Abstract
Objective
To examine the relationship between body mass index (BMI) and cognitive decline in subjects diagnosed with mild cognitive impairment (MCI).
Methods
Neuropsychologic and clinical evaluations were conducted at baseline, 6-months, and 1-year on 286 MCI subjects enrolled in the Alzheimer’s Disease Neuroimaging Initiative. A global cognitive composite score was derived (mean Z-score) from performance on 9 neuropsychologic subtests. Height and weight were assessed at baseline and used to calculate BMI. Generalized estimating equations (linear and logistic) assessed the relationships of baseline BMI with cognitive outcomes, clinician judgment of “clinically significant decline” over 1-year, and diagnostic progression from MCI to Alzheimer disease.
Results
Lower baseline BMI was associated with significant declines in cognitive performance in individuals with MCI over 1 year (Mini-Mental State Examination, Alzheimer Disease Assessment Scale-Cognitive subscale, and a global cognitive composite; all P<0.05). We observed a significant protective effect of baseline BMI in reducing the risk of a clinically significant decline in Alzheimer Disease Assessment Scale-Cognitive subscale and mini-mental state examination (P<0.05). No association was found between BMI and changes in the clinical dementia rating sum of boxes or conversion to Alzheimer disease.
Conclusions
Lower baseline BMI is associated with more rapid cognitive decline in MCI. This relationship suggests either body composition may influence the rate of cognitive decline in MCI or factors related to MCI influence body composition.
Keywords: mild cognitive impairment, Alzheimer disease, body weight, body composition, body mass index, cognitive decline
Higher body mass index (BMI) in midlife is associated with structural brain changes,1,2 cognitive decline,3 and an increased risk of Alzheimer disease (AD)4 in late life. Accumulating evidence suggests, however, that the relationship between BMI and cognitive outcomes is attenuated, and perhaps reversed, by age.5 In longitudinal studies of older adult populations (generally aged 65 y and above), low BMI is associated with an increased risk of developing dementia,6 whereas high BMI is associated with a lower risk of dementia.5,7 In addition, weight loss is associated with an increased risk of dementia5 suggesting it may be a risk factor or an early sign of AD. This is consistent with multiple studies demonstrating that weight loss is present several years before the onset of clinically recognizable AD symptoms.8–11
Mild cognitive impairment (MCI) is characterized as a transition phase between normal aging and AD with 10% to 15% of MCI patients progressing to overt AD annually. There is little prospective data on body composition in MCI. Thus, we examined the relationship between BMI and cognitive decline in individuals with MCI enrolled in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). We hypothesized that low BMI in MCI is a marker of preclinical AD and would be associated with more rapid cognitive decline and higher rates of progression to AD.
METHODS
Sample
All participant data were obtained from the ADNI database (www.loni.ucla.edu/ADNI). ADNI is an ongoing 5-year, 50 site, public-private cooperative partnership conducted by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations (PI: Michael W. Weiner, MD, VA Medical Center and University of California, San Francisco). Its primary goal is to test whether serial magnetic resonance imaging, positron emission tomography, other biologic markers, and clinical and neuropsychologic assessment can be combined to measure the progression of MCI and AD. The current report used all of the available clinical and neuropsychologic data collected at baseline, 6, and 12-month follow up (data available as of February 5, 2008).
Two hundred eighty-six ADNI participants diagnosed with MCI were included. MCI diagnostic criteria included Mini-Mental State Examination (MMSE) scores between 24 and 30, a memory complaint, objective memory loss measured by education adjusted scores on Wechsler Memory Scale (WMS) Logical Memory II, a clinical dementia rating (CDR) of 0.5, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia.
