Abstract
Background
Currently approved Alzheimer’s disease (AD) treatments have been reported to provide symptomatic benefit, without proven impact on clinical progression. We hypothesized that the loss of initial therapeutic benefit over time may be mitigated by higher doses of a cholinesterase inhibitor.
Objective
The aim of this study was to determine the effectiveness and tolerability of increasing donepezil from 10 to 23 mg/d in patients with moderate to severe AD.
Methods
This randomized, double-blind study was conducted at 219 sites in Asia, Europe, Australia, North America, South Africa, and South America from June 6, 2007, to March 27, 2009. Patients aged 45 to 90 years with probable AD, Mini-Mental State Examination score 0 to 20 (moderate to severe impairment), and who were receiving donepezil 10 mg once daily for ≥12 weeks before the start of the study were eligible. Patients (n = 1467) were randomly assigned to receive high-dose donepezil (23 mg once daily) or standard-dose donepezil (10 mg once daily) for 24 weeks. Coprimary effectiveness measures were changes in cognition and global functioning, as assessed using least squares mean changes from baseline (LSM [SE] Δ) scores (last observation carried forward) on the Severe Impairment Battery (SIB; cognition) and the Clinician’s Interview-Based Impression of Change Plus Caregiver Input scale (CIBIC+; global function rating) overall change score (mean [SD]) at week 24. Treatment-emergent adverse events (TEAEs) were assessed using spontaneous patient/caregiver reporting and open-ended questioning; clinical laboratory testing (hematology, biochemistry, and urinalysis panels analyzed by a central laboratory); 12-lead ECG; and physical and neurologic examinations, including vital sign measurements.
Results
The effectiveness analyses included 1371 patients (mean age, 73.8 years; 62.8% female; 73.5% white; weight range, 34.0–138.7 kg). A total of 296 of 981 patients (30.2%) withdrew from the donepezil 23-mg/d group; 87 of 486 patients (17.9%) withdrew from the donepezil 10-mg/d group. At study end (week 24), the LSM (SE) Δ in SIB score was significantly greater with donepezil 23 mg/d than with donepezil 10 mg/d (+2.6 [0.58] vs +0.4 [0.66], respectively; difference, 2.2; P < 0.001). The between-treatment difference in CIBIC+ score was nonsignificant (4.23 [1.07] vs 4.29 [1.07]). In post hoc analysis, LSM Δ in SIB score and CIBIC+ treatment effect at end point were greater with donepezil 23 mg/d than 10 mg/d in patients with more advanced AD compared with less impaired patients (SIB, +1.6 [0.78] vs −1.5 [0.88], respectively [P < 0.001]; CIBIC+, 4.31 [1.09] vs 4.42 [1.10] [P = 0.028]). TEAEs were reported in 710 of 963 patients (73.7%) who received donepezil 23 mg/d and in 300 of 471 patients (63.7%) who received donepezil 10 mg/d. With donepezil 23 mg/d, mild, moderate, and severe TEAEs were reported in 297 (30.8%), 332 (34.5%), and 81 (8.4%) patients, respectively; with donepezil 10 mg/d, these proportions were 147 (31.2%), 119 (25.3%), and 34 (7.2%). The 3 most common severe AEs reported with the 23-mg/d dose were nausea (9 patients [0.9%] vs 1 [0.2%] with the 10-mg/d dose), dizziness (7 [0.7%] vs 1 [0.2%]), and vomiting (6 [0.6%] vs 0). The most commonly reported TEAEs considered probably related to treatment with the 23-mg/d dose were nausea (59 patients [6.1%] vs 9 [1.9%] with the 10-mg/d dose), vomiting (48 [5.0%] vs 4 [0.8%]), and diarrhea (31 [3.2%] vs 7 [1.5%]). Thirteen deaths were reported during the study or within 30 days of study discontinuation (23 mg/d, 8 patients [0.8%]; 10 mg/d, 5 patients [1.1%]); all were considered unrelated to the study medication.
Conclusions
In this study in patients with moderate to severe AD, donepezil 23 mg/d was associated with greater benefits in cognition compared with donepezil 10 mg/d. The between-treatment difference in global functioning was not significant in the overall population. Patients with more advanced AD appeared to benefit from donepezil 23 mg/d on the assessment of global functioning, but this observation requires additional studies for confirmation.
Keywords: Alzheimer’s disease, cognitive disorders, dementia, randomized controlled clinical trials
INTRODUCTION
As Alzheimer’s disease (AD) advances, cognitive and functional capacities become progressively impaired1,2 and caregiver burden increases. Approved pharmaco-therapies for AD, such as donepezil hydrochloride3; other acetylcholinesterase inhibitors (AChEIs), such as rivastigmine and galantamine4; and memantine, an N-methyl-D-aspartate receptor antagonist,5 have been reported to provide symptomatic benefits that are lost with disease progression over time despite continued treatment.6–8 To sustain symptomatic cognitive and functional benefits, clinicians might prescribe combination therapy, such as donepezil plus memantine.5,9,10 There are no other established evidence-based options in patients with disease progression.2,7 Because clinical decline in AD has been associated with the deterioration of cholinergic neurons,11,12 it is unclear whether patients with moderate to severe AD can benefit from higher doses of AChEIs.13
Donepezil hydrochloride is a selective, reversible AChEI believed to enhance central cholinergic function.14 The current maximum daily dose (10 mg) approved by the US Food and Drug Administration (FDA), the UK Medicines and Healthcare Products Regulatory Agency, and other regulatory authorities is available in an immediate-release (IR) tablet formulation.3 A matrix-type (sustained-release [SR]) tablet of 23 mg was developed to provide a higher once-daily dose while avoiding a sharp daily increase in peak concentration. The drug exposure with the SR formulation is ~92% (95% CI, 89.1–94.7; dose adjusted) that of the IR formulation, with a Tmax that is <2-fold greater (6–9 hours with SR vs 3–4 hours with IR) and an AUC0–∞ that is >2-fold greater (data on file, Eisai Inc., study no. E2020-G000-326, 2009).
The currently approved doses of 5 and 10 mg/d of donepezil have been associated with 20% to 30% inhibition of cortical AChE activity.15,16 In a study in 61 Japanese patients with AD who were receiving a stable dose of donepezil 5 mg/d, a dose increase to 10 mg/d for 24 weeks was associated with more effective prevention of deterioration in severe AD, as measured using the Revised Hasegawa Dementia Scale and Mini-Mental State Examination (MMSE).17,18 Based on those findings, doses of AChEIs that are higher than those currently approved might provide greater stabilization and/or symptomatic improvement in later stages of AD. A finding that increasing cholinesterase inhibition confers further benefits in moderate to severe disease, including in patients receiving combination therapy, would have implications for extending and/or improving AD treatment and significant value for public health.19
The objective of this study was to compare the effectiveness and safety profile of high-dose donepezil (23 mg/d) and standard-dose donepezil (10 mg/d) in patients with moderate to severe AD.
PATIENTS AND METHODS
This randomized, double-blind study was conducted at 219 sites in Asia, Europe, Australia, North America, South Africa, and South America from June 6, 2007, to March 27, 2009. The protocol and informed-consent form were approved by the independent ethics committee/institutional review board for each independent research site and conformed to the principles of the World Medical Association Declaration of Helsinki and all local regulations. The study design was reviewed and deemed appropriate by the FDA and other regulatory agencies.
