Abstract
Background
The pathogenesis of inflammatory bowel disease is unknown; however, the disorder is aggravated by psychological stress and is itself psychologically stressful. Chronic intestinal inflammation, moreover, has been reported to activate forebrain neurons. We tested the hypotheses that the chronically inflamed bowel signals to the brain through the vagi and that administration of a combination of secretin (S) and oxytocin (OT) inhibits this signaling.
Methods
Three daily enemas containing 2,4,6-trinitrobenzene sulfonic acid (TNBS), which were given to rats produced chronic colitis and ongoing activation of Fos in brain neurons.
Key Results
Fos was induced in neurons in the paraventricular nucleus of the hypothalamus, basolateral amygdala, central amygdala, and piriform cortex. Subdiaphragmatic vagotomy failed to inhibit this activation of Fos, suggesting that colitis activates forebrain neurons independently of the vagi. When administered intravenously, but not when given intracerebroventricularly, in doses that were individually ineffective, combined S/OT prevented colitis-associated activation of central neurons. Strikingly, S/OT decreased inflammatory infiltrates into the colon and colonic expression of tumor necrosis factor-α and interferon-γ.
Conclusions & Inferences
These observations suggest that chronic colonic inflammation is ameliorated by the systemic administration of S/OT, which probably explains the parallel ability of systemic S/OT to inhibit the colitis-associated activation of forebrain neurons.
It is possible that S and OT, which are endogenous to the colon, might normally combine to restrict the severity of colonic inflammatory responses and that advantage might be taken of this system to develop novel means of treating inflammation-associated intestinal disorders.
Keywords: autism, gut-brain signaling, inflammatory bowel disease, interferon- γ (IFNγ), neuropeptides, tumor necrosis factor-α (TNFα)
Introduction
Bidirectional signaling occurs between the brain and the gut and can disturb function in both organs. Anxiety, for example, often leads to gastrointestinal (GI) discomfort, and GI disorders, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) share a high degree of co-morbidity with psychiatric illness.1,2 Intestinal discomfort, furthermore, occurs frequently in individuals with an autistic spectrum disorder (ASD).3–5 Gastrointestinal symptoms occurring in a series of autistic children and adopted orphans have successfully been treated with intensive family nurture. 6,7 Nurture is known to be associated with the release of secretin (S) and oxytocin (OT).8,9
Secretin is best known for its role as a duodenal hormone released in response to acidification of the intestinal lumen. 10 Secretin, however, can also activate vagal sensory nerves; 11,12 moreover, S is synthesized within the brain and can activate hypothalamic neurons.13–16 Mice lacking S receptors, furthermore, exhibit defects in social and cognitive behaviors, suggesting that the central actions of S are significant.17 Although S treatment was reported to be beneficial in autism and associated GI abnormalities,18 its efficacy was not confirmed in subsequent clinical trials;19 moreover, studies have cast doubt on the existence of a specific relationship between autism and IBD. 19 Conceivably, S may have to act in concert with another regulatory hormone.
Oxytocin is best known for its ability to stimulate milk let down and uterine contraction. Oxytocin, however, also promotes adaptive affiliative behaviors that oppose stress. 20–23 Oxytocin also delays gastric emptying and slows intestinal transit. 24–27 Enteric neurons produce OT, and OT receptors have been identified on enteric neurons and the intestinal epithelium. 28 The gastrointestinal effects of OT may be relevant to its behavioral actions; breastfeeding, which is associated with the delivery of significant quantities of OT to neonates,29 confers long-term protection against GI inflammation. 30
Because of the ability of S to stimulate the vagi and that of OT to oppose stress, we tested the hypothesis that S and OT in combination modulate transmission of signals from the inflamed bowel to the brain. To do so, chronic intestinal inflammation was induced by rectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS)31–33 and we determined the effects of subdiaphragmatic vagotomy and S and OT combinations on the inflammation-associated activation of neurons in the paraventricular nucleus of the hypothalamus (PVH), central amygdala (CeA), basolateral amygdala (BLA), and piriform cortex (PIR). Observations suggest that signals from the TNBS-inflamed gut are transmitted to PVH, CeA, BLA, and PIR independently of the vagi; this signaling is antagonized by peripheral, but not central, administration of S/OT, which also decreases the intensity of TNBS-induced colitis.
Methods
Animals
Male Sprague–Dawley rats (250–300 g) were obtained from Hilltop Lab Animals, Inc. (Scottdale, PA, USA) and housed at the New York State Psychiatric Institute Housing Facility. All procedures were approved both by the Institutional Animal Care and Utilization Committees of Columbia University and that of the New York State Psychiatric Institute.
Haptene-induced colitis
After fasting overnight, animals were anesthetized with ketamine [60 mg kg−1 intraperitoneally (i.p.)] and xylazine (7 mg kg−1 i.p.). A glass microsyringe equipped with a gastric intubation needle was used to administer a 1, 0.5, or 0.5 mL enema of TNBS in ethanol/PBS (1: 1: 2), respectively, on three consecutive days. The final concentration of TNBS was 1.25%. An equal volume of phosphate buffered saline (PBS) was administered identically to controls.
Systemic S/OT
Four days after the final TNBS/control enemas, animals were anesthetized with ketamine and xylazine. An Alzet™ (DURECT Corp., Cupertino, CA, USA) osmotic pump (delivery rate = 0.25 μL h−1) was implanted intraperitoneally (i.p.) in control and experimental animals to infuse combinations of S, OT, and S/OT (100 μg each; Phoenix Pharmaceuticals, Burlingame, CA, USA) in 250 μL of saline or equivolume saline into the femoral vein for 21 days. Wounds were sutured, anesthesia was discontinued, bacitracin was applied as a topical antiseptic jelly, and animals were returned to individual cages for postoperative care and observation.
Intracerebroventricular S/OT
Four days after the final TNBS administration, animals were anesthetized with ketamine/xylazine. An Alzet™ osmotic pump was surgically implanted under the neck to infuse equivolume saline or S/OT (50 μg of each peptide in 250 μL of saline; Phoenix Pharmaceuticals) intracerebro-ventricularly (ICV) 0.5 μL h−1 for 21 days.