Clinical Assessment
Standard clinical and neuropsychologic evaluations (common to all ADNI sites) were performed at baseline, 6, and 12-months including a CDR712 and battery of 2 cognitive screening instruments and 9 neuropsychologic tests (MMSE, Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog), WMS-Revised Logical Memory I and II, Auditory Verbal Learning Test, Boston Naming Test, Trail Making A and B, Digit Symbol, Clock Drawing Test, and Category Fluency). We used height and weight measures to calculate BMI (kg body weight/m2 height). After clinical evaluation and review of medical history, CDR, neuropsychologic performance, and laboratory tests evaluating clinicians rendered diagnosis consistent with National Institute of Neurological Disorders and Stroke/Alzheimer Disease and Related Disorders Association criteria for each participant (normal, MCI, AD; ADNI protocol available at http://www.adni-info.org). Cognitively normal participants had MMSE scores between 24 and 30 (inclusive), a CDR of 0, were nondepressed, non-MCI, and nondemented. Participants diagnosed with MCI had MMSE scores between 24 and 30 (inclusive), a memory complaint and objective memory loss measured by education-adjusted scores on WMS-Revised Logical Memory II, a CDR of 0.5, largely preserved activities of daily living, and an absence of dementia. Participants diagnosed with AD had MMSE scores between 20 and 26 (inclusive), CDR of 0.5 or 1.0, and met National Institute of Neurological Disorders and Stroke/Alzheimer Disease and Related Disorders Association criteria for probable AD. All data records were reviewed by a Central Review Committee to insure the uniform application of eligibility and diagnostic criteria across sites (including conversion from MCI to AD).
Cognitive Outcomes
We used 4 summary scores to index global cognitive performance: MMSE,13 ADAS-Cog,14 CDR sum of boxes,15 and a global Z-score composite derived by averaging standard scores within an individual across all 9 subtests of the ADNI neuropsychologic battery (Logical Memory II, Digit Span Forward, Digit Span Backward, category fluency animals, category fluency vegetables, Trail Making B, Boston naming test, Auditory Verbal Learning test, and Digit Symbol). The MMSE is a brief cognitive impairment-screening instrument. ADAS-Cog is a slightly longer cognitive impairment instrument used frequently in clinical trials. The CDR assesses cognitive function along 5 levels of impairment (rated as 0, 0.5, 1, 2, or 3) in each of 6 domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. CDR sum of boxes is the sum of the ratings in each of the 6 domains.
In follow up assessments, 3 of these quantitative measures (ADAS-Cog, MMSE, and the CDR sum of boxes scores) were rated by the clinician as representing either a “clinically significant or nonsignificant” decrement in performance (relative to the participant’s previous assessment; yes or no). Given the entire clinical-cognitive profile of each participant, the evaluating clinician also rendered a diagnostic appreciation whether that individual had progressed from MCI to dementia (yes or no). As per the ADNI protocol, the threshold for “clinically significant” was left to the judgment of the ADNI physician.
Statistical Analyses
Generalized estimating equations16 (GEE; SPSS 15, 1 tailed α<0.05) were used to assess the relationship between all cognitive outcomes and baseline BMI, controlling for age, sex, and education. GEE is a repeated measures regression that additionally accounts for serial autocorrelation of test scores over time (within-subject). Dependent variables included the 4 quantitative indices of global cognition and the 5 binomial qualitative appreciations of clinically significant change from prior assessment. For quantitative outcomes linear GEE was used. For qualitative (binomial) outcomes logistic GEE was used. In all analyses, predictor variables included baseline BMI, time, and a BMI by time interaction. Resultant slope estimates reflect the influence of baseline BMI on cognition over time. GEE analyses included all 3 time points (baseline, 6, and 12 mo), thus using all available data to maximize statistical efficiency whereas also controlling for any existing baseline differences in performance. For all GEE analyses, we used a model fitting technique described in Singer and Willett17 to determine which of 4 within-subject correlation structures (independent, exchangeable, autoregressive, and unstructured) best represented the autocorrelation of the MCI participant scores. The exchangeable correlation structure best fit both the linear and binomial data.
RESULTS
Sample Characteristics
The MCI cohort (N=286) had a mean BMI of 26.0 (SE=4.0), age of 75.0 years (SE=7.5 y), and education of 15.8 (SE=3.0); 34.3% were female (n=98). Cognitive outcome characteristics for the ADNI MCI cohort are reported in Table 1.
TABLE 1.