Study Population
Eligible patients were 45 to 90 years of age and had a diagnosis of probable dementia of the Alzheimer’s type, as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition1 (code 290.00 or 290.10) and based on the National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer’s Disease and Related Disorders Association criteria20; had an MMSE score of 0 to 20 (moderate to severe impairment) and a Severe Impairment Battery (SIB)21,22 score ≤90; had a Cornell Scale for Depression in Dementia23 score <12; were otherwise physically healthy and ambulatory or ambulatory aided; and had clinical laboratory values within normal limits or, if abnormal, considered by the investigator to be clinically nonsignificant. Patients with the following comorbidities were considered eligible if the condition was deemed by the investigator to be stable and well controlled: hypertension (supine diastolic blood pressure <95 mm Hg); cardiovascular disease (stable on appropriate medication for ≥12 weeks before screening); diabetes mellitus (stable with no hospitalizations for diabetic ketoacidosis, hyperosmolar coma, or hypoglycemia within 12 weeks before screening); non–insulin-dependent diabetes (controlled with diet and/or oral medications); and hypothyroidism (controlled on a stable dose of medication for ≥12 weeks before screening, normal thyroid-stimulating hormone and free T4 concentrations at screening, and considered euthyroid). Eligible patients were also receiving a stable, single daily dose of donepezil 10 mg for ≥12 weeks before the start of the study. Donepezil use was confirmed by the presence of detectable plasma donepezil concentrations.
Patients were excluded if they had an additional neurologic disorder that might, in the investigator’s opinion, affect cognition or the assessment of cognition, even if the disorder was distinguishable from AD (including Parkinson’s disease, multi-infarct dementia, dementia due to cerebrovascular disease, Huntington’s disease, frontotemporal dementia, Creutzfeldt-Jakob disease, Lewy body dementia, normal-pressure hydrocephalus, brain tumor, progressive supranuclear palsy, seizure disorder, subdural hematoma, or multiple sclerosis). Magnetic resonance imaging or computed tomography within a year before screening was used to rule out causes of dementia other than probable AD.
Before conducting study procedures, investigators obtained written informed consent from each patient, if possible, or from the patient’s legal guardian or representative. If a patient was unable to provide written consent, he or she was required to provide verbal assent to participate in the study, with documentation of assent noted in the study record, and a caregiver was required to separately provide written informed consent for his/her own participation in the study. Caregivers were required to be sufficiently familiar with the patient to provide data on global functioning, with regular contact ≥10 hours/week, and could not be clinically depressed (>15 on the Center for Epidemiologic Studies–Depression scale)24 or cognitively impaired (MMSE <27 or <25 if illiterate).
Study Drug Administration
Patients were randomly assigned, in a 2:1 ratio using computer-generated randomization codes, to receive donepezil 23 mg (test) or donepezil 10 mg (reference) once daily for 24 weeks. Patients, caregivers, and study personnel were blinded to treatment assignment. Block size was 6, with site as the unit. Stratification was based on whether a patient was concurrently taking memantine at baseline. If a patient was taking memantine at a stable dose of ≤20 mg/d for ≥12 weeks before screening, use was allowed to continue. All additional prescriptions for AD, including other cholinesterase inhibitors, were required to have been discontinued for ≥12 weeks before screening. Atypical antipsychotics and selective serotonin reuptake inhibitors were permitted, the latter provided that doses were less than or equal to the approved range for therapeutic effectiveness as specified in the Physicians’ Desk Reference25 or regional equivalent, and that the dose was stable for ≥12 weeks before screening. The use of any medication known to interfere with the clinical effects of donepezil (eg, anticholinergics such as oxybutynin) or that could substantially impact cognition, either by enhancing alertness or causing sedation, was not permitted.
Because the treatments were not identical in appearance, a double-dummy design was used to maintain blinding. Study medication was provided free of charge and was administered at any time of day, provided that the time was consistent.
To determine compliance, unused tablets were counted and recorded by a designated staff member at the study site at each clinic visit (weeks 6, 12, 18, and 24), with the number of days since the last visit recorded as the treatment period. The number of tablets remaining was subtracted from the number of tablets dispensed; this value was divided by the number of days in the treatment period.
Effectiveness and Tolerability Assessments
Patients were asked to return to the clinic for effectiveness and tolerability assessments at treatment weeks 3 (tolerability only), 6, 12, 18, and 24. The coprimary effectiveness measures were changes in cognition and global functioning, as assessed using the SIB (cognition) and the Clinician’s Interview-Based Impression of Change Plus Caregiver Input scale (CIBIC+; global function rating). The SIB is a 40-item instrument administered to the patient that assesses cognitive function in patients with more advanced dementia.21,22 Total scores range from 0 (most impaired) to 100 (least impaired). The CIBIC+ is a semistructured tool administered by an independent clinician interviewing the patient and caregiver that assesses overall change and change in various domains of patient functioning26,27 on a 7-point scale (1 = marked improvement; 2 = moderate improvement; 3 = minimal improvement; 4 = no change; 5 = minimal worsening; 6 = moderate worsening; and 7 = marked worsening). The CIBIC+ rating uses baseline disease severity as a point of reference, captured by the Clinician’s Interview-Based Impression of Severity Plus Caregiver Input scale (CIBIS+).
Secondary effectiveness variables were scores on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living scale (severe version) (ADCS-ADL)28 and the MMSE. The ADCS-ADL is a 19-item instrument with total scores ranging from 0 (most impaired) to 54 (least impaired). The MMSE is a 30-item test of cognitive function, with total scores ranging from 0 (most impaired) to 30 (least impaired).17
All of the effectiveness assessments were conducted by systematically trained site raters after meeting qualification standards, including education and years of experience with the patient population.
Tolerability assessments included clinical laboratory testing (hematology, biochemistry, and urinalysis panels analyzed by a central laboratory that met regulatory certification requirements), 12-lead ECG read by a cardiologist or physician with advanced training, and physical and neurologic examinations, including vital sign measurements, at all clinic visits. Blood pressure was measured in triplicate after ≥5 minutes in the supine position; after ≥2 minutes in the standing position, blood pressure measurements were repeated. Heart rate was determined in the supine and standing positions at all clinic visits using the radial pulse, auscultation over the heart with a stethoscope, or other accepted means. Temperature, respiratory rate, and weight were also determined at all clinic visits. Height was recorded only at screening. Treatment-emergent adverse events (TEAEs) were recorded throughout the study and were determined using spontaneous reports from patients and/or caregivers and open-ended questioning. The investigators determined the severity of each TEAE (mild, moderate, or severe) and its relationship to the study treatment (unrelated, possibly related, or probably related).
Statistical Analysis
Sample size was calculated based on the findings from the randomized, placebo-controlled, parallel-group study by Tariot et al,5 in which memantine 20 mg/d or placebo was added to an existing regimen of donepezil 5 to 10 mg/d in 404 patients with probable AD, with meaningful treatment differences found. In the current study, ~1200 patients (donepezil 23 mg/d, 800; donepezil 10 mg/d, 400) were planned to be enrolled in the study to provide an overall power of ≥80% to find a significant difference between treatment groups (least squares mean change from baseline [LSM Δ], 3.0 on the SIB; 0.20 at week 24 on the CIBIC+). To assess whether the higher dose was associated with new or substantially increased safety concerns compared with the 10-mg dose, an interim tolerability analysis was conducted without statistical testing after the first 400 subjects in the intent-to-treat (ITT) population (patients who received ≥1 dose of study medication and in whom either [1] the SIB total score was available at baseline and ≥1 SIB total score was available after the administration of the first dose of study medication or [2] the CIBIS+ score was available at baseline and ≥1 CIBIC+ overall change score was available after the administration of the first dose of study medication) had completed or discontinued from the study. An independent safety monitoring board determined that the study could proceed as planned. All statistical tests were conducted using SAS version 8.0 or higher (SAS Institute Inc., Cary, North Carolina) and were 2-sided at a significance level of 0.05.29
Effectiveness was analyzed in the ITT population. Tolerability was analyzed in the safety population (all randomized patients who received ≥1 dose of study medication and who had data available from ≥1 post-baseline tolerability assessment).