Brain preparation
Animals were euthanized at the treatment endpoint by rapid i.p. injection of ketamine/xylazine. The animals were then transcardially perfused, sequentially with heparinized physiological saline and a 4% solution of formaldehyde (freshly prepared from paraformaldehyde) in sodium phosphate buffer (pH 7.4). The brains were removed, post-fixed for 2–3 h in the same solution and cryoprotected overnight at 4 °C in a solution of 20% sucrose in 0.1 mol L−1 PBS. Frozen sections were cut on a sliding microtome at 30 μm in the transverse plane. Every fourth section was processed immunocytochemically to demonstrate Fos. Control and experimental tissues were processed simultaneously in the same solutions in order to control for potential variability in immunocytochemistry. Incubations were carried out in separate test wells on a rotator table. Tissues were collected in 0.1 mol L−1 PBS (pH 7.4) in spot test wells and washed in Tris-buffered saline (TBS) between each step. Non-specific binding sites were blocked by pre-incubating with 1% bovine serum albumin (BSA) in TBS for 30 min. Sections were incubated overnight at room temperature with rabbit primary antibodies to Fos (diluted 1: 10 000) (Oncogene, Cambridge, MA, USA) in TBS containing 0.1% BSA, to which 0.25% Triton X-100 was added to facilitate antibody penetration. Sections were washed with TBS for 10 × 3 min and exposed for 1 h to biotinylated goat antibodies to rabbit IgG (1: 200). Sections were again washed for 10 × 3 min and incubated for 45 min with a preformed complex of avidin-biotin peroxidase (1: 100) (ABC Elite Kit, Vector Labs, Burlingame, CA, USA). Sites of primary antibody binding were visualized by demonstrating peroxidase activity with H 2O 2 and 3, 3′-diamonobenzidine (DAB).
Assessment of Fos activation
Digital images of tissue sections were obtained with a SPOT-RT Slider camera (Diagnostic Instruments, Sterling Heights, MI, USA) mounted on a Nikon Microphot microscope (Nikon Instruments, Melville, NY, USA) interfaced with a Dell Pentium III computer (Dell, Round Rock, TX, USA). Images were not altered except to adjust brightness and contrast. Plates were arranged using Adobe Photoshop CS3 software (Adobe, New York, NY, USA). Topographic distribution and density patterns of immunocytochemically labeled neurons were mapped microscopically. Fos-ir nuclei counts were obtained in the PIR, CeA, BLA, and PVH, the boundaries of which corresponded to those of Swanson’s Atlas. 34 Quantitative data were collected from images of three sections, located 1.08, 1.53, and 2.00 mm behind the Bregma, using a two-dimensional counting paradigm. The fields through the CeA that were counted measured 0.5 × 0.5 mm, while those through the PVH measured 0.5 × 0.625 mm. The PIR was considered to include three samples, each 0.3 × 0.3 mm, enclosed within an area defined by a line running from the rhinal fissure to the midpoint on the ventral surface of the cortex and which were located within 0.5 mm of the outer edge of the section.
Colon damage and histopathology scores
After euthanasia, the distal 8 cm of the colon was opened longitudinally along the mesenteric border, cleaned of luminal content, and gently rinsed in saline. Segments were blocked for histological assessment. Inflammatory cells infiltrating the colonic mucosa and submucosa were identified morphologically in paraffin sections stained with hematoxylin and eosin (H&E). Two animal histopathologists, who were blinded to the treatment of the animals, scored severity of inflammatory lesions. Submucosal edema, tissue damage/necrosis, inflammatory cell infiltration, and vasculitis were rated on a scale of 0–3 (0 = none; 3 = severe); perforation was scored as 0 (absent) or 1 (present). 35
Statistics
Data for counts of Fos-ir nuclei in brain regions, macroscopic, and histological colon damage scores were reported as means ± standard error (SEM). One-way analysis of variance (ANOVA) or Student’s t-test were used to compare means. Values of P < 0.05 were regarded as significant. Calculations were carried out with the assistance of SPSS (Version 15.0; SPSS Inc., Chicago, IL, USA) or GRAPHPAD PRISM software (La Jolla, CA, USA).
Vagotomy
Subdiaphragmatic vagotomy was carried out on rats anesthetized with ketamine/xylazine. A 2 cm incision was made extending from the midline immediately below and parallel to the ribcage. The liver was gently retracted with a Q-tip. Using a stereomicroscope, the right and then left vagus nerves were visualized along the lower esophagus and a length (0.5 cm) of the nerve was excised. In control animals, the incision was made and the vagi were visualized but left intact. Following the vagotomy in experimental animals or the sham procedure in controls, wounds were sutured, anesthesia was discontinued, bacitracin was applied topically to the surgical site and animals were returned to individual cages for postoperative care and observation. Both vagotomized and sham-operated control rats were placed on a liquid diet following vagotomy. The animals were found to lose little weight on the diet, which enabled them to tolerate the procedure without recourse to pyloroplasty. The effectiveness of vagotomy was monitored by demonstrating the accumulation of NADPH diaphorase activity in the neurons of the dorsal motor nucleus of the vagus (Fig. S1). This activity has been demonstrated to increase in the cell bodies of the vagal motor neurons following axotomy.36
Reverse-transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from segments of rat gut with Trizol (Invitrogen, Carlsbad, CA, USA) and stored for further use at −80 °C. Complementary DNA (cDNA) was prepared from 3 μg of total RNA by reverse transcription in a 30 μL reaction volume with 0.5 μg random hexamer primers, 0.5 mmol L−1 dNTPs, 40 units of RNAsin™, and 400 units of Maloney Murine Leukemia Virus reverse transcriptase (MMLV; Promega, Madison, WI, USA). Real-time PCR was used to quantify enteric transcripts encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), S, interleukin-6 (IL-6), interferon-gamma (IFNγ), and tumor necrosis factor-alpha (TNFα). Transcripts encoding S, IL-6, IFNγ, and TNFα were normalized to those of GAPDH. The real-time PCR reaction mixture contained 5 μL of the cDNA along with the primers for S, IL-6, IFNγ, and TNFα and PCR master mix [Applied Biosystems (ABI), Foster City, CA, USA]. cDNA levels were quantified using a GeneAmp 7500 sequence detection system (ABI). Duplicate reactions of each standard or sample were incubated for 2 min at 50 °C, denatured for 10 min at 95 °C, and subjected to 40 cycles of annealing at 60 °C for 20 s, extension at 60 °C for 1 min, and denaturation at 95 °C for 15 s. Data were analyzed with computer assistance employing the TAQMAN 7500™ software (Applied Biosystems).
