Abstract
Animal research supports a central role for corticotropin releasing factor (CRF) in actions of ethanol on brain function. An examination of alcohol consumption in adolescents reported a significant genotype × environment (G × E) interaction involving rs1876831, a CRHR1 polymorphism, and negative events. CRHR1 and at least 4 other genes are located at 17q21.31 in an extremely large block of high linkage disequilibrium resulting from a local chromosomal inversion; the minor allele of rs1876831 is contained within the H2 haplotype. Here we examine whether G × E interactions involving this haplotype and childhood sexual abuse (CSA) are associated with risk for alcohol consumption and dependence in Australian participants (N=1128 respondents from 476 families) of the Nicotine Addiction Genetics project. Telephone interviews provided data on DSM-IV alcohol dependence diagnosis and CSA and enabled calculation of lifetime alcohol consumption factor score (ACFS) from 4 indices of alcohol consumption. Individuals reporting a history of CSA had significantly higher ACFS and increased risk for alcohol dependence. A significant G × E interaction was found for ACFS involving the H2 haplotype and CSA (p<0.017). A similar G × E interaction was associated with protective effects against alcohol dependence risk (odds ratio 0.42; 95%CI 0.20 – 0.89). For each outcome, no significant CSA-associated risk was observed in H2 haplotype carriers. These findings support conducting further investigation of the H2 haplotype to determine the gene(s) responsible. Our results also suggest that severe early trauma may prove to be an important clinical covariate in the treatment of alcohol dependence.
Keywords: alcohol dependence, association, childhood sexual abuse, CRHR1, haplotype, interaction
INTRODUCTION
Alcohol use disorders are common illnesses that profoundly impact the lives of affected individuals, their families, and those with whom they interact (World Health Organization 2004; Hasin et al. 2007). The heritability of alcohol dependence has been estimated to be at least 50% by large twin studies (Heath et al. 1997; Prescott & Kendler, 1999; Tsuang et al. 2001; Knopik et al. 2004) with the remaining contribution to liability attributed to individual-specific environmental sources. Included among genes implicated in genetic studies are those whose products are involved in the metabolism [ADH clusters (Luo et al. 2005b; Edenberg et al. 2006; Luo et al. 2006; Macgregor et al. 2009)] and effects [GABRA2 (Covault et al. 2004; Edenberg et al. 2004; Lappalainen et al. 2005; Fehr et al. 2006), CHRM2 (Wang et al. 2004; Luo et al. 2005a)] of ethanol; however, replicated findings to date explain only a minority of the overall genetic risk.
Caspi and colleagues’ influential examinations (Caspi et al. 2002; Caspi et al. 2003) of other psychiatric disorders have stimulated more recent investigations of genotype × environment (G × E) interactions targeting alcohol-related phenotypes (Blomeyer et al. 2008; Kaufman et al. 2007; Covault et al. 2007; Armeli et al. 2008; Ducci et al. 2008; van der Zwaluw et al. 2009). These studies have focused on environmental covariates occurring during important periods of brain development [physical abuse, childhood sexual abuse (CSA) (Kaufman et al. 2007; Ducci et al. 2008)] or acquisition of risky drinking patterns [parental rule-setting (van der Zwaluw et al. 2009), negative life events during adolescence (Blomeyer et al. 2008) or college (Covault et al. 2007; Armeli et al. 2008)].
One longitudinal investigation (Blomeyer et al. 2008) recruited as infants a high risk sample enriched for those whose births involved severe obstetric problems or whose families experienced substantial psychosocial adversity. At age 15, these individuals (N=280) completed an assessment that included measures of alcohol consumption and severe stressors experienced during the past three years. The authors found evidence of a significant G × E interaction involving a corticotropin-releasing hormone receptor 1 (CRHR1) polymorphism, rs1876831, and negative life events. Specifically, one or more copies of the minor allele was associated with reduced risk for (any) lifetime history of binge drinking and maximum amount of alcohol consumed per occasion in those who had experienced severe stressors. No evidence of a similar G × E interaction was seen for a second CRHR1 polymorphism, rs242938. A prior examination (Treutlein et al. 2006) of their sample reported significant main effects on alcohol consumption for both of these CRHR1 polymorphisms with confirmation in an older alcohol dependent clinical sample. The potential importance of these findings is underscored by an extensive animal literature (Le et al. 2000; Hansson et al. 2006; Sommer et al. 2008; Heilig & Koob, 2007; Pastor et al. 2008) supporting CRF1 receptor involvement in diverse effects of ethanol including sensitization (Pastor et al. 2008), consumption (Hansson et al. 2006), withdrawal (Sommer et al. 2008), and stress-induced relapse (Le et al. 2000).