Characteristics of MCI Subjects at Baseline, 6, and 12 Months (N = 286)
| Baseline | 6 mo Follow-up | 12 mo Follow-up | “Clinically Significant” Change (n) | |
|---|---|---|---|---|
| BMI (kg body weight/m2 height) | 26.0 (4.0) | 26.1 (4.2) | 26.0 (4.0) | — |
| MMSE (range: 0 to 30) | 26.9 (1.8) | 26.5 (2.6) | 26.6 (2.5) | 70 |
| ADAS-Cog (range: 0 to 70)* | 9.5 (3.7) | 14.5 (3.2) | 14.9 (3.6) | 54 |
| CDR Box Score (range: 0 to 18)* | 1.6 (0.9) | 1.8 (1.1) | 1.9 (1.2) | 86 |
| Global composite (Z-score, −1 to +1) | −0.97 (0.6) | −0.89 (0.68) | −0.91 (0.71) | — |
| Diagnostic progression from MCI to AD | — | n=15 | n=30 | 54 |
Clinically significant change was assessed by ADNI clinicians for MMSE, ADAS-cog, CDR scores (yes or no). Data presented represents the number of subjects deemed to have significant change in these tests or progressing to a diagnosis of AD.
Reverse scored: less impaired to more impaired performance, otherwise high scores indicate better performance.
AD indicates Alzheimer disease; ADAS-cog, Alzheimer’s Disease Assessment Scale-cognitive subscale; ADNI, Alzheimer’s Disease Neuroimaging Initiative; BMI, body mass index; CDR, clinical dementia rating; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Examination.
Relationship of BMI to Cognitive Performance
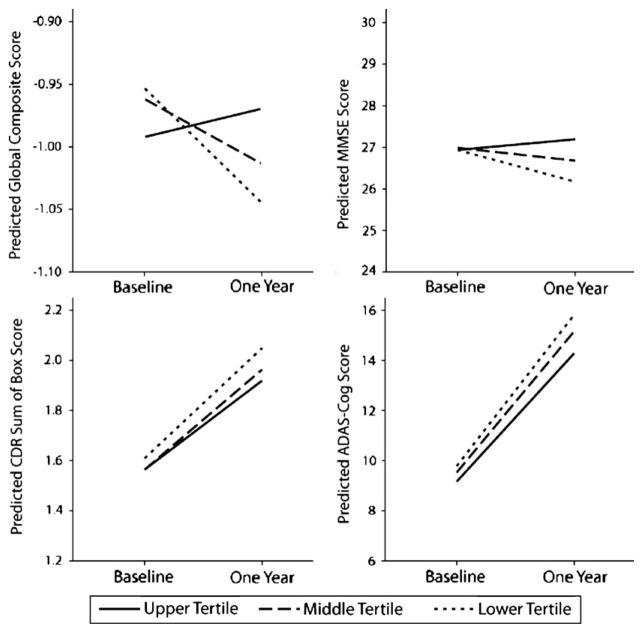
Results from the linear GEE analyses (adjusted for age, education, and sex) indicate that although BMI was not associated with cognitive performance at baseline, lower BMI (at baseline) was associated with an increased rate of cognitive decline in MMSE (Wald X2=15.4, df=2; P<0.001), ADAS-cog (Wald X2=6.7, df=2; P=0.02), and the global composite (Z-score; Wald X2=8.2, df=2; P=0.02) over the 3 assessments in the 1-year study period (baseline, 6, and 12 mo). No association was observed between baseline BMI and change in CDR sum of boxes over the 12-month follow-up (Wald X2=3.5, df=2; P=0.17). Inspection of Table 1 reveals that most of the cognitive decrement occurred at the 6-month follow-up and then persisted at relatively constant levels in the 12th month. Figure 1 graphically represents the relationship between baseline BMI and 1-year change in global cognition. Tertiles of BMI participant data were plotted against cognitive performance over time (adjusted for covariates). Across all 3 measures presented, less cognitive decline is apparent in the highest BMI tertile compared with participants in the lowest baseline BMI tertile.
FIGURE 1.