Results
Macroscopic and microscopic evaluation of colonic lesions
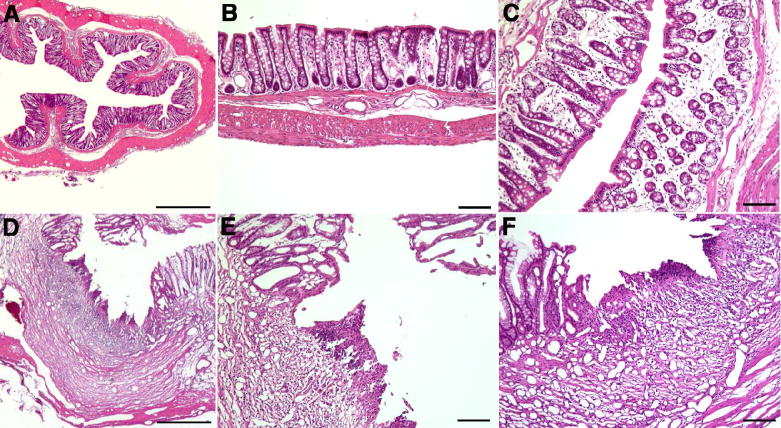
The colons of rats given enemas containing PBS (control; Fig. 1A,B) or TNBS (Fig. 1C–F) were fixed and stained with H&E in order to evaluate colonic inflammation. Relatively little residual evidence of inflammation was found in the colons of most of the rats, which were examined 21 days after the last treatment with TNBS. Microscopic damage scores averaged 0.08 ± 0.05, which was not significantly different from 0 and there was no evidence of vasculitis or perforation of the bowel wall. In saline-treated rats that had been subjected to enemas containing TNBS (Fig. 1D–F), however, there was significant evidence of edema (score 0.9 ± 0.1; P < 0.0001) along with infiltration of neutrophils and macrophages into the mucosa and submucosa (score 0.5 ± 0.1; P < 0.001). Cratered ulcers were observed in 2/8 of TNBS-exposed rats that were treated with saline (Fig. 1D–F).
Figure 1.
Moderate inflammation is morphologically detectable in the colons of rats 3 weeks following intra-colonic adminstration of 2,4,6-trinitrobenzene sulfonic acid (TNBS). Animals received enemas containing phosphate buffered saline (PBS, control) or TNBS and were treated for 3 weeks with saline administered via an osmotic minipump. (A) Control colon. No evidence of inflammation is apparent. The marker = 500 μm. (B) Control colon. There is no evidence of mucosal disruption, edema, or infiltration of leukocytes. The marker = 100 μm. (C) Colon subjected to TNBS enemas. There is mucosal edema but the epithelium is intact. The marker = 100 μm. (D, E) Colon subjected to TNBS enemas. A large ulcer disrupts the mucosal epithelium. Fibrosis and infiltration of leukocytes are evident in the ulcer crater. The markers D = 500 μm; E = 100 μm. (F) Colon subjected to TNBS enemas. Another ulcer is illustrated. Again the epithelium is disrupted and the presence of many new blood vessels (empty of blood because the preparation was fixed by perfusion) indicates that the ulcer is a chronic one. The marker = 100 μm.
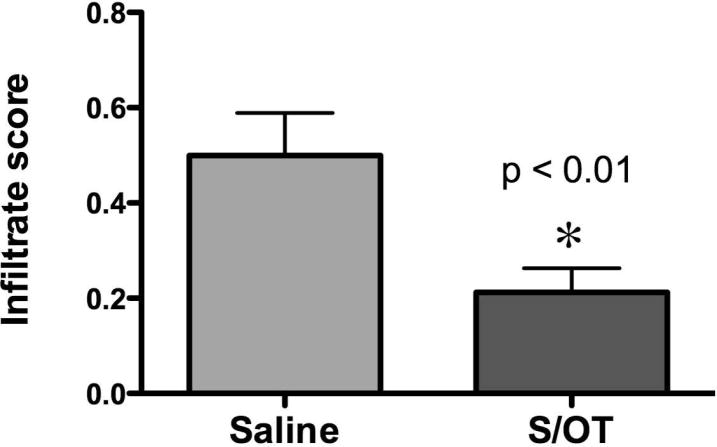
Considerable fibrosis was evident under the denuded surfaces of the ulcers and an extensive ingrowth of new blood vessels were seen in the adjacent submucosa. Similar ulcers were never observed in the colons of rats that received PBS enemas instead of TNBS or in animals that were subjected to TNBS-containing enemas but which were treated with S/OT, although residual edema was observed in S/OT-treated animals (0.8 ± 0.1; P < 0.0001). No residual ulcers were detected in any of 11 TNBS-treated colons after infusion of S/OT (score 0; P = 0.001; Fisher’s exact test) vs control rats in which TNBS-induced colitis was treated with saline (n = 8). The edema score in S/OT-treated rats was not significantly different from that found in the colons of animals given PBS-containing enemas. Secretin/OT infusion, however, significantly reduced the infiltration of the mucosa and submucosa by neutrophils and macrophages (Fig. 2; P < 0.01 vs infusion of saline). These observations suggest that inflammatory infiltrates and edema were still present in the colons of rats 21 days after exposure to TNBS and a subset had residual ulcers, although other histological signs of inflammation had resolved. After infusion of S/OT there was a significant reduction in inflammatory infiltrates and residual ulcers.
Figure 2.
Treatment of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis with the secretin/oxytocin (S/OT) combination reduces the extent of mucosal infiltration with leukocytes. Two pathologists who were blinded as to the treatment scored the degree to which the colonic wall was infiltrated with neutrophils and macrophages. Animals that were subjected to TNBS-induced colitis were treated either with saline (n = 8) or with the S/OT combination (n = 11) and the resulting infiltration scores were compared. Treatment with S/OT significantly reduced the inflammation scores (*).