CRHR1 is located in a region, 17q21.31, which was recently described (Pennisi, 2008) as “one of the most structurally complex and evolutionarily dynamic regions of the genome.” A nearby gene, microtubule-associated protein tau (MAPT), first (Hutton et al. 1998) drew research attention to this area as a result of accumulating evidence (Pittman et al. 2006; Pastor et al. 2004) for association with risk of progressive supranuclear palsy (PSP), a neurodegenerative disease in which tau-positive neurofibrillary tangles are present. Additional examination (Pastor et al. 2004; Stefansson et al. 2005) revealed the existence of an extremely large linkage disequilibrium (LD) block spanning ~1.5 Mb extending across 5 adjacent genes (including MAPT and CRHR1). Two haplotypes, termed H1 and H2, have been described; the H2 haplotype contains an ~970 kb inversion that prevents recombination in H1/H2 heterozygotes (Stefansson et al. 2005). The H1 haplotype, present in all populations [H2 is found predominately in those of European ancestry (Stefansson et al. 2005)], is associated with increased risk for PSP and other neurologic disorders (Pastor et al. 2004; Skipper et al. 2004; Cruts et al. 2005; Pittman et al. 2006; Sundar et al. 2007; Webb et al. 2008). Evidence of positive selection for the H2 haplotype was observed in a large Icelandic sample (Stefansson et al. 2005); however, a predisposition to a microdeletion syndrome resulting in mental retardation and neurologic symptoms has also been associated with the H2 haplotype (Koolen et al. 2006).
A recent study (Tantisira et al. 2008) found an association with inhaled corticosteroid response in asthma for four SNPs that tag the H2 haplotype in samples from populations in which a similar association had been previously attributed (Tantisira et al. 2004) to rs1876828, a CRHR1 SNP. Similarly, the minor allele of rs1876831, the CRHR1 polymorphism for which a significant G × E interaction associated with risk for alcohol consumption was previously reported (Blomeyer et al. 2008), is also contained within the H2 haplotype. Research on this region has also not yet been integrated into the psychiatric literature.
The current report examines whether a similar G × E interaction is observed involving the H2 haplotype and history of CSA that is protective against alcohol consumption and DSM-IV alcohol dependence in the Australian sample of the Nicotine Addiction Genetics (NAG) project (Saccone et al. 2007; Agrawal et al. 2008). Our large sample, drawn from a heavy-drinking population, enriched for regular smokers [rates of regular smoking in clinical samples of alcoholics approach 90% (Hurt et al. 1996)], and having largely survived the period of greatest risk for the onset of alcohol dependence, is particularly well-suited for this investigation.
MATERIALS AND METHODS
Sample Ascertainment and Recruitment
The Australian component of the NAG project (Saccone et al. 2007), a collaboration between Queensland Institute of Medical Research (QIMR) and Washington University School of Medicine (WUSM) investigators, used data collection procedures approved by both institutions’ institutional review boards. Detailed descriptions of study methods have been reported (Saccone et al. 2007; Agrawal et al. 2008). In brief, prior reports by index cases of smoking status and family structure (in surveys of two large Australian Twin Panel cohorts and of spouses of the older twin cohort) were used to ascertain families with a sib pair [containing at most one monozygotic (MZ) twin] concordant for heavy smoking. Index cases were contacted to confirm smoking history and to obtain permission to contact family members. Families with two available parents were prioritized. When both parents were not available, at least one unaffected sibling was targeted for recruitment. After confirmation of family eligibility, study materials (including consent form) were mailed and telephone interviews scheduled with individual family members. Interviewing was conducted from 2001-2006. Data from only one member of any MZ twin pair, the designated index case, were included in analyses.
Assessment
All participants first provided verbal consent. A computer-assisted telephone interview adapted from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al. 1994) was then administered. Data collected included demographic information, DSM-IV diagnoses of psychiatric and substance dependence disorders, and other non-diagnostic sections. To reduce respondent time commitment, younger cohort twins (born 1964 – 1975) and spouses of older cohort twins were not readministered some interview items identical to their prior assessment.
Two primary outcome measures are used in analyses reported here: (1) a quantitative alcohol consumption factor score (ACFS) (Agrawal et al. 2009; Grant et al. 2009); and (2) a binary DSM-IV diagnosis of alcohol dependence. Individuals who denied any lifetime alcohol use were coded as missing for the alcohol consumption measure and for alcohol dependence. Respondents who endorsed at least 3 of 7 diagnostic criteria in a single year were given a DSM-IV alcohol dependence diagnosis. The ACFS was created from four indices of alcohol consumption queried in the alcohol use disorders section: (1) lifetime maximum 24 hour alcohol consumption (log-transformed to adjust for skewness); and for the heaviest drinking period of at least one year’s duration, (2) weekly alcohol consumption (log-transformed); (3) frequency of drinking to intoxication; (4) frequency of drinking ≥5 drinks per day. Because the NAG sample is enriched for heavy smokers, data from BigSib, a general community sample ascertained from the Australian Twin Registry on the basis of large sibship size [see (Saccone et al. 2007) for more information] were used to generate scoring coefficients more representative of the general population. An option in the Factor Procedure of SAS (SAS Institute 2004) enabled separate scoring coefficients to be calculated for women and men using BigSib sample data. The SAS Score Procedure was then used to apply these scoring coefficients to data from NAG project participants to obtain factor scores. Consistently high factor loadings for all component items were found for women (0.68-0.85) and men (0.63-0.92).