An ANCOVA model with terms for baseline score, country, and treatment was used as the primary model for testing treatment effects on SIB score, with LSM (SE) Δ used to compare treatment groups. Similar analyses were conducted for the ADCS-ADL and MMSE. For the categoric end point, CIBIC+ score at week 24, a nonparametric ANCOVA method combined with a Cochran-Mantel-Haenszel test component was used.30 The analysis was adjusted for baseline CIBIS+ score, with a stratification adjustment for country. Additional prespecified analyses were based on whether patients were receiving memantine concurrently at baseline.
To further examine the coprimary end points, post hoc sensitivity analyses of the impact of baseline disease severity on treatment response were conducted in patients with baseline MMSE scores from 0 to 16 (more severe impairment) versus 17 to 20 (less severe impairment) and in the subgroup of patients from the United States (the country that randomized the largest number of patients [31.7% vs ≤8.0% from any other country]), which allowed a meaningful post hoc analysis of a sizable subpopulation (n = 432) with a more uniform basis of health care and clinical practice. A similar approach has been used by others.31
The primary effectiveness analyses used the last-observation-carried-forward (LOCF) method to impute missing values. The observed-cases (OC) population (patients who provided data at a given visit) at week 24 was also assessed to support the findings from the coprimary analyses.
RESULTS
Patient Disposition and Baseline Characteristics
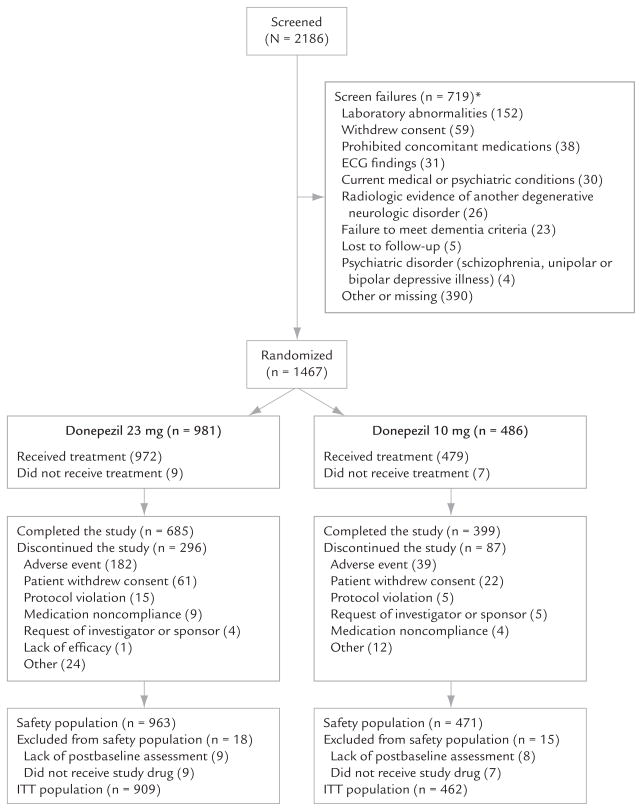
Patient disposition is presented in Figure 1. Site and patient distributions by country are shown in Table I. The baseline demographic and clinical characteristics of the safety population are presented in Table II (donepezil 23 mg/d, n = 963; donepezil 10 mg/d, n = 471; female, 63.0% and 62.4%, respectively). All baseline characteristics were comparable between the 2 treatment groups. Concurrent use of medication classes appeared proportionally similar between the 2 treatment groups. The medication classes most frequently concurrently used during the study were psychoactive agents (memantine and antidepressants, 50.3%), lipid-lowering agents (32.6%), and antithrombotic agents (31.9%). Memantine was prescribed concurrently in 36.6% and 35.7% of patients in the 23- and 10-mg/d groups, respectively; antidepressants in 25.1% and 26.3%; and antipsychotics in 11.1% and 10.0%. The mean durations of prior treatment with donepezil were 112.17 and 104.76 weeks.
Figure 1.
Patient disposition in this study of the effectiveness and tolerability of donepezil 23 or 10 mg/d in patients with moderate to severe Alzheimer’s disease. ITT = intent-to-treat (patients who received ≥1 dose of study medication and in whom either [1] the Severe Impairment Battery [SIB]21,22 total score was available at baseline and ≥1 SIB total score was available after the administration of the first dose of study medication or [2] the Clinician’s Interview-Based Impression of Severity Plus Caregiver Input scale [CIBIS+] score was available at baseline and ≥1 Clinician’s Interview-Based Impression of Change Plus Caregiver Input scale [CIBIC+]26,27 overall change score was available after the administration of the first dose of study medication). *If a patient failed screening for multiple reasons, he or she was counted under each reason.
Table I.
Global enrollment statistics in this study of the effectiveness and tolerability of donepezil 23 or 10 mg/d in patients with moderate to severe Alzheimer’s disease.
| Country | No. of Sites (n = 209) | No. of Randomized Patients (n = 1467) |
|---|---|---|
| United States | 61 | 465 |
| India | 18 | 113 |
| Poland | 14 | 86 |
| Germany | 12 | 76 |
| South Africa | 10 | 77 |
| South Korea | 9 | 92 |
| Spain | 9 | 62 |
| Chile | 5 | 77 |
| Argentina | 5 | 67 |
| Other* | 66 | 352 |
Countries with <50 randomized patients (sites/patients): Australia (10/37); Italy (8/31); France (8/26); Israel (7/44); United Kingdom (6/32); Austria (5/35); Lithuania (4/36); Croatia (4/35); Romania (4/19); Taiwan (3/31); Hong Kong (2/15); Sweden (2/7); Singapore (2/2); and Denmark (1/2).
Table II.