S/OT combined treatment reduces pro-inflammatory cytokines TNFα and IFNγ
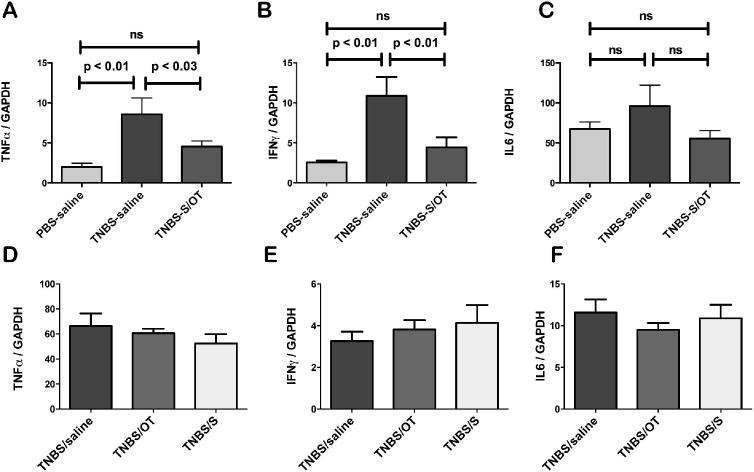
Further studies were carried out to determine whether an indicator that was more sensitive than histological scoring might confirm whether or not residual inflammation was still present in the gut 3 weeks after administration of TNBS enemas. Expression of the pro-inflammatory cytokines TNFα, IFNγ, and IL-6 was therefore quantified 3 weeks after subjection of the animals to enemas containing PBS or TNBS. Infusions of saline (control) or S/OT were then given for 3 weeks to each group of rats. Transcripts encoding TNFα and those encoding IFNγ were each found to be significantly more abundant in the colons of rats exposed to TNBS (n = 8) than in those exposed to saline (n = 7) (Fig. 3A,B). However, the abundance of transcripts encoding IL-6, which is known to decrease in chronic TNBS-induced colitis, 37 was not significantly different in the two groups of rats (Fig. 3C). These data suggest that, despite the relatively normal macroscopic and microscopic damage scores, significant inflammation remains in the colon 3 weeks following administration of TNBS. The infusion of the S/OT combination reduced the abundance of transcripts encoding TNFα and those encoding IFNγ to the level found in the control animals that received saline enemas (Fig. 3A,B), but had no significant effect on the abundance of transcripts encoding IL-6 (Fig. 3C). When infused individually, neither infusion of S (n = 4) nor that of OT (n = 4) was able to reduce the abundance of transcripts encoding TNFα (Fig. 3D), IFNγ (Fig. 3E), or IL-6 (Fig. 3F) in the colons of rats subjected to enemas containing TNBS.
Figure 3.
Combined, but not individual, treatment with secretin (S) and oxytocin (OT) inhibits the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-colitis evoked increase in expression of pro-inflammatory cytokines. (A–C) Animals were given enemas containing phosphate buffered saline (PBS, control) or TNBS and treated with saline or S/OT in combination. (A) TNBS-induced colitis is associated with a significant increase in the abundance of transcripts encoding TNFα; treatment with S/OT prevents this increase. (B) TNBS-induced colitis is associated with a significant increase in the abundance of transcripts encoding IFNγ; treatment with S/OT prevents this increase. (C) TNBS-induced colitis is not associated with a significant change in the abundance of transcripts encoding IL-6; treatment with S/OT does not alter this abundance. (D–F) All animals were subjected to enemas containing TNBS. Treatment with saline was compared with that with OT or S, which were administered individually. No significant differences were found in the abundance of transcripts encoding TNFα (D), IFNγ (E) or IL-6 (F) between animals treated with saline, OT, or S, when these hormones were administered individually.
Colitis-induced excitation of central neurons
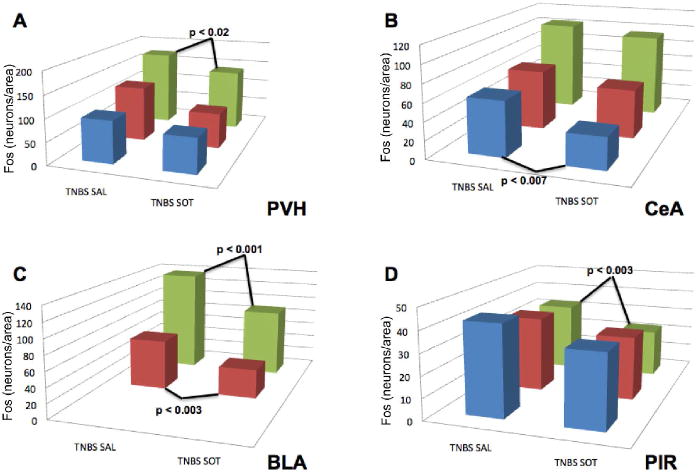
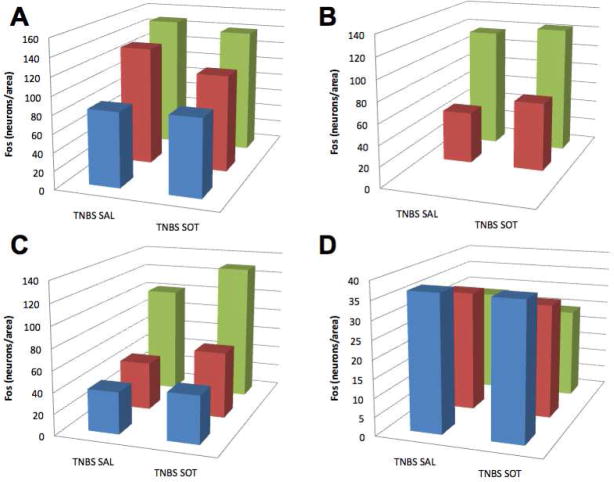
The ability of TNBS-induced colitis to activate neurons in the hypothalamus, amygdala, and piriform cortex has previously been reported. 38 2,4,6-trinitrobenzene sulfonic acid-containing enemas were administered to rats, which were then treated for 3 weeks with saline or the S/OT combination and Fos-IR was demonstrated (Fig. 4A,B). The numbers of neurons with Fos-ir nuclei were then determined in the PVH (Fig. 4C), CeA (Fig. 4A,B,D), BLA (Fig. 4E), and PIR (Fig. 4F). Systemic treatment of rats with the S/OT combination instead of saline reduced the number of Fos-ir neurons in these areas.
Figure 4.
Treatment with the systemic secretin/oxytocin (S/OT) combination inhibits the colitis-evoked activation of neurons in regions of the brain concerned with arousal (PVH), visceral nociception (CeA), reward (BLA), and odor aversion (PIR). Animals were subjected to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis and treated with saline or the S/OT combination. Fos immunoreactivity was demonstrated and the numbers of Fos-immunoreactive neurons/unit area was determined at multiple levels (blue bar = level 23/anterior, red bar = level 25, green bar = level 27/posterior) in four regions of the brain. (A) TNBS-induced colitis activates Fos in neurons of the CeA. (B) Systemic treatment with S/OT decreases the number of neurons in the CeA in which Fos is activated in response to TNBS-induced colitis. The density of activated neurons in saline-treated animals was quantified and compared with that of S/OT-treated rats at each of the four levels of the brain that were analyzed. Significantly fewer neurons with Fos-ir neurons were found in at least one level in each of the four brain regions examined. (C) PVH; (D) CeA; (E) BLA; (F) PIR.