The primary covariate, CSA, is derived from a question in the conduct disorder section of the interview (not administered to parents, for whom data were thus not included in the current analyses): “Before age 18, were you ever forced into sexual intercourse or any other form of sexual activity?” A follow-up question determined the age at which forced sexual activity first occurred. Two respondents (N=2) who endorsed the forced sex question, but reported its first occurrence at an age ≥18, were excluded from analyses (Nelson et al. 2002; Nelson et al. 2006). Seven individuals whose responses to this item at subsequent assessment were inconsistent (3 endorsed and then denied; 4 denied and then endorsed) were also excluded from further analyses. As we have done previously (Nelson et al. 2002; Nelson et al. 2006), those reporting forced sexual activity with first occurrence before age 18 (N=151) or who did not report (N=5) age of first occurrence were coded as having a history of CSA.
SNP genotyping and haplotype assignment
DNA was extracted from blood samples by salting out. Sequenom MassArray iPLEX technology was used for SNP genotyping. PCR primers, extension primers, and multiplexing capabilities were determined with Sequenom MassARRAY Assay Designer software v3.1.2.2. Standard procedures were used to amplify PCR products; unincorporated nucleotides were deactivated with shrimp alkaline phosphatase. A single base pair extension step was completed with the mass extension primer and the terminator (iPLEX). The primer extension products were cleaned with resin and spotted onto a silicon SpectroChip. The chip was scanned with a mass spectrometry workstation (Bruker). The resulting genotype spectra were analyzed with Sequenom SpectroTYPER software v3.4.
The 13 genotyped CRHR1 SNPs included those from prior reports (Blomeyer et al. 2008; Treutlein et al. 2006; Bradley et al. 2008) and nearby SNPs identified from dbSNP. HWE p values were >0.1 for all SNPs; no evidence of substantial Mendelian errors was found. The call rate for rs3029044, an insertion/deletion polymorphism, was 94%; call rates for the SNPs otherwise ranged from 0.95 -0.98 (TABLE 1). We examined the linkage disequilibrium (LD) relationships of the 13 SNPs (shown in FIGURE 1 as pairwise r2 values) and recognized that the large block of 8 SNPs (including rs1876831) in very strong LD with minor allele frequencies (MAFs) ranging from 0.21 to 0.22 (TABLE 1) are part of the H2 haplotype. The other 5 SNPs forming two small additional LD blocks apart from the H2 haplotype are not included in the current analyses. Data were coded consistent with the prior report (Blomeyer et al. 2008) to enable comparison of individuals with one or more copy of the H2 haplotype to individuals homozygous for the H1 haplotype (ie, consistent with a dominant mode of inheritance).
Table 1.
Genotyped CRHR1 SNPs, minor alleles, MAFs, and call rates
| CRHR1 SNP | Minor allele | MAF | Call rate |
|---|---|---|---|
| rs1876830 | T | 0.22 | 0.98 |
| rs16940674 | T | 0.22 | 0.98 |
| rs1876831 | T | 0.22 | 0.97 |
| rs3029044 | Insert | 0.22 | 0.94 |
| rs1396862 | A | 0.22 | 0.98 |
| rs1912151 | T | 0.21 | 0.97 |
| rs242938 | A | 0.06 | 0.98 |
| rs2316764 | G | 0.22 | 0.98 |
| rs242939 | C | 0.06 | 0.98 |
| rs2316763 | T | 0.22 | 0.98 |
| rs242924 | T | 0.42 | 0.98 |
| rs110402 | A | 0.41 | 0.95 |
| rs7209436 | T | 0.40 | 0.95 |
Key: white (bolded)= H2 haplotype SNPs; gray= SNPs in 2 LD blocks (light and dark) outside of H2 not included in analyses
Figure 1.