Predicted means of cognitive outcomes and BMI in tertiles at baseline and 1-year follow up for mild cognitive impairment participants. The predicted means of cognitive outcomes are presented for tertiles of BMI adjusted for age, education, and sex. Lower scores represent worse performance for the global composite score and Mini-Mental Status Examination (MMSE), whereas higher scores represent decline in clinical dementia rating (CDR) sum of boxes and Alzheimer Disease Assessment Scale-Cognitive subscale. Less cognitive decline over 1-year is apparent in the highest BMI tertile across the measures. BMI indicates body mass index.
Baseline BMI and Clinically Significant Cognitive Change
Results from the logistic GEE adjusted for age, education, and sex show a protective effect of higher BMI. Higher BMI is associated with lower rates of declining cognitive scores in both MMSE [odds ratio (OR)=0.93; confidence interval (CI)=0.86–1.00; P=0.03] and ADAS-Cog performance (OR=0.91; CI=0.83–0.99; P=0.02). No association was found between BMI and CDR Box Score (OR=0.98; CI=0.91–1.05; P=0.27) or between BMI and diagnostic progression to AD (OR=0.98; CI=0.92–1.05; P=0.31).
DISCUSSION
This assessment of 286 MCI participants enrolled in ADNI suggests that lower BMI at baseline is associated with increased rates of cognitive decline over 1 year as measured by the MMSE, ADAS-Cog, and a global composite score derived from the ADNI neuropsychologic battery. Lower BMI was also associated with increased risk of clinically significant decline on the MMSE and ADAS-cog suggesting that these associations are clinically recognizable and important. The data indicate that BMI remained stable through this short follow-up whereas cognition declined, suggesting that low BMI predisposes an individual to more rapid disease progression. Alternatively, factors associated with MCI and cognitive decline may influence body composition. Longer follow-up with time-lagged or change-score designs in the ADNI sample is warranted to investigate the causal relationship between weight loss and cognition.
BMI is a commonly used measure of adiposity that is associated with adverse health outcomes including mortality,18–20 cardiovascular disease,21–23 diabetes,24 and hypertension.25,26 Several longitudinal studies have demonstrated that lower BMI at baseline and loss of BMI over time is associated with an increased risk of developing AD.10,11,27,28 Individuals who develop dementia have weight loss in the 4 to 10 years leading up to diagnosis with accentuation of this weight loss at the time of diagnosis, depending on diagnostic criteria.10,11 Although clinical studies suggest that weight loss may be present in the clinical and preclinical stages of AD, autopsy data suggests that the loss of BMI in older adults may be in part related to the accumulation of AD pathology.29 In a clinical-pathologic study of 298 individuals, BMI in the years before death was associated with AD pathologic burden, even in nondemented individuals.29 As many, if not most, individuals with MCI are in the earliest clinical stages of AD,30,31 our data is consistent with prior studies suggesting that decreased BMI may be an early systemic manifestation of the AD process.10,27,28 In this study, however, BMI did not predict progression to a diagnosis of AD, although the power to assess this relationship is limited by the small number of individuals progressing to AD (n=54) over the short time frame (1 y). This issue is likely compounded by the subjectivity of the diagnostic appreciation across centers and individual clinicians.
Although MCI is a heterogeneous condition and can be related to static or reversible causes (ie, depression, medications, and medical illness), the MCI criteria identify a population enriched with individuals in the earliest clinical stages of AD. Although our analyses did not demonstrate increased rates of progression to overt clinical AD, the relationship between BMI and cognitive decline is consistent with prior studies suggesting that AD neuropathology, which likely begins accumulating years before the clinical onset, may be in part responsible for lower BMI.27 Greater atrophy in the medial temporal lobe in AD is associated with lower BMI suggesting that brain change and body weight occur in tandem.32 Psychosocial hypotheses for weight loss such as behavioral changes influencing caloric intake (forgetting to eat or the inability to plan and prepare adequate meals) are possible, although the studies suggest against this possibility10,33 and MCI participants have limited functional changes.
