Abstract
Aims
The aim of the study was the development of a sensitive human specific quantitative real-time PCR (qPCR) assay for microbial faecal source tracking (MST) in alpine spring water. The assay detects human specific faecal DNA markers (BacH) from 16S rRNA gene sequences from the phylum Bacteroidetes using TaqMan® minor groove binder (MGB) probes.
Methods and Results
The qualitative and quantitative detection limits of the PCR assay were 6 and 30 marker copies, respectively. Specificity was proven by testing 41 human faeces and waste water samples and excluding cross-amplification from 302 animal faecal samples from Eastern Austria. Marker concentrations in human faecal material were in the range from 6.6 × 109 to 9.1 × 1010 marker equivalents per gram. The method was sensitive enough to detect a few hundred pg of faeces in faecal suspensions. The assay was applied on water samples from an alpine karstic spring catchment area and the results reflected the expected levels of human faecal influence.
Conclusions
The method exhibited sufficient sensitivity to allow quantitative source tracking of human faecal impact in the investigated karstic spring water.
Significance and Impact of Study
The developed method constitutes the first quantitative human specific MST tool sensitive enough for investigations in ground and spring water.
INTRODUCTION
Water from mountainous karst springs is an important resource in drinking water supply. Due to the characteristics of karst aquifers their discharge is occasionally prone to faecal contamination from point sources such as leaking septic systems and non-point sources such as grazing livestock. Classical faecal indicator parameters such as Escherichia coli or intestinal enterococci allow the sensitive detection of this faecal input in spring water habitats (Farnleitner et al. 2005). However, these indicators do not allow source identification or microbial source tracking (MST) as they occur in animal as well as human faeces. Efforts to type strain libraries of traditional faecal indicators by genotypical or phenotypical methods are hindered by poor host adaptation of these bacteria and laborious procedures (Simpson et al. 2002). Thus, there is a strong demand for more practical approaches to facilitate the management of vulnerable spring catchment areas.
The dominant anaerobic bacterial inhabitants of the intestine are a promising target group for modern MST methods. Members of the phylum Bacteroidetes, highly abundant in faeces (Suau et al. 1999), have been proposed as faecal indicator organisms (Allsop and Stickler 1985; Fiksdal et al. 1985) and exhibit host adaptation on the genetic level (Dick et al. 2005). Due to this property these bacteria have recently become the target of MST research efforts. Previous studies identified human specific genetic markers in dominant Bacteroidetes populations and developed methods for their qualitative and quantitative detection by conventional and quantitative PCR (Bernhard and Field 2000a; 2000b; Seurinck et al. 2005; Layton et al. 2006). However, all these methods have so far failed to be fully specific for their target sources, human and bovine faeces. In addition, detection of the relatively low levels of faecal contamination occasionally encountered in spring water demands for MST methods with high sensitivity not offered by the already developed PCR approaches. The aim of this study was to establish a method meeting these requirements for the specific detection and quantification of human faecal pollution in spring and groundwater from alpine karstic regions important for public water supply. The developed quantitative MST method represents a potentially promising tool for monitoring and evaluating management activities for spring water quality. Furthermore it might serve source specific system assessment in the context of water safety plans as suggest by WHO (WHO 2004).
MATERIALS AND METHODS
Sampling and DNA extraction of faeces and water
A total of 343 faecal and waste water samples were collected in a large alpine catchment area in the Northern Calcareous Alps (area of 100 km2, described by (Farnleitner et al. 2005)). For pooled faecal samples ten single samples were combined and homogenised. Samples were collected in sterile faecal sampling tubes and stored at −20 °C. DNA was extracted as previously described (Reischer et al. 2006) and stored at −20 °C. Water sampling, filtration and DNA extraction was performed as previously described (Reischer et al. 2006). All analysed DNA extracts of faecal and aquatic origin contained amplifiable bacterial DNA as checked by applying a universal bacterial PCR assay (Farnleitner et al. 2005).