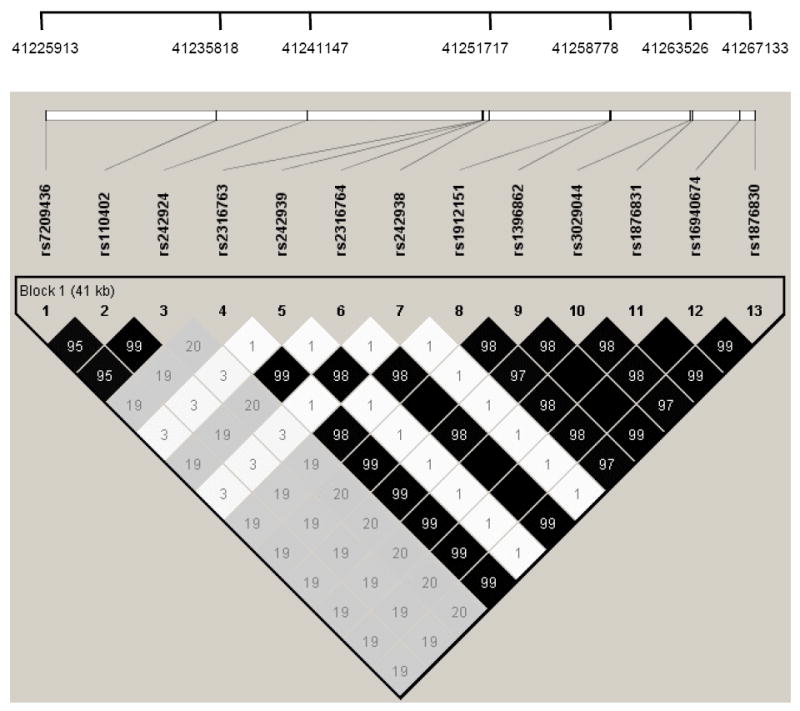
Linkage disequilibrium (LD) pattern (r2 values shown) and physical location of genotyped CRHR1 SNPs
Genotyping results were entirely consistent for 1119 participants: for 671, no minor alleles were detected at any successfully genotyped locus (H2 haplotype absent); for 448, a minor allele was detected at each locus (H2 haplotype present). For another 9 individuals, genotyping results included minor inconsistencies that primarily involved the more difficult to genotype rs3029044 insertion/deletion: 6 who were homozygous for the major allele at all but one successfully genotyped locus were coded as H2 haplotype absent; 3 others who were homozygous for the major allele at a single locus (with minor alleles present at the other successfully genotyped loci) were coded as H2 haplotype present. Finally, 3 individuals who had intermediate genotypic results were coded as indeterminate for H2 haplotype and excluded from subsequent analyses. Haplotypes were similarly assigned for all additional family members (including parents) with genotypic data available and Pedcheck (O’Connell & Weeks, 1998) was used to look for Mendelian errors (total N=1814). No Mendelian errors in haplotype assignment were identified. The final sample (N=1128 individuals from 476 families) consisting of all those with data available for H2 haplotype, CSA, and outcome measures included 565 women [mean age 41.0, (SD 8.6)] and 563 men [mean age 43.1, (SD 9.4)]. These individuals almost exclusively report Anglo-Celtic or other European ancestry.
Statistical Analyses
Analyses were performed using the SAS statistical software package version 9.1 (SAS Institute, 2004). The primary analyses examine whether G × E interactions involving the H2 haplotype and a history of CSA are protective against alcohol consumption and DSM-IV alcohol dependence. For linear regression analyses, the SurveyReg Procedure was used to control for inclusion of data from multiple members of families. For logistic regression analyses, the SurveyLogistic Procedure provided similar control. We performed T-tests to determine if mean ACFS differed by CSA status when controlling for gender and genotype. A significance threshold of alpha=0.05 was used for all analyses.
RESULTS
Descriptive Analyses
121 women (21.4%) and 35 men (6.2%) reported a history of CSA; mean age at first CSA occurrence was 11.0 years (SD 4.3). A lifetime DSM-IV diagnosis of alcohol dependence was more common in men (40.7%) than women (21.2%). The mean ACFS values for women and men were 0.42 (SD 1.01) and 0.43 (SD 0.91) respectively. ACFS were correlated (p<0.0001) with lifetime alcohol dependence diagnoses in both women (r=0.54) and men (r=0.45). Those with a history of CSA had significantly higher ACFS [mean values: CSA+ 0.67 (SD 1.10); CSA- 0.39 (SD 0.94); p<0.0031].
Linear Regression Analyses Examining ACFS
In linear regression analyses with ACFS as the dependent variable, we first confirmed that a history of CSA is associated with higher lifetime alcohol consumption (main effect for CSA, p<0.003). We performed a similar analysis and found no significant main effect for H2 haplotype (p>0.77). We then examined whether a G × E interaction involving CSA and H2 haplotype was observed in analyses that also included terms for main effects of CSA, gender, and H2 haplotype. We found a significant G × E interaction with the H2 haplotype protecting against CSA-associated effects on alcohol consumption (see TABLE 2). To demonstrate the protective effects of the G × E interaction more clearly, we compared mean ACFS by CSA and H2 haplotype status. We initially confirmed that mean ACFS did not vary by gender for either H1 homozygotes (p>0.69) or individuals with the H2 haplotype (p>0.88). We then found that significantly higher (p=0.0006) mean ACFS was associated with a history of CSA only in H1 homozygotes; in individuals with the H2 haplotype, the mean ACFS varied minimally (p=0.77) with CSA status (FIGURE 2).
Table 2.
Contribution to ACFS of H2 haplotype, CSA, and interaction controlling for gender (N=1128)
| Parameter | Beta (95% CI) |
|---|---|
| Intercept | 0.33 (0.23 to 0.43) |
| Male | 0.06 (- 0.06 to 0.17) |
| H2 haplotype | 0.07 (- 0.05 to 0.20) |
| CSA | 0.47 (0.22 to 0.71) |
| CSA × H2 haplotype | - 0.41* (- 0.75 to - 0.08) |
Significant results are bolded;
p<0.017
Figure 2.