Baseline demographic and clinical characteristics of the safety population in this study of the effectiveness and tolerability of donepezil 23 or 10 mg/d in patients with moderate to severe Alzheimer’s disease.*†
| Characteristic | Patients With Baseline MMSE Score 0–16 |
US Patients |
All Patients |
|||
|---|---|---|---|---|---|---|
| Donepezil 23 mg/d (n = 676) | Donepezil 10 mg/d (n = 338) | Donepezil 23 mg/d (n = 308) | Donepezil 10 mg/d (n = 146) | Donepezil 23 mg/d (n = 963) | Donepezil 10 mg/d (n = 471) | |
| Age, mean (SD), y | 73.4 (8.65) | 73.4 (8.74) | 74.5 (8.74) | 75.0 (8.20) | 73.9 (8.53) | 73.8 (8.56) |
| Sex, no. (%) | ||||||
| Female | 437 (64.6) | 224 (66.3) | 194 (63.0) | 94 (64.4) | 607 (63.0) | 294 (62.4) |
| Male | 239 (35.4) | 114 (33.7) | 114 (37.0) | 52 (35.6) | 356 (37.0) | 177 (37.6) |
| Race, no. (%) | ||||||
| White | 489 (72.3) | 234 (69.2) | 268 (87.0) | 129 (88.4) | 708 (73.5) | 346 (73.5) |
| Asian/Pacific Islander | 117 (17.3) | 68 (20.1) | 0 | 1 (0.7) | 161 (16.7) | 87 (18.5) |
| Hispanic | 47 (7.0) | 24 (7.1) | 18 (5.8) | 7 (4.8) | 67 (7.0) | 26 (5.5) |
| Black | 19 (2.8) | 9 (2.7) | 22 (7.1) | 9 (6.2) | 22 (2.3) | 9 (1.9) |
| Other | 4 (0.6) | 3 (0.9) | 0 | 0 | 5 (0.5) | 3 (0.6) |
| Weight | ||||||
| Group, no. (%) | ||||||
| <55 kg | 162 (24.0) | 88 (26.0) | 43 (14.0) | 26 (17.8) | 218 (22.6) | 111 (23.6) |
| 55–<65 kg | 176 (26.0) | 94 (27.8) | 60 (19.5) | 34 (23.3) | 245 (25.4) | 129 (27.4) |
| 65–<75 kg | 162 (24.0) | 73 (21.6) | 94 (30.5) | 32 (21.9) | 240 (24.9) | 110 (23.4) |
| ≥75 kg | 175 (25.9) | 83 (24.6) | 111 (36.0) | 54 (37.0) | 259 (26.9) | 121 (25.7) |
| Data unavailable | 1 (0.1) | 0 | 0 | 0 | 1 (0.1) | 0 |
| Range, kg | 34.0–129.3 | 35.0–111.6 | 37.7–138.7 | 43.2–111.6 | 34.0–138.7 | 35.0–112.0 |
| Living arrangements, no. (%) | ||||||
| Lives with caregiver | 561 (83.0) | 269 (79.6) | 249 (80.8) | 107 (73.3) | 780 (81.0) | 365 (77.5) |
| Lives with relative or friend | 67 (9.9) | 27 (8.0) | 26 (8.4) | 12 (8.2) | 97 (10.1) | 45 (9.6) |
| Lives alone | 17 (2.5) | 18 (5.3) | 13 (4.2) | 8 (5.5) | 34 (3.5) | 30 (6.4) |
| Residence, no. (%) | ||||||
| Assisted-living facility | 14 (2.1) | 16 (4.7) | 13 (4.2) | 17 (11.6) | 20 (2.1) | 20 (4.2) |
| Senior residence or retirement home | 6 (0.9) | 4 (1.2) | 3 (1.0) | 2 (1.4) | 14 (1.5) | 5 (1.1) |
| Skilled nursing care facility | 5 (0.7) | 1 (0.3) | 3 (1.0) | 0 | 7 (0.7) | 2 (0.4) |
| Intermediate nursing care facility | 2 (0.3) | 0 | 0 | 0 | 3 (0.3) | 0 |
| Other | 4 (0.6) | 3 (0.9) | 1 (0.3) | 0 | 8 (0.8) | 4 (0.8) |
| Duration of prestudy donepezil 10 mg/d treatment, mean (SD), wk | 122.01 (114.97) | 109.84 (106.15) | 167.52 (120.69) | 166.67 (117.96) | 112.17 (108.18) | 104.76 (98.98) |
| Concurrent memantine use, no. (%) | 272 (40.2) | 137 (40.5) | 231 (75.0) | 108 (74.0) | 352 (36.6) | 168 (35.7) |
| CIBIS+ score group, no. (%) | ||||||
| Borderline mentally ill | 1 (0.1) | 1 (0.3) | 1 (0.3) | 0 | 7 (0.7) | 5 (1.1) |
| Mildly mentally ill | 30 (4.4) | 27 (8.0) | 30 (9.7) | 13 (8.9) | 99 (10.3) | 55 (11.7) |
| Moderately mentally ill | 266 (39.3) | 136 (40.2) | 136 (44.2) | 69 (47.3) | 451 (46.8) | 217 (46.1) |
| Markedly mentally ill | 275 (40.7) | 120 (35.5) | 98 (31.8) | 46 (31.5) | 300 (31.2) | 138 (29.3) |
| Severely mentally ill | 96 (14.3) | 52 (15.4) | 38 (12.3) | 17 (11.6) | 97 (10.1) | 53 (11.3) |
| Among the most extremely mentally ill | 4 (0.6) | 2 (0.6) | 4 (1.3) | 1 (0.7) | 4 (0.4) | 2 (0.4) |
| Data unavailable | 4 (0.6) | 0 | 1 (0.3) | 0 | 5 (0.5) | 1 (0.2) |
MMSE = Mini-Mental State Examination17; CIBIS+ = Clinician’s Interview-Based Impression of Severity Plus Caregiver Input scale.
The final total patient number was higher than planned due to an unexpected surge in enrollment during the final weeks of the recruitment period.
Percentages may not total 100 due to rounding.
Baseline disease severity as assessed using the MMSE and SIB was not notably different between the 2 treatment groups. Patients with more severe impairment (baseline MMSE 0–16) and US patients had lower mean SIB and poorer CIBIS+ scores at baseline, longer prior treatment with donepezil, and higher rates of concomitant memantine use than did the overall ITT population.
Treatment compliance rates were 93.2% in the high-dose group and 97.3% in the standard-dose group.
Effectiveness
SIB
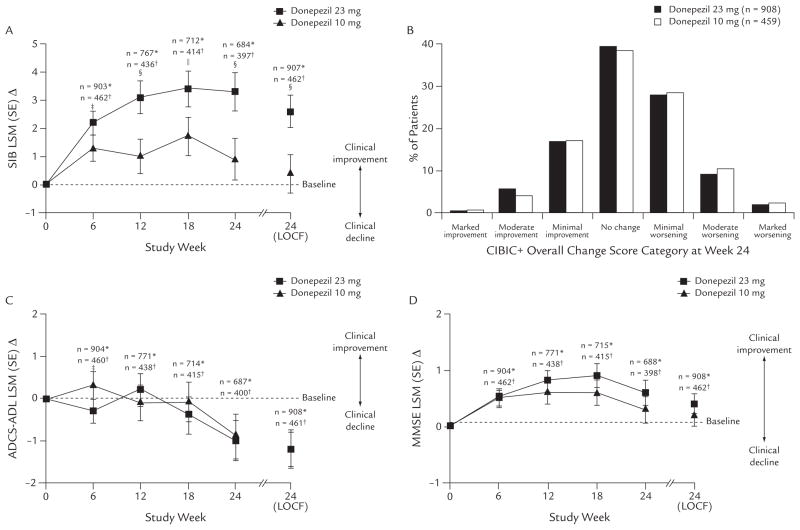
At study end (week 24), the LSM (SE) Δ in SIB score (ITT-LOCF) was significantly greater with donepezil 23 mg/d than with donepezil 10 mg/d (+2.6 [0.58] vs +0.4 [0.66], respectively; difference, 2.2; P < 0.001). Similar results were found in the OC population, with a 2.4-point LSM difference between the 2 treatment groups (P < 0.001) (Figure 2 and Table III).
Figure 2.
Effectiveness of donepezil 23 or 10 mg/d in patients with moderate to severe Alzheimer’s disease. (A) Changes from baseline in Severe Impairment Battery (SIB)21,22 total score (observed cases [OC] and intent-to-treat [ITT], last observation carried forward [LOCF]). (B) Frequency distribution of Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC+)26,27 scores at week 24 (ITT-LOCF). (C) Changes from baseline in Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL)28 total score (OC and ITT-LOCF). (D) Changes from baseline in Mini-Mental State Examination (MMSE)17 total score (OC and ITT-LOCF). LSM = least squares mean. *Donepezil 23 mg; †donepezil 10 mg; ‡P < 0.05 between treatment groups; §P < 0.001 between treatment groups; and ||P < 0.01 between treatment groups.
Table III.