Assay development
Primers were designed by aligning the following published 16S rRNA gene sequences using the Vector NTI software package (InforMax Inc., Frederick, USA): AF233408, AF233409, AF233410, AF233411, AF233412, AF233413 (Bernhard and Field 2000a). Primers were designed from the derived consensus sequence with the Primer Express® software (Applied Biosystems, Foster City, USA). The primers were designated BacH_f (5′- CTTGGCCAGCCTTCTGAAAG -3′) and BacH_r (5′-CCCCATCGTCTACCGAAAATAC-3′). PCR was performed on an iCycler (Biorad, Hercules, USA) using the following program: 95 °C for 3 min, 30 cycles of 95 °C for 15 s, 61 °C for 15 s and 72 °C for 45 s and finally 72 °C for 3 min. Reaction mixtures (25 μl) contained 2.5 μl of template DNA dilution, 200 nmol l−1 BacH_f, 200 nmol l−1 BacH_r, 10 μg BSA (Boehringer Mannheim, Mannheim, Germany), 12.5 μl of iQ Supermix (Biorad).
A total of 14 conventional PCR assays with the newly developed primers were performed from 10−2 DNA dilutions of three human faecal samples, one pooled sample composed of five human samples, one pooled sample containing samples from three cesspits, three samples from individual cesspits and six samples of municipal waste water (populations of 20, 600, 1500, 7000, 23000 and 2 million, respectively). Cloning and sequencing were performed as previously described (Reischer et al. 2006). 16S rRNA gene sequences gained from faecal DNA (GenBank accession numbers DQ384022 - DQ384054) were included in the sequence alignment.
For the detection and quantification of both dominant sequence types two separate but identically labelled TaqMan® MGB probes were developed. The probes named BacH_pC (FAM-5′-TCATGATCCCATCCTG-3′-NFQ-MGB) and BacH_pT (FAM-5′-TCATGATGCCATCTTG-3′-NFQ-MGB; FAM, 6-carboxyfluorescein; NFQ-MGB, non-fluorescent quencher plus attached minor groove binder) (Table 1) were designed using Primer Express®and purchased from Applied Biosystems (Warrington, UK). The amplicon was analysed for intra- and intermolecular secondary structures using the mfold software (http://www.bioinfo.rpi.edu/applications/mfold/dna/form1.cgi).
TABLE 1.
Sequence types C and T found in human faecal clones and the respective TaqMan® MGB probes for quantification
| Name | Sequence * |
|---|---|
| Sequence Type C | 5′–CAGGATGGGATCATGA–3′ |
| Probe BacH_pC | 3′–MGB–NFQ–GTCCTACCCTAGTACT–FAM–5′ |
| Sequence Type T | 5′–CAAGATGGCATCATGA–3′ |
| Probe BacH_pT | 3′–MGB–NFQ–GTTCTACCGTAGTACT–FAM–5′ |
Highlighted bases, substitutions in the probe binding sites.
BacH qPCR
qPCR was monitored on an iQ Real-Time System (Biorad). Optimised reaction mixture composition was (total volume of 25 μl): 2.5 μl of sample DNA dilution, 200 nmol l−1 BacH_f, 200 nmol l−1 BacH_r, 100 nmol l−1 BacH_pC, 100 nmol l−1 BacH_pT, 10 μg bovine serum albumin (Boehringer Mannheim), 12.5 μl of iQ Supermix (Biorad). The PCR program was identical to the one described above. All reactions were performed in triplicates and in two ten-fold dilution steps of the template DNA. Two of the specific clones (GenBank numbers DQ384034 for BacH_pC and DQ384048 for BacH_pT) were used for the generation of plasmid standards for qPCR. The concentration of the undiluted plasmid standard solution was measured photometrically (photometric accuracy ±1%). The standard was ten-fold serially diluted in a 5 ng μl−1 poly d(I-C) solution as unspecific DNA background (Roche Diagnostics, Mannheim, Germany). For absolute quantification in qPCR a total of six ten-fold serial dilutions of the BacH_pT standard (7 to 7 × 105 gene copies) were run in triplicate on every wellplate as well as no-template and no-amplification controls. To assess the presence of PCR inhibitors water and faecal sample DNA were analysed in several ten-fold dilution steps.