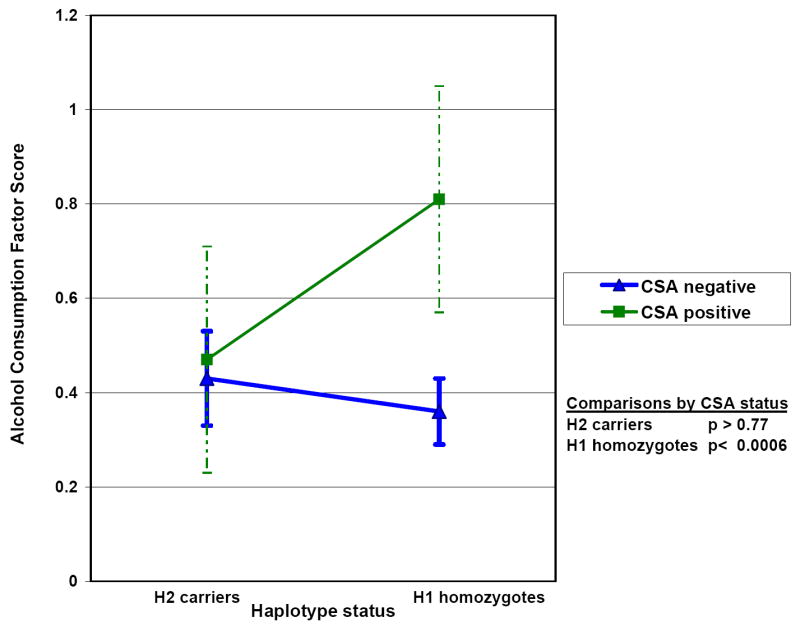
Mean Alcohol Consumption Factor Score by H1/H2 haplotype and CSA status (error bars indicate 95% confidence intervals for each mean)
Logistic Regression Analyses Examining Alcohol Dependence
We conducted a series of logistic regression analyses, controlling for gender, with alcohol dependence diagnosis as the dependent variable. We first confirmed that a history of CSA is associated with lifetime alcohol dependence risk (OR 2.03; 95%CI 1.40 – 2.92); we again found no evidence of a significant main effect for H2 haplotype on risk for alcohol dependence (OR 0.92; 95%CI 0.70 – 1.21). We next included a term for the G × E interaction involving CSA and the H2 haplotype in a model that also contained terms for main effects of CSA history, gender, and H2 haplotype. We found evidence of a significant G × E interaction for the H2 haplotype protecting against CSA-associated effects on alcohol dependence risk (OR 0.42; 95%CI 0.20 – 0.89; p=0.023). We calculated alcohol dependence risk by CSA status, controlling for gender, separately for individuals with and without the H2 haplotype. For H1 homozygotes (N=677), significant alcohol dependence risk was associated with CSA (OR 3.37; 95%CI 2.03 – 5.59). For those with the H2 haplotype (N=451), no evidence was found of CSA-associated risk (OR 1.04; 95%CI 0.57 – 1.90).
DISCUSSION
Our results provide evidence that CSA-associated risk for alcohol-related outcomes is moderated by the H2 haplotype. We first confirmed that a history of CSA is associated with significant risk for alcohol consumption and lifetime DSM-IV alcohol dependence in our sample; the H2 haplotype was not associated with risk for either of these outcomes. We found that, for both alcohol consumption and dependence, a G × E interaction involving CSA and the H2 haplotype is associated with significant protective effects. In those with the H2 haplotype, we observed no significant CSA-associated risk for either higher alcohol consumption or alcohol dependence.
Our results extend and clarify a prior report (Blomeyer et al. 2008) in which a significant G × E interaction involving severe stressors over the prior three years and the minor allele of rs1876831, a CRHR1 SNP, led to protection against alcohol consumption in adolescents. We found evidence of a similar interaction and demonstrated that it involves the ~1.5 Mb H2 haplotype spanning several genes in this region of chromosome 17. rs1876831 is one of many informative markers that can be used to tag this haplotype. Another important feature that distinguishes our study from the prior report is that our larger, substantially older sample has already passed through the period of greatest risk for problematic alcohol use enabling an examination lifetime measures, peak ACFS and DSM-IV alcohol dependence diagnosis, that characterize respondents’ mature drinking patterns. Both of these measures have excellent psychometric properties and have been shown to be at least moderately heritable (Heath et al. 1997; Bucholz et al. 1994; Agrawal et al. 2009; Grant et al. 2009). Our findings demonstrate that the protection against CSA-associated risk for problematic alcohol use associated with the H2 haplotype is persistent into adulthood. Overall, our results suggest that one or more of the 5 adjacent genes within the H2 haplotype play a major role in the risk for alcohol-related outcomes associated with severe life stressors.