Effectiveness of 24-week treatment with donepezil 23 or 10 mg/d in patients with moderate to severe Alzheimer’s disease (AD).
| Parameter | Baseline, ITT Population,a Mean (SD) |
24 Weeks, ITT Population, LSM (SE) Δ (LOCF) |
24 Weeks, OC Population,b LSM (SE) Δ |
|||||
|---|---|---|---|---|---|---|---|---|
| Donepezil 23 mg/d | Donepezil 10 mg/d | Donepezil 23 mg/d | Donepezil 10 mg/d | P | Donepezil 23 mg/d | Donepezil 10 mg/d | P | |
| SIBc | ||||||||
| All patientsd | ||||||||
| n | 907 | 462 | 907 | 462 | – | 684 | 397 | – |
| Value | 74.2 (17.58) | 75.6 (16.28) | +2.6 (0.58) | +0.4 (0.66) | <0.001 | +3.3 (0.69) | +0.9 (0.75) | <0.001 |
| Concurrent memantinee | ||||||||
| Yes | ||||||||
| n | 338 | 163 | 338 | 163 | – | 246 | 139 | – |
| Value | 72.0 (20.13) | 74.7 (17.68) | −0.2 (1.27) | −3.0 (1.36) | 0.003 | +0.9 (1.20) | −2.3 (1.35) | 0.003 |
| No | ||||||||
| n | 569 | 299 | 569 | 299 | – | 438 | 258 | – |
| Value | 75.4 (15.75) | 76.1 (15.47) | +3.1 (0.61) | +1.3 (0.72) | 0.007 | +3.6 (0.71) | +1.7 (0.79) | 0.008 |
| Baseline AD severityf | ||||||||
| More advanced (baseline MMSE 0–16) | ||||||||
| n | 641 | 331 | 641 | 331 | – | 490 | 285 | – |
| Value | 70.1 (19.03) | 72.3 (17.75) | +1.6 (0.78) | −1.5 (0.88) | <0.001 | +2.1 (0.80) | −1.3 (0.91) | <0.001 |
| Less impairment (baseline MMSE 17–20) | ||||||||
| n | 265 | 131 | 265 | 131 | – | 265 | 131 | – |
| Value | 83.8 (6.96) | 84.0 (6.31) | +4.3 (0.54) | +4.3 (0.63) | 0.939 | +5.0 (0.62) | +4.8 (0.69) | 0.764 |
| US Populationf | ||||||||
| n | 292 | 141 | 292 | 141 | – | 211 | 123 | – |
| Value | 73.1 (10.31) | 76.6 (15.43) | +2.7 (0.59) | −1.2 (0.85) | <0.001 | +3.0 (0.72) | −1.1 (0.95) | 0.001 |
| CIBIS+/CIBIC+g | ||||||||
| All patientsh | ||||||||
| n | 904 | 461 | 908 | 459 | – | 682 | 395 | |
| Value | 4.42 (0.85) | 4.38 (0.89) | 4.23 (1.07) | 4.29 (1.07) | 0.179 | 4.18 (1.11) | 4.28 (1.09) | 0.059 |
| Concomitant memantinee | ||||||||
| Yes | ||||||||
| n | 336 | 163 | 338 | 161 | 245 | 136 | ||
| Value | 4.58 (0.88) | 4.60 (0.84) | 4.40 (1.02) | 4.52 (0.94) | 0.137 | 4.38 (1.05) | 4.54 (0.97) | 0.107 |
| No | ||||||||
| n | 568 | 298 | 570 | 298 | 437 | 259 | ||
| Value | 4.32 (0.82) | 4.26 (0.89) | 4.12 (1.09) | 4.16 (1.12) | 0.380 | 4.07 (1.12) | 4.15 (1.13) | 0.204 |
| Baseline AD severityf | ||||||||
| More advanced (baseline MMSE 0–16) | ||||||||
| n | 639 | 331 | 642 | 329 | 488 | 284 | ||
| Value | 4.67 (0.80) | 4.58 (0.87) | 4.31 (1.09) | 4.42 (1.10) | 0.028 | 4.29 (1.13) | 4.42 (1.11) | 0.021 |
| Less impairment (baseline MMSE 17–20) | ||||||||
| n | 264 | 130 | 265 | 130 | 194 | 111 | ||
| Value | 3.80 (0.63) | 3.88 (0.70) | 4.02 (1.01) | 3.95 (0.91) | 0.595 | 3.91 (0.99) | 3.93 (0.97) | 0.921 |
| US populationf | ||||||||
| n | 291 | 141 | 292 | 140 | 211 | 120 | ||
| Value | 4.50 (0.90) | 4.45 (0.83) | 4.38 (0.97) | 4.57 (0.89) | 0.033 | 4.38 (1.01) | 4.59 (0.91) | 0.051 |
| ADCS-ADLi | ||||||||
| n | 908 | 461 | 908 | 461 | 687 | 400 | ||
| Value | 34.1 (10.88) | 34.5 (11.19) | −1.2 (0.40) | −1.2 (0.45) | 0.882 | −1.0 (0.48) | −0.9 (0.52) | 0.827 |
| MMSEi | ||||||||
| n | 908 | 462 | 908 | 462 | 688 | 398 | ||
| Value | 13.1 (4.99) | 13.1 (4.72) | +0.4 (0.18) | +0.2 (0.20) | 0.244 | +0.6 (0.22) | +0.3 (0.23) | 0.127 |
ITT = intent-to-treat; LSM = least squares mean; Δ = change from baseline to week 24; LOCF = last observation carried forward; OC = observed cases; SIB = Severe Impairment Battery21,22; MMSE = Mini-Mental State Examination17; CIBIS+/CIBIC+ = Clinician’s Interview-Based Impression of Severity Plus Caregiver Input scale/Clinician’s Interview-Based Impression of Change Plus Caregiver Input26,27; ADCS-ADL = Alzheimer’s Disease Cooperative Study–Activities of Daily Living (severe version).28
Patients who received ≥1 dose of study medication and in whom either (1) the SIB total score was available at baseline and ≥1 SIB total score was available after the administration of the first dose of study medication or (2) the CIBIS+ score was available at baseline and ≥1 CIBIC+ overall change score was available after the administration of the first dose of study medication.
The OC population analysis was exploratory for all end points.
Analysis method for the SIB was an ANCOVA model with terms for baseline, country, and treatment for the change from baseline to week 24.
Primary end point.
Preplanned exploratory end point.
Post hoc end point.
For CIBIS+/CIBIC+, values are mean (SD) at week 24 (nonparametric ANCOVA combined with a Cochran-Mantel-Haenszel test, adjusted for CIBIS+ at baseline with a stratification adjustment for country, for CIBIC+ scores at week 24). CIBIS+ scores were collected at baseline and established a point of reference for subsequent CIBIC+ assessments.
Coprimary end point.
Secondary end point. ANCOVA with terms for baseline, country, and treatment for the change from baseline to week 24.
In the OC population, the LSM Δ was significantly greater with donepezil 23 mg/d at weeks 6 (P < 0.05), 12 (P < 0.001), 18 (P < 0.01), and 24 (P < 0.001) (Figure 2). In both subgroups of patients concurrently taking and not taking memantine, the LSM Δ was significantly greater with donepezil 23 mg/d (P = 0.003 and P = 0.007, respectively, vs 10 mg) (Table III).
CIBIC+
In the ITT population, mean (SD) CIBIC+ scores at week 24 (LOCF) were 4.23 (1.07) with donepezil 23 mg/d and 4.29 (1.07) with donepezil 10 mg/d; the difference was not statistically significant. In the CIBIC+ OC population analysis, global scores were 4.18 (1.11) with 23 mg/d versus 4.28 (1.09) with 10 mg/d (P = NS). In the subgroups of patients receiving and not receiving concurrent memantine, CIBIC+ overall change scores were not statistically significantly different between treatment groups (Table III).
Secondary End Points
No incremental benefit of high-dose donepezil was found in ADCS-ADL or MMSE total scores in the ITT population. There was little change from baseline on the ADCS-ADL scale at study end (LSM Δ, −1.2 in both groups). The MMSE score was numerically increased compared with baseline (LSM, +0.4 and +0.2 with 23 and 10 mg/d, respectively) (Figure 2), with no statistically significant incremental benefit with the 23-mg/d dose (Table III).