The S/OT combination, therefore, reduces both the manifestations of inflammation in the intestine and the accompanying excitation of central neurons 3 weeks after the induction of chronic TNBS-induced colitis. Despite the relatively normal appearance of the colons of the animals 3 weeks after the induction of colitis with TNBS, therefore, the brains still display evidence of neuronal excitation, which is sensitive to reduction by systemic administration of S/OT. In contrast to systemic treatment, ICV administration of S/OT failed to alter the numbers of neurons with Fos-immunoreactivity in any of the brain regions examined (Fig. 5).
Figure 5.
Intracerebro-ventricular (ICV) administration of the secretin/oxytocin (S/OT) combination does not inhibit the colitis-evoked activation of neurons in any of the regions of the brain where activation was opposed by the systemic administration of S/OT. Animals were subjected to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis and treated with ICV saline or S/OT. Fos immunoreactivity was again demonstrated and the numbers of neurons/unit area was determined at multiple levels. The density of activated neurons in ICV saline-treated animals was compared with that of ICV S/OT-treated rats at each level. No significant differences were found in any of the brain regions that were examined. (A) PVH; (B) CeA; (C) BLA; (D) PIR.
Vagotomy
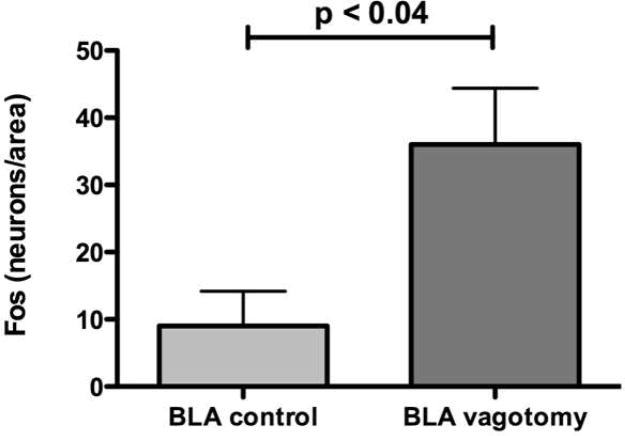
Bilateral subdiaphragmatic vagotomy and sham operations were carried out to determine whether the vagi carry the signals generated by an inflamed colon to the brain. Again, the numbers of neurons with Fos-ir nuclei were determined in the PVH, CeA, BLA, and PIR. Vagotomy did not reduce the number of neurons displaying nuclear Fos immunoreactivity significantly below that found in rats receiving sham operations in any of these regions (Table S1). Interestingly, vagotomy itself increased the excitation of neurons, as evidenced by their Fos-ir nuclei, in the BLA (Fig. 6; P < 0.04). These observations suggest that inflammation associated signaling is transmitted from the colon to the brain via a pathway that is not totally vagus-dependent. The data are also consistent with the idea that vagal activity constitutively inhibits activity in the BLA, which then increases when the vagi are cut.
Figure 6.
Animals were subjected to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis and to vagotomy (n = 4) or sham (n = 4) operation. Fos immunoreactivity was again demonstrated and the numbers of neurons/unit area was determined at multiple levels. Except in the anterior basolateral amygdala (BLA), no significant differences were found in the brain regions that were examined. Unlike the paraventricular nucleus of the hypothalamus (PVH), central amygdala (CeA), and piriform cortex (PIR), the density (number/unit area) of Fos-ir neurons at this level of the basolateral amygdala (BLA) was significantly increased, not decreased by subdiaphragmatic vagotomy, suggesting that tonic vagal inhibition of the activity of neurons occurs in the BLA.
Secretin in the colon
Transcripts encoding S were found in the colon; however, their abundance in the duodenum was >20-fold greater than in the colon (the ratio to GAPDH was 550 ± 63 in the duodenum and 25 ± 5; P < 0.0001).
Discussion
Prior studies established that chronic TNBS-induced inflammation in the colon activates neurons in brain regions involved in emotion and autonomic regulation. 38 Although the systemic treatment of animals with S and OT was not found to influence the inflammation-associated activation of central neurons when these agents were administered individually, treatment with the peptides in combination was not evaluated. The current study was carried out to determine whether the systemic administration of a S/OT combination would oppose the colitis-associated activation of neurons in PVH, CeA, BLA, and PIR. Systemic, but not ICV, administration of S/OT to rats with TNBS-induced colitis significantly reduced the numbers of Fos-ir neurons in the PVH, CeA, BLA, and the PIR. The efficacy of the S/OT combination in inhibiting the colitis-associated activation of neurons in the brain thus appears to be related to peripheral effects of these hormones. Because vagotomy did not prevent colitis-associated activation of neurons in any region, it seems unlikely that S/OT inhibits the activation of central neurons by interfering with vagal afferents. It seems more likely that S/OT’s ability to decrease colitis-associated activation of central neurons is related to its parallel ability to inhibit chronic TNBS-induced colitis. Although neither S nor OT, by itself, modulates the severity of chronic TNBS-induced colitis, the S/OT combination reduces the number of residual ulcers, the degree to which neutrophils and macrophages infiltrate the intestinal mucosa, and the expression of the pro-inflammatory cytokines, TNFα and IFNγ. The ability of systemic S/OT to attenuate TNBS-induced colonic inflammation thus provides an adequate explanation for the ability of systemic S/OT to oppose the colitis-associated activation of neurons in the brain.
The ability of enteric neuropeptides/neurotransmitters to influence intestinal inflammation is well known. 39 Some, such as vasoactive intestinal peptide (VIP), exert anti-inflammatory effects.40–45 Secretin is a member of the same peptide family as VIP. Receptors, both for S and VIP (VPAC1), are family B class II guanine nucleotide-binding proteins that are coupled to the production of cAMP. 46,47 VPAC1, which binds VIP with high affinity, also binds S, albeit with lower affinity. 48,49 Similarly, the S receptor binds S and VIP, but with equal affinity. 48,50 VPAC1 and S receptors, moreover, form oligomeric complexes when co-expressed 51,52 and both utilize ‘a hidden endogenous agonist’ within the receptor that is exposed when the receptor binds a ligand. 53 Although the conformation of the VPAC1 ectodomain normally restricts access of S to the binding site, it is possible that conditions of inflammation facilitate an interaction of S with VPAC1.49 The resultant action of S on VPAC1 could be anti-inflammatory. Alternatively, the activation of shared signal transduction pathways downstream of cAMP may allow S to amplify the anti-inflammatory effects of endogenous VIP. The OT receptor is also positively coupled to the generation of cAMP; 54 therefore, the synergy observed in the combined administration of S/OT may be a phenomenon of cross-talk at the signal transduction level.