Additional research will be necessary to clarify which gene or genes are responsible for these protective effects. Animal studies (Le et al. 2000; Shaham et al. 2000; Funk et al. 2003; Hansson et al. 2006; Sommer et al. 2008; Heilig & Koob, 2007; Pastor et al. 2008; Funk et al. 2003) published to date provide evidence that alterations in CRHR1 expression are involved in important facets of alcohol consumption, dependence, and relapse. Marchigian-Sardinian Preferring (msP) rats, selectively bred for high alcohol preference, have a CRHR1 promoter polymorphism that results in increased CRHR1 expression in limbic regions (Hansson et al. 2006). Alcohol self-administration by non-dependent msP rats is suppressed by CRF1 antagonists; similar effects are not seen in unselected Wistar (control) rats. msP rats also display greater sensitivity to inhibition of foot-shock-induced ethanol reinstatement by a CRF1 antagonist than do control rats.(Hansson et al. 2006) Rodents (not limited to those bred for ethanol preference) experience a prolonged period of increased anxiety and stress responsivity when withdrawn from ethanol after having been made dependent, symptoms that can be blocked by administration of a CRF1 antagonist (Sommer et al. 2008). In these animals, upregulation of CRHR1 expression has been found in the basolateral and medial amygdalar nuclei (Sommer et al. 2008) as have elevated CRF levels in their central amygdalar nucleus. In rats previously dependent on ethanol (similar findings have been reported with heroin and cocaine) (Shaham et al. 2000), relapse can be induced by foot-shock stress. For each drug, this reinstatement can be blocked by intracerebroventricular infusion of CRF1 antagonists (Le et al. 2000; Shaham et al. 2000). Direct CRF administration into involved brain regions also induces relapse in these animals (Le et al. 2000) and has been shown to block the increase in c-fos expression in the central nucleus of the amygdala that follows footshock (Funk et al. 2003).
A recent report (Barr et al. 2009) provides evidence that findings from the rodent literature are also applicable to non-human primates. The authors describe a functional CRH promoter polymorphism in rhesus macaques that is associated with greater stress responsivity and report interactions between genotype and rearing condition with significantly greater ACTH and cortisol responses to social stress observed in peer-reared carriers of this polymorphism. Most importantly, they report a genotype-environment interaction in which significantly greater alcohol consumption was found only for carriers of this polymorphism who had experienced early life stress (peer-rearing). This interaction, involving a CRH promoter polymorphism and a severe early life stressor associated with greater alcohol consumption in the absence of a significant main effect, is strikingly similar to the findings of the current report.
Other studies in rodents have demonstrated that exposure to severe, early life stressors results in epigenetically-mediated alterations in gene expression (Weaver et al. 2004; Weaver et al. 2005; Champagne et al. 2006; Weaver et al. 2007). Although admittedly speculative at present, it is possible that the protective effect which we observed could result from one or more functional polymorphisms limiting CSA-related epigenetically-mediated changes. Epigenetic modifications of gene expression due to ethanol are known to occur through a variety of different mechanisms (Pandey et al. 2008; Pietrzykowski et al. 2008; Shukla et al. 2008) and thus might not be susceptible to a similar protective effect (consistent with the lack of a significant main effect for H2 haplotype in our sample).
Our failure to confirm the earlier report (Treutlein J et al. 2006) of a significant main effect for rs1876831 on lifetime prevalence of (any) binge drinking and drunkenness in their adolescent sample and on alcohol intake in clinically-ascertained alcoholic adults may have resulted from disparity in the two studies’ outcome measures. The ACFS (Agrawal et al. 2009; Grant et al. 2009) used in this study is an estimate of consumption that is primarily based on the period of heaviest lifetime use. The main effects reported in the adolescent sample (Treutlein et al. 2006) were for two very early drinking career milestones, any episode of binge drinking or drunkenness. In their clinical sample, the significant main effect was for consumption of >250 g ethanol daily prior to admission, a binary measure of daily consumption much later in the course of the disorder that could be affected by various covariates (eg, gender, ADH genotype, alcoholic liver disease). Another study (Dahl et al. 2005) examined whether CRHR1 polymorphisms were associated with alcohol dependence risk in European American alcoholics found no association for any SNPs including those informative for the H2 haplotype.