Evaluation setup
Raw water from an alpine karst spring was filtered through a 0.22 μm Steritop™ filter (Millipore). Seven single faecal samples from humans living in Eastern Austria and five waste water samples from cesspits and sewers of mountain huts (number of inhabitants ranging from 10 to 100) were analysed. One gram of wet faeces or waste water from each sample was suspended in 50 ml of filtered spring water and diluted in 100-fold steps. One millilitre from each dilution step was filtered as described for water samples, DNA was extracted and BacH qPCR was performed. Additionally 500 ml of the spring water used for the suspensions were filtered and analysed as a negative control. E. coli CFU per gram sample were determined from suspensions as previously described (Farnleitner et al. 2005b). To assess the occurrence of marker in the study area samples were taken from the following water sources in the study area (short description, sampling dates and filtered volumes in parentheses): Spring KPAS (well protected spring site; November 2005, 4.5 l); Spring LKAS2, (relatively vulnerable karstic spring site, mean water residence time 0.8 - 1.5 years, quick discharge response after precipitation, described in detail in (Farnleitner et al. 2005); winter 2004, 4.5 l, and summer 2005 during a strong flood event, 1.5 l); River (small river in the study area; December 2005, 0.3 l); River below WWTP (the same river below the effluent pipe of a waste water treatment plant of a village, 600 population equivalents; December 2005, 0.6 l); Watering brook (running through a remote game-feeding compound; December 2005, 1.5 l); Cesspit (pit latrine of a large mountain hut with 10-100 visitors per day in summer; Summer 2005). The value from the cesspit sample was derived from the detection limit experiment.
RESULTS
Primer and probe design
The 16S rRNA gene sequence alignment generated from published Bacteroidetes sequences led to the development of the new primers BacH_f and BacH_r. These primers were tested in a conventional PCR assay on a small setup of DNA extracts from selected faecal samples from Eastern Austria to confirm the preliminary specificity of the primers (data not shown) and to obtain additional sequence information for qPCR probe design. The 33 sub-cloned sequences belonged to two sequence types (Table 1), 1/3 showing type C and 2/3 showing type T. Based on this information the TaqMan® MGB probes BacH_pC and BacH _pT were designed. For evaluation purposes the probes BacH_pC and BacH_pT were tested separately with their respective serial qPCR standard dilution series. The PCR efficiency was equally high for both independent assays (between 97 and 100 %). For the final assay both probes were combined in one reaction and tested by measuring both standards separately and in mixtures. The probes showed no reduction in their capacity to quantify the sum of the respective sequence types down to the detection limit in any mixing ratio.
Characteristics of the BacH qPCR assay
Quantification of the copy number of the human-specific marker in the BacH qPCR assay was achieved by analysing a plasmid standard dilution series in every measurement run. The marker copy number of any sample could be determined from the respective standard regression curves (PCR efficiencies between 97% and 100% for all runs, R2 > 0.99). The lower qualitative detection limit was in the range of a few copies of the marker per reaction volume (RV) demonstrated by the fact that the standard containing 7 marker copies per RV was detectable in almost all (> 95%) runs. Linear quantification was possible in the range from 30 to 107 marker copies per RV.
Source specificity of the BacH qPCR assay
The specificity of the assay was tested on DNA extracted from human faecal, cesspit and waste water samples as well as animal faecal samples (most cesspit and animal samples from the studied catchment area (~100km2), all human single samples and several waste water and animal samples from all over Eastern Austria (~25000 km2)) (Table 2). The marker sequence was detected in 100% of the municipal waste water and cesspit samples from mountain huts and in 95% of the analysed faecal samples from human individuals. The marker was not detectable in any animal faecal sample tested (n = 302) with the exception of one sample from a domestic cat (Table 2).
TABLE 2.