Little is currently known about the colonic functions of S or OT; nevertheless, S, 55 OT,56 the S receptor, 28,46,57 and the OT receptor 28 are all endogenous to the colon. Secretin is produced in the duodenum by the ‘S’ subtype of enteroendocrine cell, but these cells are rare 58 or absent 57 in adult colon. Colonic S cells are more common during development. 55 Transcripts encoding S were found in the colon; however, their duodenal abundance was >20-fold that of the colon. Lymphocytes, which are abundant in the colon, express both S and its receptor; moreover, transgenic ablation of S-expressing lymphocytes leads to the sudden onset of colitis. 59 This colitis is associated with the specific loss of the S-expressing subset of CD4 + lymphocytes, which has been postulated to be the cause of the colitis. Colitis, however, could equally well be caused by the concurrent loss of S that is expressed by the subset of colonic enterochromaffin cells that derive from progenitors common to S cells. 55 The importance of duodenal S in the regulation of pancreatic ductal secretion is very well known, as is its ability to stimulate vagal afferent nerves. 60–62 Neither of these properties would seem to account for the expression of S in the colon. The known functions of OT in milk let-down and uterine contraction also fail to account for its colonic presence. Within the colon, OT is present in neurons, primarily in the myenteric plexus, but OT receptors are epithelial and neuronal. 28 It is conceivable that endogenous S/OT interact to exert an anti-inflammatory effect that restrains inflammation in the colon. This possibility is supported by the very small amount of exogenous S and OT that have to be combined to oppose inflammation.
Microbial provocation of innate and acquired immunity is intense in the colon; 63–65 therefore, a multiplicity of regulatory mechanisms, perhaps involving S/OT, might have evolved in that organ to prevent excessive inflammation-induced tissue damage. Oxytocin receptors, moreover, are expressed selectively in junctional complexes of intestinal epithelial cells, 28 a location that is consistent with the possibility that OT receptor signaling is involved in the maintenance of the colonic epithelial barrier. Such an action might impair the translocation of microbes from the lumen to the intestinal wall. 66 The sudden onset of colon inflammation that accompanies ablation of S-expressing cells is consistent with the possibility that S, which promotes growth of the intestinal epithelium in the duodenum and might also do so in the colon, 67 interacts with OT to help maintain mucosal integrity. An interaction between endogenous S and OT in the colon might contribute to the prevention of IBD.
Both OT68 and S receptors59 are expressed by T lymphocytes. Their effects on lymphocytes have not been extensively studied; nevertheless, the lymphocytic expression of these receptors is consistent with the possibility that immune or inflammatory responses could be affected directly by an action of S/OT on lymphocytes. Whether or not endogenous S and OT combine to regulate mucosal integrity or inflammatory responses, it is of considerable interest that the anti-inflammatory effect of the combination of these agents might be useful in the treatment of IBS or IBD.
Because vagotomy failed to decrease the colitis-associated activation of central neurons, it is apparent that the vagi are not solely responsible for this signaling. The vagus nerves have previously been shown to modulate inflammation and vagotomy to aggravate colitis. 69 Vagal afferents protect against TNBS-induced colitis 70 and vagal efferents modulate immune effectors. 71 In fact, we observed a significant increase in neurons displaying activated Fos in the BLA after vagotomy. This increase in activity may well be due to the loss of vagal attenuation of the colitis. Whatever effect the vagi exert on the colon, however, it remains clear that the persistence of central signaling from the colon following vagotomy rules the vagus nerves out as the sole conduit of this information to the brain. Although the S/OT combination reduced the colitis-associated activation of neurons within the PVH, CeA, BLA, and PIR, this action cannot be attributed to an effect of these molecules on the vagi. The S/OT combination was ineffective after ICV administration; moreover, the ability of the S/OT combination to inhibit the activation of the brain in response to chronic TNBS-induced colitis would seem logically to follow from the parallel S/OT amelioration of the colitis. Cytokines, such as TNFα, the expression of which is diminished by S/OT administration, cross the blood–brain barrier72–74 especially in the region of the hypothalamus. 75 Transport of colitis-liberated cytokines from the blood to the brain might thus be responsible for the colitis-associated activation of neurons in the PVH, CeA, BLA, and PIR. Because these regions are concerned with stress adaptation and visceral-emotional responses,76–80 their activation during chronic colitis could contribute to adverse central manifestations of intestinal inflammation. The anti-inflammatory effects of S/OT would thus seem to be centrally beneficial even if they are the result of a peripheral action.
In conclusion, our experimental data shows that administration of S and OT in combination inhibits chronic colitis. Anti-inflammatory efficacy, manifested as significant reductions in the colon of leukocyte invasion and expression of the inflammatory cytokines, TNFα, and IFNγ, requires that S/OT be combined because neither hormone individually reduces leukocytic infiltration or cytokine expression. Because intracerebroventricular administration of S/OT did not affect colitis or the activation of central neurons consequent to it, the anti-inflammatory actions of S/OT are peripheral, not central. Both S and OT, and their receptors, are endogenous to the colon. These observations are thus consistent with the ideas that colonic S and OT might normally combine to restrict the severity of inflammation and that advantage could be taken of this system to develop novel means of treating IBD or other inflammation-associated intestinal disorders.
Supplementary Material
The effectiveness of vagotomy is reflected in the ipsilateral accumulation of NADPH diaphorase activity in neurons of the dorsal motor nucleus of the vagus (DMX).
Effect of sham operations and vagotomy on activation of Fos in brain neurons in association with TNBS-induced colitis.