Several additional issues should be considered when interpreting our results. We substituted CSA for the measure of multiple severe stressors in adolescence used in the prior report (Blomeyer et al. 2008). Previous reports of G × E interactions have included either multiple classes of stressors (Caspi et al. 2003) or substituted stressors for which data were available in replication studies (Kaufman et al. 2004; Gillespie et al. 2005; Kendler et al. 2005; Surtees et al. 2006). Additional study will be necessary to delineate whether our findings will generalize to different types of stressors or those occurring during other developmental periods. CSA is also associated (Nelson et al. 2002) with risk for subsequent stressors further complicating definitive attribution. Although use of binary outcome variables for examination of G × E interactions may predispose to spurious positive findings (Eaves, 2006), the association we observed with ACFS, a continuous measure, is less susceptible to this source of error. Given concerns about the sensitivity of inferences from G × E interactions to scaling, we ran an additional analysis using Huber robust regression which down-weights outlier observations: the G × E interaction term remained significant (p=0.026) with a modest reduction in effect size (beta= -0.37 versus beta= -0.41 in the linear regression analyses). The association with alcohol-related phenotypes could be bidirectional, however, the mean age (11.0y) of first CSA occurrence suggests that CSA typically precedes alcohol problems. The use of a binary variable for CSA combines diverse abuse experiences encompassing a wide range of severity; a stronger association might have been observed if a quantitative covariate incorporating information on abuse severity and duration were available. Retrospective assessment of CSA in adults may raise concerns regarding the introduction of bias. The CSA measure used in this study has been found to have reasonable concordance within female like-sex twin pairs and significant association with psychiatric sequelae and parental risk factors (Dinwiddie et al. 2000; McLaughlin et al. 2000). Examination of discordant pairs found no evidence for retrospective bias in the association of CSA with parental rejection (McLaughlin et al. 2000). The prevalence of CSA in our sample is higher than is typical for community samples, a likely result of enrichment for heavy smoking. Because of this enrichment, additional studies will be necessary to determine whether our results will generalize to samples representative of the general population. Post-hoc analyses of genetically informative data on ancestry from a partially overlapping GWAS revealed that two families (N=4 individuals in total) are outliers on the basis of ancestry. The ACFS of these families does not differ significantly from the remainder of the sample; dropping these 4 individuals has very minimal effect on either interaction term point estimates or p values.
Our data suggest that the H2 haplotype is protective against CSA-associated risk for higher lifetime alcohol consumption and alcohol dependence. The extent of these protective effects suggests that one or more of the genes within the H2 haplotype are playing an important role in stress-associated risk for alcohol consumption and dependence. Although the evidence (Le et al. 2000; Hansson et al. 2006; Sommer et al. 2008; Heilig & Koob, 2007; Pastor et al. 2008) from animal research is quite strong, it is premature to conclude that these protective effects are due to a CRHR1 polymorphism. Additional research is needed to determine definitively the gene or genes responsible for these protective effects. Gene expression studies have provided strong evidence in favor of MAPT (Caffrey et al. 2006; Caffrey et al. 2008) versus CRHR1 (Campdelacreu et al. 2006) involvement in PSP risk, although other research (Cruchaga et al. 2009) suggests that multiple genes may be involved. However, since our finding involves a G × E interaction rather than a main effect, it will be considerably more difficult to conduct this type of investigation. If the effects that we observe are a consequence of a CRHR1 polymorphism, our results may have immediate clinical relevance. Researchers have recently developed (Gehlert et al. 2007) improved CRF1 antagonist drugs which are already scheduled for pharmaceutical company-sponsored clinical trials of alcohol dependence treatment. Our findings would predict that H1 homozygotes with a history of severe early trauma exposure will preferentially respond to these agents. More generally, our findings emphasize the potential utility of screening for severe early trauma exposure in individuals presenting for alcohol dependence treatment.
Acknowledgments
The Nicotine Addiction Genetics (NAG) project is supported by DA012854, a grant from the National Institute on Drug Abuse for which Dr. Madden is the Principal Investigator (PI). Additional funds for the current report’s SNP genotyping were provided by a grant from ABMRF/ The Foundation for Alcohol Research (PI Dr. Agrawal). Support was also received from National Institute on Alcohol Abuse and Alcoholism grants AA013446 (PI Dr. Nelson) and AA017688, AA011998, and AA007728 (PI Dr. Heath) and the Australian NHMRC Fellowship Scheme (GWM).
Footnotes
Financial Disclosures: Drs. Goate, Rice, Saccone, and Wang are listed as inventors on a patent (US 20070258898) held by Perlegen Sciences, Inc., covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction.