Specificity of the BacH q PCR assay tested on human and animal faecal samples and cesspits and waste water samples
| Source | Sample type * | Number of samples ** |
BacH positive/negative |
|---|---|---|---|
| Cesspits and wastewater *** | Wastewater | 20 | 20/0 |
| Human**** | single | 21 | 20/1 |
| Cattle | pooled | 6 (60) | 0/6 |
| Deer | pooled | 4 (40) | 0/4 |
| Chamois | pooled | 4 (40) | 0/4 |
| Roe deer | pooled | 1 (10) | 0/1 |
| Sheep | pooled | 3 (30) | 0/3 |
| Goat | pooled | 3 (30) | 0/3 |
| Horse | pooled | 3 (30) | 0/3 |
| Fox | single | 2 | 0/2 |
| Dog | single | 3 | 0/3 |
| Cat | single | 3 | 1/2 |
| Pig | pooled | 2 (20) | 0/2 |
| Chicken | pooled | 1 (10) | 0/1 |
| Turkey | pooled | 1 (10) | 0/1 |
| Swan | single | 1 | 0/1 |
| Duck | pooled | 1 (10) | 0/1 |
| Black grouse | single | 3 | 0/3 |
single, single faecal sample; pooled, sample pooled from 10 single faecal samples of the respective source or waste water sample;
original number of samples before pooling in brackets;
cesspits from mountain huts in the study area with 10 to 100 inhabitants, municipal waste water from settlements with 300 to 2 million inhabitants from all over Eastern Austria;
persons from Eastern Austria.
Limit of detection in applied use
The Bacteroidetes strain carrying the marker has not been isolated yet. Therefore it was not possible to relate the copy numbers of our marker directly to a cell count. The results were expressed as marker equivalents (ME) detected in qPCR. ME concentrations were determined for human faeces, waste water and cesspit samples suspended in water as the environmental matrix of interest. Faeces from seven human single samples and five waste water or cesspit samples was suspended and diluted in pre-filtered spring water in 100-fold dilution steps. After filtration and DNA extraction the ME concentrations were determined, signifying the actual copy number of marker still detectable after the losses incurred by the preceding steps. In addition, CFU of E. coli were determined from the same samples.
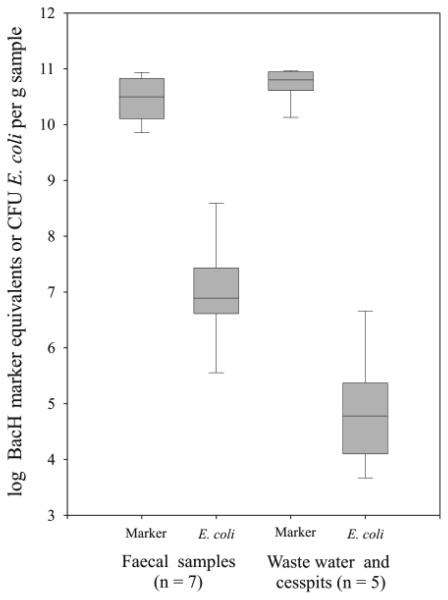
ME concentrations detected in human faecal samples ranged from 6.6 × 109 − 8.7 × 1010 ME per g wet faeces, for waste water samples the range was 1.4 × 1010 − 9.1 × 1010 ME per g (Fig. 1). These values were determined from the most diluted faecal suspensions showing positive and quantifiable qPCR results. The marker was not detected in any higher dilutions nor in the pre-filtered spring water used for diluting. The faecal samples contained cultivable E. coli in concentrations between 2.0 × 105 and 7.5 × 108 CFU per g wet faeces, while cesspit and waste water samples contained between 4.7 × 103 to 4.5 × 106 CFU E. coli per gram sample (Fig. 1). In order to give a realistic estimate of the detectable ME concentration a Poisson distribution in the samples was assumed and the detection frequency was defined as 95% ( S = Xm ± √Xm where S is the 95% confidence interval and Xm the arithmetic average (Reischer et al. 2006). It follows that the reliable detection of the marker can only be expected in PCR reactions containing an average of 6 or more copies of the marker. Taking this into account, the average detection limit of human faecal input in water was 3.2 × 10−10 g of wet faeces and 1.6 × 10−10 g of waste water per filtered volume.
FIG. 1.