Impact of AD Severity on Treatment Response
In the post hoc analysis of the impact of baseline disease severity on treatment response based on the positive SIB results in the overall study population (Table III), greater treatment effects were found in patients with more impairment at baseline (MMSE score 0–16), representing >70% of the study population. In these patients, the between-treatment differences in LSM Δ in SIB score were 3.1 (ITT, P < 0.001) and 3.4 (OC, P < 0.001), which were numerically greater than the treatment differences in the overall population (2.2 and 2.4, respectively). In patients with more impairment at baseline, those in the 23-mg/d group showed improvement compared with baseline (+1.6), whereas in patients continuing on the 10-mg dose, the score declined (−1.5). Treatment differences in SIB scores were not found in patients with less impairment at baseline (MMSE score 17–20) (Table III). Significantly greater treatment differences in LSM Δ SIB scores were found in the sub-population of US patients (more impaired at the start of the study compared with the overall population) (ITT, 3.9 [P < 0.001]; OC, 4.1 [P = 0.001]).
Similarly, on the global functioning measure, the between-treatment difference in CIBIC+ overall change score at week 24 was not significant in the overall patient population, but was significant in patients with more impairment at baseline (MMSE score 0–16) (P = 0.028) and in the US patients (P = 0.033) (Table III).
Tolerability
TEAEs occurring in ≥2% of patients in the 23-mg/d group that were also reported at a higher frequency than in the 10-mg/d treatment group are presented in Table IV. Overall, 710 of 963 patients (73.7%) who received the 23-mg/d dose and 300 of 471 (63.7%) in the 10-mg/d group experienced ≥1 AE during the study. In both treatment groups, gastrointestinal (GI) TEAEs occurred at the highest frequency within the first month after starting study medication (23 mg/d, 21.0% of patients reporting first onset of any GI TEAE in the first month, ~3% thereafter; 10 mg/d, 5.9% and ~2% thereafter). In both treatment groups, most patients reported TEAEs that were mild or moderate in severity (23 mg/d, 297 [30.8%] mild, 332 [34.5%] moderate, and 81 [8.4%] severe; 10 mg/d, 147 [31.2%] mild, 119 [25.3%] moderate, and 34 [7.2%] severe). More patients in the 23-mg/d dose group had TEAEs that were classified by the investigators as possibly or probably related to treatment (301 [31.3%] and 173 [18.0%], respectively) compared with the 10-mg/d dose group (97 [20.6%] and 33 [7.0%]). The 3 most common severe AEs reported with the 23-mg/d dose were nausea (9 patients [0.9%] vs 1 [0.2%] with the 10-mg/d dose), dizziness (7 [0.7%] vs 1 [0.2%]), and vomiting (6 [0.6%] vs 0). The most commonly reported TEAEs considered probably related to treatment with the 23-mg/d dose were nausea (59 patients [6.1%] vs 9 [1.9%] with the 10-mg/d dose), vomiting (48 [5.0%] vs 4 [0.8%]), and diarrhea (31 [3.2%] vs 7 [1.5%]). There were no notable findings or clinically meaningful differences between treatment groups in clinical laboratory assessments, vital signs, or ECGs. Although no notable changes in mean weight were observed in either treatment group, decreased weight as an AE was reported in 45 patients (4.7%) in the 23-mg/d group and 12 patients (2.5%) in the 10-mg/d group. Compared with baseline weight, 11.3% of patients in the donepezil 23-mg group had a weight decrease of ≥7% at any time during the study (79 [8.4%] at the end of the study) compared with 7.4% at any time in the group who received 10 mg (23 [4.9%] at the end of the study). Patients in the 23-mg/d treatment group with lower weight at baseline (<55 kg) had a higher incidence of TEAEs (178/218 [81.7%]) than did patients with higher weight (531/744 [71.4%]).
Table IV.
Treatment-emergent adverse events* (TEAEs) in patients with moderate to severe Alzheimer’s disease who received ≥1 dose of treatment with donepezil 23 or 10 mg/d. Data are number (%) of patients.
| Parameter | Donepezil 23 mg/d (n = 963) | Donepezil 10 mg/d (n = 471) |
|---|---|---|
| Patients with ≥1 TEAE | 710 (73.7) | 300 (63.7) |
| TEAE | ||
| Nausea | 114 (11.8) | 16 (3.4) |
| Vomiting | 89 (9.2) | 12 (2.5) |
| Diarrhea | 80 (8.3) | 25 (5.3) |
| Anorexia | 51 (5.3) | 8 (1.7) |
| Dizziness | 47 (4.9) | 16 (3.4) |
| Weight decrease | 45 (4.7) | 12 (2.5) |
| Urinary tract infection | 42 (4.4) | 19 (4.0) |
| Headache | 41 (4.3) | 15 (3.2) |
| Fall | 39 (4.0) | 18 (3.8) |
| Agitation | 38 (3.9) | 18 (3.8) |
| Insomnia | 33 (3.4) | 11 (2.3) |
| Bradycardia and sinus bradycardia | 27 (2.8) | 3 (0.6) |
| Aggression | 26 (2.7) | 12 (2.5) |
| Urinary incontinence | 24 (2.5) | 6 (1.3) |
| Fatigue | 23 (2.4) | 4 (0.8) |
| Asthenia | 20 (2.1) | 3 (0.6) |
| Somnolence | 20 (2.1) | 5 (1.1) |
| Contusion | 20 (2.1) | 1 (0.2) |
Medical Dictionary for Regulatory Activities preferred terms.32 TEAEs that occurred in ≥2% of patients who received donepezil 23 mg/d and that occurred at a higher frequency with donepezil 23 mg/d than with donepezil 10 mg/d are shown.
Thirteen patients died during the study or within 30 days after the administration of the last dose (8 [0.8%] in the 23-mg/d group and 5 [1.1%] in the 10-mg/d group); none of the deaths were considered related to the study medication. Serious TEAEs occurred in 80 patients (8.3%) in the 23-mg/d group and 45 patients (9.6%) in the 10-mg/d group (Table V); the majority (53/80 [66.3%] in the 23-mg/d group and 34/45 [75.6%] in the 10-mg/d group) were considered not related to treatment. More patients in the 23-mg/d group (182 [18.6%]) discontinued due to TEAEs than patients in the 10-mg/d group (39 [7.9%]); of the total discontinuations due to AEs in the higher-dose group, the majority of those discontinuations occurred during the first month of treatment (108 patients [60.3%]). The most common (occurring in ≥1% in either group) TEAEs that led to discontinuation in the 23- and 10-mg/d groups were vomiting (28 [2.9%] and 2 [0.4%], respectively), nausea (18 [1.9%] and 2 [0.4%]), diarrhea (16 [1.7%] and 2 [0.4%]), and dizziness (11 [1.1%] and 0).
Table V.
Serious treatment-emergent adverse events* (TEAEs) occurring in ≥0.5% of patients with moderate to severe Alzheimer’s disease who received ≥1 dose of treatment with donepezil 23 or 10 mg/d. Data are number (%) of patients.
| Parameter | Donepezil 23 mg/d (n = 963) | Donepezil 10 mg/d (n = 471) |
|---|---|---|
| Patients with ≥1 serious TEAE | 80 (8.3) | 45 (9.6) |
| Serious TEAE | ||
| Fall | 6 (0.6) | 2 (0.4) |
| Urinary tract infection | 6 (0.6) | 2 (0.4) |
| Pneumonia | 3 (0.3) | 3 (0.6) |
| Syncope | 2 (0.2) | 5 (1.1) |
| Aggression | 2 (0.2) | 4 (0.8) |
| Confusional state | 1 (0.1) | 3 (0.6) |
Medical Dictionary for Regulatory Activities preferred terms.32
DISCUSSION
In this large-scale, randomized, double-blind study in patients with moderate to severe AD who were already receiving 10 mg/d of donepezil IR, statistically significant benefit on 1 of the 2 prespecified coprimary outcome measures was found with donepezil 23 mg/d compared with continued 10-mg/d treatment. Although benefit was found in cognition as measured on the SIB, no incremental benefit above that achieved with 10-mg/d treatment was found on global functioning as measured using the CIBIC+.