Acknowledgments
We would like to acknowledge the contributions of Robert Ludwig, T. Bramwell Welch-Horan, Nargis Anwar, Lawrence McGill, D. Glen Esplin, Sara Glickstein, Jason Keune and Farrukh Jafri. Funding Columbia Grant #15665, Einhorn Family Charitable Trust, F. Fairman.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med. 2005;2:146–54. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- 2.Tillisch K, Mayer EA. Pain perception in irritable bowel syndrome. CNS Spectr. 2005;10:877–82. doi: 10.1017/s1092852900019830. [DOI] [PubMed] [Google Scholar]
- 3.Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder [see comments] J Pediatr. 1999;135:559–63. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 4.Horvath K, Perman JA. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14:583–7. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7:165–71. doi: 10.1177/1362361303007002004. [DOI] [PubMed] [Google Scholar]
- 6.Welch MG, Ruggiero DA. Predicted role of secretin and oxytocin in the treatment of behavioral and developmental disorders: implications for autism. Int Rev Neurobiol. 2005;71:273–315. doi: 10.1016/s0074-7742(05)71012-6. [DOI] [PubMed] [Google Scholar]
- 7.Welch MG, Northrup RS, Welch-Horan TB, Ludwig RJ, Austin CL, Jacobson JS. Outcomes of prolonged parent-child embrace therapy among 102 children with behavioral disorders. Complement Ther Clin Pract. 2006;12:3–12. doi: 10.1016/j.ctcp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Guilloteau P, Chayvialle JA, Toullec R, Grongnet JF, Bernard C. Early-life patterns of plasma gut regulatory peptide levels in calves: effects of the first meals. Biol Neonate. 1992;61:103–9. doi: 10.1159/000243537. [DOI] [PubMed] [Google Scholar]
- 9.Uvnas-Moberg K. Neuroendocrinology of the mother-child interaction. Trends Endocrinol Metab. 1996;7:126–31. doi: 10.1016/1043-2760(96)00036-7. [DOI] [PubMed] [Google Scholar]
- 10.Chey WY, Chang T. Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology. 2001;1:320–35. doi: 10.1159/000055831. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wu X, Yao H, Owyang C. Secretin activates vagal primary afferent neurons in the rat: evidence from electrophysiological and immunohistochemical studies. Am J Physiol Gastrointest Liver Physiol. 2005;289:G745–52. doi: 10.1152/ajpgi.00039.2005. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Wang L, Wu SV, et al. Peripheral secretin-induced Fos expression in the rat brain is largely vagal dependent. Neuroscience. 2004;128:131–41. doi: 10.1016/j.neuroscience.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Welch MG, Keune JD, Welch-Horan TB, et al. Secretin: hypothalamic distribution and hypothesized neuroregulatory role in autism. Cell Mol Neurobiol. 2004;24:219–41. doi: 10.1023/B:CEMN.0000018618.59015.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuxe K, Andersson K, Hokfelt T, et al. Localization and possible function of peptidergic neurons and their interactions with central catecholamine neurons, and the central actions of gut hormones. Fed Proc. 1979;38:2333–40. [PubMed] [Google Scholar]
- 15.Itoh N, Furuya T, Ozaki K, Ohta M, Kawasaki T. The secretin precursor gene. Structure of the coding region and expression in the brain. J Biol Chem. 1991;266:12595–8. [PubMed] [Google Scholar]
- 16.Samson WK, Lumpkin MD, McCann SM. Presence and possible site of action of secretin in the rat pituitary and hypothalamus. Life Sci. 1984;34:155–63. doi: 10.1016/0024-3205(84)90586-1. [DOI] [PubMed] [Google Scholar]
- 17.Nishijima I, Yamagata T, Spencer CM, et al. Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior. Hum Mol Genet. 2006;15:3241–50. doi: 10.1093/hmg/ddl402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J Assoc Acad Minor Phys. 1998;9:9–15. [PubMed] [Google Scholar]
- 19.Williams KW, Wray JJ, Wheeler DM. Intravenous secretin for autism spectrum disorder. Cochrane Database Syst Rev. 2005;3:CD003495. doi: 10.1002/14651858.CD003495.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Insel TR. Oxytocin – a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 21.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 22.Penney D, McGee G. Chemical trust: oxytocin oxymoron? Am J Bioeth. 2005;5:1–2. doi: 10.1080/15265160590961040. [DOI] [PubMed] [Google Scholar]
- 23.Young LJ, Winslow JT, Wang Z, et al. Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm Behav. 1997;31:221–31. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
- 24.Hashmonai M, Torem S, Argov S, Barzilai A, Schramek A. Prolonged post-vagotomy gastric atony treated by oxytocin. Br J Surg. 1979;66:550–1. doi: 10.1002/bjs.1800660809. [DOI] [PubMed] [Google Scholar]
- 25.Ohlsson B, Bjorgell O, Ekberg O, Darwiche G. The oxytocin/vasopressin receptor antagonist atosiban delays the gastric emptying of a semisolid meal compared to saline in human. BMC Gastroenterol. 2006;6:11. doi: 10.1186/1471-230X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CL, Hung CR, Chang FY, Pau KY, Wang JL, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflugers Arch. 2002;445:187–93. doi: 10.1007/s00424-002-0925-7. [DOI] [PubMed] [Google Scholar]
- 27.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:406–13. doi: 10.1007/s00210-003-0690-y. [DOI] [PubMed] [Google Scholar]
- 28.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol. 2009;512:256–70. doi: 10.1002/cne.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda S, Kuwabara Y, Mizuno M. Concentrations and origin of oxytocin in breast milk. Endocrinol Jpn. 1986;33:821–6. doi: 10.1507/endocrj1954.33.821. [DOI] [PubMed] [Google Scholar]
- 30.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis – the importance of breast milk. J Pediatr Surg. 1974;9:587–95. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 31.Adam B, Liebregts T, Gschossmann JM, et al. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–86. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 33.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–93. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam, The Netherlands: Elsevier Science B.V; 1998. (2nd rev.) [Google Scholar]
- 35.Jahovic N, Ercan F, Gedik N, Yuksel M, Sener G, Alican I. The effect of angiotensin-converting enzyme inhibitors on experimental colitis in rats. Regul Pept. 2005;130:67–74. doi: 10.1016/j.regpep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez MF, Sharp FR, Sagar SM. Axotomy increases NADPH-diaphorase staining in rat vagal motor neurons. Brain Res Bull. 1987;18:417–27. doi: 10.1016/0361-9230(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 37.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–52. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch MG, Welch-Horan TB, Anwar M, Anwar N, Ludwig RJ, Ruggiero DA. Brain effects of chronic IBD in areas abnormal in autism and treatment by single neuropeptides secretin and oxytocin. J Mol Neurosci. 2005;25:259–74. doi: 10.1385/JMN:25:3:259. [DOI] [PubMed] [Google Scholar]