BacH Marker equivalent (ME) concentrations and E. coli numbers found in human faeces (single faecal samples) and in waste water and cesspit samples. Results are given as log10 of ME or CFU E. coli per g wet faeces or waste water, respectively (Whiskers, ranges; boxes, 25th and 75th percentiles; lines within the box, median; n, number of samples)
Occurrence of the marker throughout the study area
To investigate the quantitative occurrence of the human-specific marker in natural aquatic systems, water samples from locations chosen according to a presumptive faecal pollution gradient were taken in the study area. This gradient ranged from well protected springs to waste water. The marker could be found at concentrations ranging from not detectable in 4.5 litres to 1013 ME per litre. It was not detectable in 4.5 litres of water from the well protected spring KPAS, the spring LKAS2 sample taken during winter and the watering brook inside a remote fenced game feeding compound. The concentration was 6.5 × 102 ME per litre both in the spring LKAS2 sample taken during a strong summer flood event and the river water. Higher marker concentrations were found in the river water sample taken right downstream of a waste water treatment plant of a small village in the study area (1.1 × 106 ME per litre). With a value of 1.4 × 1013 ME per litre the highest marker concentrations were detected in a cesspit sample from a mountain hut.
DISCUSSION
Advantages of BacH qPCR assay for MST
Human faeces are influencing aquatic systems by leaks in sewer systems and cesspits, as effluent from waste water treatment plants and, in rare cases, direct deposition of faeces. Taking this into consideration, the sensitivity of the presented assay was investigated using faecal samples as well as various types of waste water. Even after the intermediate steps of filtration and DNA extraction faecal material was detectable in the extremely low range from 68 to 909 pg in any filtered volume of water. The high sensitivity of the presented assay is also demonstrated by comparing it with cultivation based faecal indicators. As an example, the median value for ME concentrations in faeces were 4 × 103 times higher than the median value for cultivable E. coli concentrations. In waste water and cesspit samples, this median ratio was even higher (1 × 106, Fig. 1). On the other hand the PCR method allows no assessment of viability of faecal bacteria. Future investigation will have to establish correlations and differences between viability-based assays and direct detection methods in the investigated environment. An additional interesting observation is the much lower variability of the marker equivalent concentrations (approximately 1 order of magnitude) as compared to the variability of the E. coli counts (up to 3 orders of magnitude, Fig.1). This might be an indication of relatively stable abundances of the strains carrying the BacH marker in human faeces.
The developed BacH qPCR assay allowed detection of marker copies in the range of the theoretical limit of detection and, therefore, meets the requirement for the detection of faecal contamination in ground and spring water. All previously published quantitative detection methods for human-specific faecal markers exhibit a detection limit at least 2 orders of magnitude worse than the limit determined in this study (Seurinck et al. 2005; Layton et al. 2006). In addition, the TaqMan® qPCR assay developed by (Layton et al. 2006) had a 32% false-positive identification rate, casting doubts on the applicability of the method. In a previous study, our working group encountered similar problems when designing qPCR assays using Scorpion probes which did not exhibit the necessary specificity for the targeted sources (Stricker et al. 2006).
Applicability of the assay
The single false negative result obtained with one human faecal sample (less than 5% of the total number) suggests that there might be human individuals who are not hosts to the detected Bacteroidetes strain. However the human specific marker was detected in all waste water samples originating from all over Eastern Austria demonstrating the applicability of the assay for the source-specific detection of faecal contamination. No animal faecal samples from the study area gave positive results. One sample from a domestic cat kept in a city flat showed cross-species amplification of the marker. This could be explained by its close relationship with its human keepers which might lead to permanent or transient cross-colonisation of its gastrointestinal flora.
The specificity and high sensitivity of the presented assay could prove highly useful in source tracking studies and investigations, especially in ground and spring water sources. This assumption is supported by the detection of the marker in several natural aquatic systems in a karstic catchment region in this study. Strong differences in occurrence of the marker in water samples were obvious and in accordance with the expected different levels of human faecal influence.
The specific allocation of faecal pollution in a water source allows waterworks to implement target-oriented measures in the catchment area like the installation of treatment facilities or repair of septic systems or sewers, and thus facilitates watershed management and helps provide save drinking water. The proposed method could also offer additional insights when included in quantitative microbial risk assessment (QMRA) studies as part of water safety plans recommended by the WHO (WHO 2004). In this field it would allow the affirmation or exemption of human faecal input and might help to determine the risk of contamination with human-specific pathogens. In combination with the recently developed qPCR assay for the quantification of a ruminant-specific faecal Bacteroidetes marker, it will allow the coverage of the largest portion of potential faecal sources in many regions (Reischer et al. 2006).