This study assessed whether patients with moderate to severe dementia, who are presumed to have greater loss of brain cholinergic function and therefore reduced acetylcholine production, would respond to higher doses of a cholinesterase inhibitor. The data support the idea that patients with more advanced AD can still achieve therapeutic benefit. The post hoc analyses found greater treatment effect on the SIB when the less impaired patients were excluded from the analysis. Although these analyses were post hoc, they are also consistent with significant benefit of donepezil 23 mg/d on the CIBIC+ in more advanced patients. The magnitude of change on the SIB found in the more impaired patients was similar to that observed when memantine was added to a regimen of donepezil in the study by Tariot et al.5 The findings from that trial were used to support the approval of combination therapy with donepezil and memantine, which is now used in clinical practice.
From the standpoint of clinical use, it is expected that a higher dose of donepezil would be tried when lower doses had already been used and either did not achieve the expected effect, or after initial treatment benefit was either clinically insufficient or appeared to wane. This study was therefore designed to compare the effects of a dose increase to 23 mg/d with those of continued treatment with 10 mg/d of donepezil. Because continued treatment in practice would likely be based on tolerability, it is of interest to examine the results in those patients who were able to complete the entire 24 weeks of study treatment. In this OC population (n = 1084), the difference between treatments on the SIB and the effect of 23 mg/d on the CIBIC+ were more robust than in the ITT population. In addition, clinicians commonly prescribe memantine for an additive benefit in patients with moderate to severe AD who are already receiving donepezil 10 mg/d. Benefits on cognition were found in this study regardless of whether patients were receiving memantine concurrently. Data from the US patient population, ~32% of the total safety population, also support the view that donepezil 23 mg/d benefited patients with more advanced AD: US patients were somewhat more impaired at baseline and were more likely to be receiving memantine than was the overall patient population. In those patients, there was a treatment difference favoring the higher dose on both coprimary measures.
There was no significant incremental benefit with the 23-mg/d dose above that with the 10-mg/d dose on the secondary end points, ADCS-ADL and MMSE. The ADCS-ADL scale has shown good sensitivity to changes in function in response to treatment compared with placebo.33 However, there are limitations for this class of instruments,34 and some previous studies report no difference in ADCS-ADL even when the active drug showed significant cognitive advantage.35,36 In addition, the ADCS-ADL scale may not be sufficiently applicable in global studies; variability in cultural differences in activities of daily living, caregiving practices, or disease stage severity of the patient population being assessed may have limited the ability to measure change despite common training across all raters who conducted the assessments, which was aimed at harmonizing assessment methodology. In a previously published study in patients with severe AD who were receiving donepezil,8 little change occurred on the ADCS-ADL scale in the 10-mg/d treatment group over 24 weeks (−1.4 points from baseline). Similarly, patients in the current study had little change in ADCS-ADL during the study (−1.2 points in 24 weeks). That there was little change on the MMSE may be due in part to the fact that the patient population had moderate to severe AD, as the scale is known to exhibit floor effects and thus there would be little opportunity for change in patients with lower MMSE scores.37
When donepezil is titrated from 5 to 10 mg/d, there is an increase in AEs.3,38 The current study design involved increasing the dose to 23 mg/d from background treatment with 10 mg/d donepezil in patients who tolerated the latter dose. Thus, the observed pattern of AEs was expected. Overall, the TEAEs of nausea, vomiting, and diarrhea were the most common treatment-related events in the 23-mg/d group and also occurred more frequently than in the 10-mg/d group and in association with the dose escalation. Patients of lower initial weight (<55 kg) experienced more TEAEs than did those weighing more. The discontinuation rate due to TEAEs was numerically higher in the 23-mg/d group, mostly reflecting a higher rate of GI-related TEAEs. There were no dose-related increases in serious TEAEs, deaths, or TEAEs associated with institutionalization, such as agitation and falls.
Because of the global nature of this trial, the ability to detect treatment differences may have been confounded by different interpretations of the assessment scales and variations in the patient population with respect to baseline disease severity or duration of prior donepezil exposure, because these varied considerably in the different regions. The trial design also did not permit an assessment of the interaction between the effects of the higher donepezil dose and concurrent use of memantine, because patients were enrolled regardless of concurrent memantine use, and at baseline, patients who were concurrently receiving memantine had more severe AD compared with those who were not. The analyses based on disease severity and region were not prespecified (post hoc). In addition, the methodology for compliance assessment, while standard, cannot be used to assess actual medication intake.
Future research should further examine the suggestion that patients with more cognitive impairment are the most appropriate population for higher donepezil doses and the long-term outcome of such treatment.
CONCLUSIONS
In this study in patients with moderate to severe AD who were receiving a stable dose of donepezil 10 mg/d, donepezil 23 mg/d provided significantly greater cognitive benefit as measured using the SIB. Although no significantly greater effect on global functioning as measured by CIBIC+ was found with the dose increase in the overall population, findings from the post hoc analyses of the SIB suggested that more severely impaired patients may also experience a global benefit (CIBIC+) with an increase to the higher donepezil dose.
Acknowledgments
The design, conduct, analysis, and publication of this study were sponsored by Eisai Inc.
The authors are grateful for the assistance of the clinical investigators and their site staff, the global Eisai study team, and the patients and their caregivers who volunteered their time and effort for this clinical program. The authors acknowledge Lisa Pierchala, MPH, MMS Holdings Inc., for her editorial assistance, including technical editing, text revision, and document formatting.
Dr. Farlow has received research funding from Bristol-Myers Squibb Company, Danone, Élan Pharmaceuticals, Inc., Eli Lilly and Company, Forest Laboratories, Inc., Janssen Pharmaceutica, Inc., Medivation, Inc., Novartis Pharmaceuticals Corporation, OctaPharma AG, Pfizer Inc, and Sonexa Therapeutics, Inc.; has been a consultant for Accera, Inc., Adamas Pharmaceuticals, Inc., Adlyfe Inc., AstraZeneca Pharmaceuticals LP, Astellas Pharma US Inc., Bayer Pharmaceuticals Corporation, BioRx, CoMentis, Inc., Cortex Pharmaceuticals, Inc., Dainippon Sumitomo Pharma Co., Ltd., Eisai Ltd., Eli Lilly, GlaxoSmithKline, Medivation, Merck & Co., Inc., Novartis, Noven Pharmaceuticals, Inc., OctaPharma, QR Pharma, Inc., The sanofi-aventis Group, Schering-Plough Corp., Suven Life Sciences Limited, and Toyama Chemical Co., Ltd.; has been a member of the speakers’ bureaus at Eisai, Forest Laboratories, Janssen, Novartis, and Pfizer; and has received royalties from Élan for intellectual property and research support from US Public Health Service grant numbers NIA P30-AG10133, U01 AG 024904, U24 AG026385, and AG18379. Dr. Salloway has been a member of the scientific advisory boards at Bristol-Myers Squibb, Eisai, Élan, Pfizer, and sanofi-aventis; has been an associate editor of Journal of Neuropsychiatry and Clinical Neurosciences; has received publishing royalties for The Frontal Lobes and Neuropsychiatric Illness (American Psychiatric Publishing, Inc., 2001), The Neuropsychiatry of Limbic and Subcortical Disorders (American Psychiatric Publishing, 1997), and Vascular Dementia (Humana Press, Inc., 2004); has received honoraria from Athena Diagnostics, Inc., Eisai, Elan, Forest, Novartis, and Pfizer; holds corporate appointments with Medivation, Merck, and EMD Serono, Inc.; has received research support from Bristol-Myers Squibb, Eisai, Élan, Janssen Alzheimer’s Immunotherapy, Pfizer, and Wyeth; has received research support from Cephalon, Inc., Forest, GlaxoSmithKline, Myriad Genetics, Inc., Neurochem Inc., and Voyager Pharmaceutical Corporation; has received research support from the Alzheimer’s Disease Neuroimaging Initiative and Dominantly Inherited Alzheimer’s Network (grant no. NIA 1U01AG032438-01); and has received research support from Aging Brain: DTI, Subcortical Ischemia, and Behavior (grant no. NIA 1 R03 AG023916-01A1), The Norman and Rosalie Fain Family Foundation, and the Champlin Foundation. Dr. Tariot has received consulting fees from Acadia Pharmaceuticals Inc., AC Immune SA, Allergan Inc., Eisai, Epix Pharmaceuticals, Inc., Forest, Genentech, Inc., MedAvante, Inc., Memory Pharmaceuticals Corp., Myriad, sanofi-aventis, Schering-Plough, and Worldwide Clinical Trials, Inc.; has received consulting fees and research support from Abbott Laboratories Inc., AstraZeneca, Avid Radiopharmaceuticals, Inc., Baxter Healthcare Corporation, Bristol-Myers Squibb, Élan, GlaxoSmithKline, Eli Lilly, Medivation, Merck, Pfizer, Toyama, and Wyeth; has received educational fees from the Alzheimer’s Foundation of America; and has received research support from the National Institute on Aging, National Institute of Mental Health, Alzheimer’s Association, Arizona Department of Health Services, and the Institute for Mental Health Research. Drs. Yardley, Moline, Wang, Brand-Schieber, Hsu, and Satlin and Mr. Zou are employees of Eisai.