- 39.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide inhibits cyclooxygenase-2 expression in activated macrophages, microglia, and dendritic cells. Brain Behav Immun. 2008;22:35–41. doi: 10.1016/j.bbi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Rey E, Varela N, Chorny A, Delgado M. Therapeutical approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr Pharm Des. 2007;13:1113–39. doi: 10.2174/138161207780618966. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131:1799–811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Chorny A, Gonzalez-Rey E, Varela N, Robledo G, Delgado M. Signaling mechanisms of vasoactive intestinal peptide in inflammatory conditions. Regul Pept. 2006;137:67–74. doi: 10.1016/j.regpep.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Rey E, Delgado M. Role of vasoactive intestinal peptide in inflammation and autoimmunity. Curr Opin Investig Drugs. 2005;6:1116–23. [PubMed] [Google Scholar]
- 45.Ganea D, Delgado M. Neuropeptides as modulators of macrophage functions. Regulation of cytokine production and antigen presentation by VIP and PACAP. Arch Immunol Ther Exp (Warsz) 2001;49:101–10. [PubMed] [Google Scholar]
- 46.Chow BK. Molecular cloning and functional characterization of a human secretin receptor. Biochem Biophys Res Commun. 1995;212:204–11. doi: 10.1006/bbrc.1995.1957. [DOI] [PubMed] [Google Scholar]
- 47.Laburthe M, Couvineau A, Gaudin P, Maoret JJ, Rouyer-Fessard C, Nicole P. Receptors for VIP, PACAP, secretin, GRF, glucagon, GLP-1, and other members of their new family of G protein-linked receptors: structure-function relationship with special reference to the human VIP-1 receptor. Ann N Y Acad Sci. 1996;805:94–109. doi: 10.1111/j.1749-6632.1996.tb17476.x. discussion 110–1. [DOI] [PubMed] [Google Scholar]
- 48.Holtmann MH, Hadac EM, Ulrich CD, Miller LJ. Molecular basis and species specificity of high affinity binding of vasoactive intestinal polypeptide by the rat secretin receptor. J Pharmacol Exp Ther. 1996;279:555–60. [PubMed] [Google Scholar]
- 49.Du K, Couvineau A, Rouyer-Fessard C, Nicole P, Laburthe M. Human VPAC1 receptor selectivity filter. Identification of a critical domain for restricting secretin binding. J Biol Chem. 2002;277:37016–22. doi: 10.1074/jbc.M203049200. [DOI] [PubMed] [Google Scholar]
- 50.Holtmann MH, Hadac EM, Miller LJ. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J Biol Chem. 1995;270:14394–8. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- 51.Harikumar KG, Morfis MM, Sexton PM, Miller LJ. Pattern of intra-family hetero-oligomerization involving the G-protein-coupled secretin receptor. J Mol Neurosci. 2008;36:279–85. doi: 10.1007/s12031-008-9060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–73. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- 53.Dong M, Pinon DI, Miller LJ. Exploration of the endogenous agonist mechanism for activation of secretin and VPAC1 receptors using synthetic glycosylated peptides. J Mol Neurosci. 2008;36:254–9. doi: 10.1007/s12031-008-9058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jans DA, Pavo I, Fahrenholz F. Oxytocin induced cAMP-dependent protein kinase activation and urokinase-type plasminogen activator production in LLC-PK1 renal epithelial cells is mediated by the vasopressin V2-receptor. FEBS Lett. 1993;315:134–8. doi: 10.1016/0014-5793(93)81149-t. [DOI] [PubMed] [Google Scholar]
- 55.Lopez MJ, Upchurch BH, Rindi G, Leiter AB. Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J Biol Chem. 1995;270:885–91. doi: 10.1074/jbc.270.2.885. [DOI] [PubMed] [Google Scholar]
- 56.Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135:7–11. doi: 10.1016/j.regpep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Andersson A, Sundler F, Ekblad E. Expression and motor effects of secretin in small and large intestine of the rat. Peptides. 2000;21:1687–94. doi: 10.1016/s0196-9781(00)00318-1. [DOI] [PubMed] [Google Scholar]
- 58.Ku SK, Lee JH, Lee HS, Park KD. The regional distribution and relative frequency of gastrointestinal endocrine cells in SHK-1 hairless mice: an immunohistochemical study. Anat Histol Embryol. 2002;31:78–84. doi: 10.1046/j.1439-0264.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 59.Rindi G, Civallero M, Candusso ME, et al. Sudden onset of colitis after ablation of secretin-expressing lymphocytes in transgenic mice. Exp Biol Med (Maywood) 2004;229:826–34. doi: 10.1177/153537020422900816. [DOI] [PubMed] [Google Scholar]
- 60.Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12(Suppl 2):S63–8. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- 61.Li P, Chang TM, Chey WY. Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am J Physiol. 1998;275:G22–8. doi: 10.1152/ajpgi.1998.275.1.G22. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Owyang C. Secretin at physiological doses inhibits gastric motility via a vagal afferent pathway. Am J Physiol. 1995;268:G1012–6. doi: 10.1152/ajpgi.1995.268.6.G1012. [DOI] [PubMed] [Google Scholar]
- 63.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 64.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quigley EM. Germs, gas and the gut; the evolving role of the enteric flora in IBS. Am J Gastroenterol. 2006;101:334–5. doi: 10.1111/j.1572-0241.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 66.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 67.Morisset J, Genik P. Effects of acute and chronic administration of secretin and caerulein on rat duodenal and gastric growth. Regul Pept. 1983;5:111–23. doi: 10.1016/0167-0115(83)90119-2. [DOI] [PubMed] [Google Scholar]
- 68.Ndiaye K, Poole DH, Pate JL. Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biol Reprod. 2008;78:786–93. doi: 10.1095/biolreprod.107.065938. [DOI] [PubMed] [Google Scholar]
- 69.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazelin L, Theodorou V, More J, Fioramonti J, Bueno L. Protective role of vagal afferents in experimentally-induced colitis in rats. J Auton Nerv Syst. 1998;73:38–45. doi: 10.1016/s0165-1838(98)00113-1. [DOI] [PubMed] [Google Scholar]
- 71.Van Der Zanden EP, Boeckxstaens GE, De Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 72.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–76. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 73.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–8. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 74.Ammori JB, Zhang WZ, Li JY, Chai BX, Mulholland MW. Effect of intestinal inflammation on neuronal survival and function in the dorsal motor nucleus of the vagus. Surgery. 2008;144:149–58. doi: 10.1016/j.surg.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 75.Banks WA, Moinuddin A, Morley JE. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging. 2001;22:671–6. doi: 10.1016/s0197-4580(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 76.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–64. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–31. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 79.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–97. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 80.Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110:567–82. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effectiveness of vagotomy is reflected in the ipsilateral accumulation of NADPH diaphorase activity in neurons of the dorsal motor nucleus of the vagus (DMX).
Effect of sham operations and vagotomy on activation of Fos in brain neurons in association with TNBS-induced colitis.