For future applications, it will be necessary to estimate the persistence of the marker in the investigated aquatic environment as well as the occurrence of the marker in soils and sediments potentially influencing the water body of interest. It can be assumed that the source specific organisms detected by this assay are highly adapted to the intestinal tract of humans and are thus unlikely to proliferate in a soil habitat. Nevertheless, soil might be an intermittent storage reservoir for these bacteria.
The presented qPCR method is capable of specifically detecting human faecal pollution in ground and spring water. In the investigated area, the assay shows high specificity for human sources and is sensitive enough to detect as little as a few hundred (68 to 909) pg of faeces per investigated volume of water. After additional evaluation in other study areas this method might provide a valuable new tool useful for scientists doing microbial source tracking studies as well as water suppliers trying to improve source water quality.
ACKNOWLEDGEMENTS
Our thanks go to Regina Sommer and Hermann Kain.
REFERENCES
- Allsop K, Stickler DJ. An assessment of Bacteroides fragilis group organisms as indicators of human faecal pollution. J Appl Bacteriol. 1985;58:95–99. doi: 10.1111/j.1365-2672.1985.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Bernhard AE, Field KG. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl Environ Microbiol. 2000a;66:1587–1594. doi: 10.1128/aem.66.4.1587-1594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard AE, Field KG. A PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl Environ Microbiol. 2000b;66:4571–4574. doi: 10.1128/aem.66.10.4571-4574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick LK, Bernhard AE, Brodeur TJ, Santo Domingo JW, Simpson JM, Walters SP, Field KG. Host distributions of uncultivated fecal Bacteroidales bacteria reveal genetic markers for fecal source identification. Appl Environ Microbiol. 2005;71:3184–3191. doi: 10.1128/AEM.71.6.3184-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnleitner AH, Mach RL, Burtscher MM, Reischer GH, Ryzinska G, Keiblinger K, Rudnicki S, Knetsch S, Sommer R. IWA - Health related water microbiology symposium. Wales; Swansea: 2005b. “Back to the roots” - incidence and abundance of faecal indicators in faeces and sewage of potential pollution sources in an alpine karstic catchment area. [Google Scholar]
- Farnleitner AH, Wilhartitz I, Ryzinska G, Kirschner AK, Stadler H, Burtscher MM, Hornek R, Szewzyk U, Herndl G, Mach RL. Bacterial dynamics in spring water of alpine karst aquifers indicates the presence of stable autochthonous microbial endokarst communities. Environ Microbiol. 2005;7:1248–1259. doi: 10.1111/j.1462-2920.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Fiksdal L, Maki JS, LaCroix SJ, Staley JT. Survival and detection of Bacteroides spp., prospective indicator bacteria. Appl Environ Microbiol. 1985;49:148–150. doi: 10.1128/aem.49.1.148-150.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. Development of bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischer GH, Kasper DC, Steinborn R, Mach RL, Farnleitner AH. Quantitative PCR method for sensitive detection of ruminant fecal pollution in freshwater and evaluation of this method in alpine karstic regions. Appl Environ Microbiol. 2006;72:5610–5614. doi: 10.1128/AEM.00364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seurinck S, Defoirdt T, Verstraete W, Siciliano SD. Detection and quantification of the human-specific HF183 Bacteroides 16S rRNA genetic marker with real-time PCR for assessment of human faecal pollution in freshwater. Environ Microbiol. 2005;7:249–259. doi: 10.1111/j.1462-2920.2004.00702.x. [DOI] [PubMed] [Google Scholar]
- Simpson JM, Santo Domingo JW, Reasoner DJ. Microbial source tracking: state of the science. Environ Sci Technol. 2002;36:5279–5288. doi: 10.1021/es026000b. [DOI] [PubMed] [Google Scholar]
- Stricker AR, Wilhartitz I, Farnleitner AH, Mach RL. Development of a Scorpion probe-based real-time PCR for the sensitive quantification of Bacteroides sp. ribosomal DNA from human and cattle origin and evaluation in spring water matrices. Microbiol Res. 2006 doi: 10.1016/j.micres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Dore J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, editor. Guidelines for Drinking-water Quality. World Health Organisation; Geneva: 2004. [Google Scholar]