This study is limited by a short follow up period of 1 year attenuating our power to assess the impact of BMI on cognitive decline in MCI and subsequent progression of MCI to AD. Although the overall effect reported here is modest, the time period measured is short (1 y) and given the likelihood of cumulative cognitive decrements, the long-term clinical impact on cognition may be severe. Thus, the findings reported here are confined to associations with the baseline BMI and not with change in BMI over time. In addition, BMI does not differentiate the contributions from muscle mass and body fat; thus, low BMI may reflect reductions in muscle mass, fat mass, or both. Further differentiating the role of body composition using more sensitive measures that differentiate components of body composition may be important given that muscle and fat are metabolically different and have different risk implications. Future studies should explore the use of more sophisticated measures of body composition to characterize the individual relationships of lean mass and fat mass on cognitive decline through time.
Acknowledgments
This study was supported by the National Institute on Aging (K23 NS058252). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc, Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co Inc, AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the US Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Footnotes
Disclosure: There are no relevant disclosures from the investigators.
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data, but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf).
References
- 1.Gazdzinski S, Kornak J, Weiner MW, et al. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafson D, Lissner L, Bengtsson C, et al. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafson D, Rothenberg E, Blennow K, et al. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 5.Atti AR, Palmer K, Volpato S, et al. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 6.Nourhashemi F, Deschamps V, Larrieu S, et al. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. J Alzheimers Dis. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 8.BarrettConnor E, Edelstein SL, CoreyBloom J, et al. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44:1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 9.White H, Pieper C, Schmader K, et al. Weight change in Alzheimer’s disease. J Am Geriatr Soc. 1996;44:265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 11.Knopman DS, Edland SD, Cha RH, et al. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 12.Berg L. Clinical dementia rating. Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Rosen WG, Mohs RC, Davis KL, et al. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 15.Berg L, Miller JP, Storandt M, et al. Mild senile dementia of the Alzheimer type: 2. Longitudinal assessment. Ann Neurol. 1988;23:477–484. doi: 10.1002/ana.410230509. [DOI] [PubMed] [Google Scholar]
- 16.Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19:769–776. doi: 10.1023/b:ejep.0000036572.00663.f2. [DOI] [PubMed] [Google Scholar]
- 17.Singer JD, Willett JB. Applied Longitudinal Data Analysis; Modeling Change and Event Occurence. Oxford: Oxford University; 2003. p. 644. [Google Scholar]
- 18.Corrada MM, Kawas CH, Mozaffar F, et al. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Droyvold WB, Lund Nilsen TI, Lydersen S, et al. Weight change and mortality: the Nord-Trondelag Health Study. J Intern Med. 2005;257:338–345. doi: 10.1111/j.1365-2796.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Tuomilehto J, Silventoinen K, et al. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47,212 middle-aged Finnish men and women. Int J Obes (Lond) 2005;29:894–902. doi: 10.1038/sj.ijo.0802870. [DOI] [PubMed] [Google Scholar]
- 21.De Michele M, Panico S, Iannuzzi A, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 22.Jousilahti P, Tuomilehto J, Vartiainen E, et al. Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 23.Vatten LJ, Nilsen TI, Romundstad PR, et al. Adiposity and physical activity as predictors of cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2006;13:909–915. doi: 10.1097/01.hjr.0000239463.80390.52. [DOI] [PubMed] [Google Scholar]
- 24.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 25.Droyvold WB, Midthjell K, Nilsen TI, et al. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Lond) 2005;29:650–655. doi: 10.1038/sj.ijo.0802944. [DOI] [PubMed] [Google Scholar]
- 26.Selmer R, Tverdal A. Body mass index and cardiovascular mortality at different levels of blood pressure: a prospective study of Norwegian men and women. J Epidemiol Community Health. 1995;49:265–270. doi: 10.1136/jech.49.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 28.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria (comment) Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- 32.Grundman M, Corey-Bloom J, Jernigan T, et al. Low body weight in Alzheimer’s disease is associated with medial temporal cortex atrophy. Neurology. 1996;46:1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 33.Wang PN, Yang CL, Lin KN, et al. Weight loss, nutritional status and physical activity in patients with Alzheimer’s disease. A controlled study. J Neurol. 2004;251:314–320. doi: 10.1007/s00415-004-0316-4. [DOI] [PubMed] [Google Scholar]