Abstract
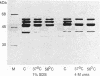
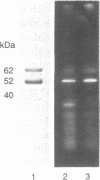
Cell lysis in presence of SDS and proteinase K followed by salting-out of residual polypeptides by dehydration and precipitation with saturated sodium chloride solution [Miller, S.A., Dykes, D.D. and Polesky, H.F., Nucleic Acids Res., 16, 1215, 1988] efficiently resolves deproteinized DNA. However, this DNA is still associated with prominent polypeptides which remain stably attached to DNA during further treatments, e.g. during repeated salting-out steps, prolonged incubation of DNA in 1% SDS or 4 M urea at 56 degrees C and ethanol precipitation. The persistent polypeptides (62, 52 and 40 kDa) released from Ehrlich ascites cell DNA were further characterized. Microsequencing indicates that the DNA binding polypeptides are not yet characterized at the sequence level. Nuclease digestion of the DNA releases stable DNA-protein complexes with the shape of globular particles (12.8 +/- 0.8 nm) and their larger aggregates in which DNA remains protected from nuclease digestion. The isolated DNA-polypeptide complexes show ATPase (Km = 7.4 x 10(-4) M) and protein kinase activity. Antibodies reveal a parallel distribution of the complexes with chromatin, however, the complexes are retained in chromatin-depleted nuclei.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Cao Q. P., Pitt S., Leszyk J., Baril E. F. DNA-dependent ATPase from HeLa cells is related to human Ku autoantigen. Biochemistry. 1994 Jul 19;33(28):8548–8557. doi: 10.1021/bi00194a021. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Kopman C. R., James C. B. DNA-stimulated protein phosphorylation in HeLa whole cell and nuclear extracts. Biochem Biophys Res Commun. 1988 Dec 15;157(2):535–540. doi: 10.1016/s0006-291x(88)80282-1. [DOI] [PubMed] [Google Scholar]
- Getzenberg R. H., Pienta K. J., Ward W. S., Coffey D. S. Nuclear structure and the three-dimensional organization of DNA. J Cell Biochem. 1991 Dec;47(4):289–299. doi: 10.1002/jcb.240470402. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Dolle A., Robertson G., Cook P. R. The attachments of chromatin loops to the nucleoskeleton. Cell Biol Int Rep. 1992 Aug;16(8):687–696. doi: 10.1016/s0309-1651(05)80013-x. [DOI] [PubMed] [Google Scholar]
- Juodka B., Pfütz M., Werner D. Chemical and enzymatic analysis of covalent bonds between peptides and chromosomal DNA. Nucleic Acids Res. 1991 Dec 11;19(23):6391–6398. doi: 10.1093/nar/19.23.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Spiess E., Werner D. Fragmentation of 'nuclear matrix' on a mica target. Eur J Cell Biol. 1983 Jul;31(1):158–166. [PubMed] [Google Scholar]
- Neuer-Nitsche B., Lu X. N., Werner D. Functional role of a highly repetitive DNA sequence in anchorage of the mouse genome. Nucleic Acids Res. 1988 Sep 12;16(17):8351–8360. doi: 10.1093/nar/16.17.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuer-Nitsche B., Werner D. Sub-set characteristics of DNA sequences involved in tight DNA/polypeptide complexes and their homology to nuclear matrix DNA. Biochem Biophys Res Commun. 1987 Aug 31;147(1):335–339. doi: 10.1016/s0006-291x(87)80126-2. [DOI] [PubMed] [Google Scholar]
- Neuer B., Plagens U., Werner D. Phosphodiester bonds between polypeptides and chromosomal DNA. J Mol Biol. 1983 Feb 25;164(2):213–235. doi: 10.1016/0022-2836(83)90076-1. [DOI] [PubMed] [Google Scholar]
- Pfütz M., Gileadi O., Werner D. Identification of human satellite DNA sequences associated with chemically resistant nonhistone polypeptide adducts. Chromosoma. 1992 Oct;101(10):609–617. doi: 10.1007/BF00360538. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yamamoto K., Sinohara H. Molecular cloning and sequence analysis of full-length cDNA coding for mouse contrapsin. J Biochem. 1990 Sep;108(3):344–346. doi: 10.1093/oxfordjournals.jbchem.a123204. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Neuer-Nitsche B. Site-specific location of covalent DNA-polypeptide complexes in the chicken genome. Nucleic Acids Res. 1989 Aug 11;17(15):6005–6015. doi: 10.1093/nar/17.15.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]