Footnotes
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: APA; 1994. (DSM-IV) [Google Scholar]
- 2.Reisberg B, Burns A, Brodaty H, et al. Diagnosis of Alzheimer’s disease. Report of an International Psychogeriatric Association Special Meeting Work Group under the cosponsorship of Alzheimer’s Disease International, the European Federation of Neurological Societies, the World Health Organization, and the World Psychiatric Association. Int Psychogeriatr. 1997;9(Suppl 1):11–38. doi: 10.1017/s1041610297004675. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed June 8, 2010];Aricept (donepezil hydrochloride) [prescribing information] http://www.pfizer.com/files/products/uspi_aricept.pdf.
- 4.Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–739. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- 5.Tariot PN, Farlow MR, Grossberg GT, et al. for the Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 6.Feldman H, Gauthier S, Hecker J, et al. for the Donepezil MSAD Study Investigators Group. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease [published correction appears. Neurology. 2001;57:2153. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]; Neurology. 2001;57:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 7.Geldmacher DS, Frolich L, Doody RS, et al. Realistic expectations for treatment success in Alzheimer’s disease. J Nutr Health Aging. 2006;10:417–429. [PubMed] [Google Scholar]
- 8.Winblad B, Kilander L, Eriksson S, et al. for the Severe Alzheimer’s Disease Study Group. Donepezil in patients with severe Alzheimer’s disease: Double-blind, parallel-group, placebo-controlled study [published corrections appear in Lancet. 2006;386:1650 and Lancet. 2006;367:1980] Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 9.Xiong G, Doraiswamy PM. Combination drug therapy for Alzheimer’s disease: What is evidence-based, and what is not? Geriatrics. 2005;60:22–26. [PubMed] [Google Scholar]
- 10.Tariot PN, Yaari R. Combination therapies for treating Alzheimer’s disease. [Accessed February 15, 2010];US Neurology. 2008 4:31–33. http://www.touchneurology.com/articles/combination-therapies-treating-alzheimer-s-disease.
- 11.Mesulam M. The cholinergic lesion of Alzheimer’s disease: Pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 12.Pappas BA, Bayley PJ, Bui BK, et al. Choline acetyltransferase activity and cognitive domain scores of Alzheimer’s patients. Neurobiol Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]
- 13.Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs. 2001;15:375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Shintani EY, Uchida KM. Donepezil: An anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54:2805–2810. doi: 10.1093/ajhp/54.24.2805. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl DE, Minoshima S, Frey KA, et al. Limited donepezil inhibition of acetylcholinesterase measured with positron emission tomography in living Alzheimer cerebral cortex. Ann Neurol. 2000;48:391–395. [PubMed] [Google Scholar]
- 16.Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Nozawa M, Ichimiya Y, Nozawa E, et al. Clinical effects of high oral dose of donepezil for patients with Alzheimer’s disease in Japan. Psychogeriatrics. 2009;9:50–55. doi: 10.1111/j.1479-8301.2009.00291.x. [DOI] [PubMed] [Google Scholar]
- 19.Farlow MR. Moderate to severe Alzheimer disease: Definition and clinicalrelevance. Neurology. 2005;65 (Suppl 3):S1–S4. [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51:41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt FA, Ashford W, Ernesto C, et al. The severe impairment battery: Concurrent validity and the assessment of longitudinal change in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 2):S51–S56. [PubMed] [Google Scholar]
- 23.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Physicians’ Desk Reference. 64. Montvale, NJ: Thomson PDR; 2010. [Google Scholar]
- 26.Joffres C, Graham J, Rockwood K. Qualitative analysis of the clinician interview-based impression of change (Plus): Methodological issues and implications for clinical research. Int Psychogeriatr. 2000;12:403–413. doi: 10.1017/s1041610200006505. [DOI] [PubMed] [Google Scholar]
- 27.Schneider LS, Olin JT. Clinical global impressions in Alzheimer’s clinical trials. Int Psychogeriatr. 1996;8:277–288. doi: 10.1017/s1041610296002645. discussion 288–290. [DOI] [PubMed] [Google Scholar]
- 28.Galasko D, Schmitt F, Thomas R, et al. for the Alzheimer’s Disease Cooperative Study. Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc. 2005;11:446–453. doi: 10.1017/s1355617705050502. [DOI] [PubMed] [Google Scholar]
- 29.Dmitrienko A, Chuang-Stein C, D’Agostino R. Pharmaceutical Statistics Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc; 2007. [Google Scholar]
- 30.Koch GG, Tangen CM, Jung JW, Amara IA. Issues for covariance analysis of dichotomous and ordered categorical data from randomized clinical trials and non-parametric strategies for addressing them. Stat Med. 1998;17:1863–1892. doi: 10.1002/(sici)1097-0258(19980815/30)17:15/16<1863::aid-sim989>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Brodaty H, Corey-Bloom J, Potocnik FC, et al. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;20:120–132. doi: 10.1159/000086613. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration. [Accessed June 21, 2010];Medical Dictionary for Regulatory Activities. [MedDRA Web site]. http://www.meddramsso.com/
- 33.Galasko D, Kershaw PR, Schneider L, et al. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. J Am Geriatr Soc. 2004;52:1070–1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- 34.Demers L, Oremus M, Perrault A, et al. Review of outcome measurement instruments in Alzheimer’s disease drug trials: Psychometric properties of functional and quality of life scales. J Geriatr Psychiatry Neurol. 2000;13:170–180. doi: 10.1177/089198870001300402. [DOI] [PubMed] [Google Scholar]
- 35.Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer’s disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 36.Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer’s disease in a Japanese population: Results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 37.Vellas B, Gauthier S, Allain H, et al. Consensus statement on dementia of Alzheimer type in the severe stage. J Nutr Health Aging. 2005;9:330–338. [PubMed] [Google Scholar]
- 38.Whitehead A, Perdomo C, Pratt RD, et al. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: A meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry. 2004;19:624–633. doi: 10.1002/gps.1133. [DOI] [PubMed] [Google Scholar]