:556–60. doi: 10.1007/BF01708618. [DOI] [PubMed] [Google Scholar]
- 9.Weightman NC, Barnham MR, Dove M. Streptococcus milleri group bacteremia in North Yorkshire, England. Indian J Med Res. 2004;119:164–7. [PubMed] [Google Scholar]
- 10.Bert F, Barion-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27:385–7. doi: 10.1086/514658. [DOI] [PubMed] [Google Scholar]
- 11.Chua D, Reinhart HH, Sobel JD. Liver abscess caused by Streptococcus milleri. Rev Infect Dis. 1989;11:197–202. doi: 10.1093/clinids/11.2.197. [DOI] [PubMed] [Google Scholar]