References
- Agrawal A, Grant JD, Littlefield A, Waldron M, Pergadia ML, Lynskey MT, Madden PA, Todorov A, Trull T, Bucholz KK, Todd RD, Sher K, Heath AC. Developing a quantitative measure of alcohol consumption for genomic studies on prospective cohorts. J Stud Alcohol Drugs. 2009;70:157–68. doi: 10.15288/jsad.2009.70.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, Statham D, Henders A, Campbell M, Garcia R, Broms U, Todd RD, Goate AM, Rice J, Kaprio J, Heath AC, Montgomery GW, Madden PAF. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Arch Gen Psychiatry. 2008;65:713–21. doi: 10.1001/archpsyc.65.6.713. [DOI] [PubMed] [Google Scholar]
- Armeli S, Conner TS, Covault J, Tennen H, Kranzler HR. A serotonin transporter gene polymorphism (5-HTTLPR), drinking-to-cope motivation, and negative life events among college students. J Stud Alcohol Drugs. 2008;69:814–23. doi: 10.15288/jsad.2008.69.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci USA. 2009;106:14593–598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–51. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15:3529–37. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Wade-Martins R. Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging. 2008;29:1923–29. doi: 10.1016/j.neurobiolaging.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campdelacreu J, Gaig C, Ezquerra M, Muñoz E, Martí MJ, Valldeoriola F, Tolosa E. No evidence of CRHR1 gene involvement in progressive supranuclear palsy. Neurosci Lett. 2006;409:61–4. doi: 10.1016/j.neulet.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–9. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AHN, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–16. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Cruchaga C, Vidal-Taboada JM, Ezquerra M, Lorenzo E, Martinez-Lage P, Blazquez M, Tolosa E, Pastor P Iberian Atypical Parkinsonism Study Group Researchers. 5’-Upstream variants of CRHR1 and MAPT genes associated with age at onset in progressive supranuclear palsy and cortical basal degeneration. Neurobiol Dis. 2009;33:164–70. doi: 10.1016/j.nbd.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Cruts M, Rademakers R, Gijselinck I, van der Zee J, Dermaut B, de Pooter T, de Rijk P, Del-Favero J, van Broeckhoven C. Genomic architecture of human 17q21 linked to frontotemporal dementia uncovers a highly homologous family of low-copy repeats in the tau region. Hum Mol Genet. 2005;14:1753–62. doi: 10.1093/hmg/ddi182. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Doyle GA, Oslin DW, Buono RJ, Ferraro TN, Lohoff FW, Berrettini WH. Lack of association between single nucleotide polymorphisms in the corticotropin releasing hormone receptor 1 (CRHR1) gene and alcohol dependence. J Psychiatr Res. 2005;39:475–9. doi: 10.1016/j.jpsychires.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Dinwiddie S, Heath AC, Dunne MP, Bucholz KK, Madden PAF, Slutske WS, Bierut LJ, Statham DB, Martin NG. Early sexual abuse and lifetime psychopathology: a co-twin-control study. Psychol Med. 2000;30:41–52. doi: 10.1017/s0033291799001373. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–47. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Eaves LJ. Genotype × Environment interaction in psychopathology: fact or artifact? Twin Res Hum Genet. 2006;9:1–8. doi: 10.1375/183242706776403073. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Chen HJ, Tian H, Wetherill LF, Dick DM, Almasy L, Bierut L, Bucholz KK, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Porjesz B, Rice J, Schuckit M, Tischfield J, Begleiter H, Foroud T. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006;15:1539–49. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Lê AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–11. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PAF, Pergadia ML, Nelson EC, Lynskey MT, Todd RD, Todorov AA, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.018. epub ahead of print 2 Jul 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–41. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in men and women. Psychol Med. 1997;27:1381–96. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. Erratum in: JAMA 1996:276(10):784. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter Joel. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–34. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–30. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R, Kooy RF, Reyniers E, Romano C, Fichera M, Schinzel A, Baumer A, Anderlid BM, Schoumans J, Knoers NV, van Kessel AG, Sistermans EA, Veltman JA, Brunner HG, de Vries BB. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet. 2006;38:999–1001. doi: 10.1038/ng1853. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–8. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Lappalainen J, Yang BZ, Gelernter J. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: results from HWD tests and case-control association studies. Neuropsychopharmacology. 2006;31:1085–95. doi: 10.1038/sj.npp.1300925. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005a;14:2421–34. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Yang BZ, Lappalainen J, Gelernter J. ADH4 gene variation is associated with alcohol and drug dependence: results from family controlled and population-structured association studies. Pharmacogenet Genomics. 2005b;15:755–68. doi: 10.1097/01.fpc.0000180141.77036.dc. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PAF, Richter MM, Montgomery GW, Martin NG, Heath AC, Whitfield JB. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Hum Mol Genet. 2009;18:580–93. doi: 10.1093/hmg/ddn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin TL, Heath AC, Bucholz KK, Madden PAF, Bierut LJ, Slutske WS, Dinwiddie S, Statham DJ, Dunne MP, Martin NG. Childhood sexual abuse and pathogenic parenting in the childhood recollections of adult twin pairs. Psychol Med. 2000;30:1293–302. doi: 10.1017/s0033291799002809. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: a twin study. Psychol Med. 2006;36:1473–83. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Madden PAF, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59:139–45. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–66. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–37. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor P, Ezquerra M, Perez JC, Chakraverty S, Norton J, Racette BA, McKeel D, Perlmutter JS, Tolosa E, Goate AM. Novel haplotypes in 17q21 are associated with progressive supranuclear palsy. Ann Neurol. 2004;56:249–58. doi: 10.1002/ana.20178. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–75. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. Genetics. 17q21.31: not your average genomic address. Science. 2008;322:842–5. doi: 10.1126/science.322.5903.842. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–87. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15(Spec No 2):R188–95. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Saccone FS, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA, Maunu H, Heikkila K, Morley KI, Rice JP, Todd RD, Kaprio J, Peltonen L, Martin NG, Goate AM, Madden PA. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet. 2007;80:856–66. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, Inc. The SAS System for Windows, Version 9.1.3. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32:1525–34. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Skipper L, Wilkes K, Toft M, Baker M, Lincoln S, Hulihan M, Ross OA, Hutton M, Aasly J, Farrer M. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am J Hum Genet. 2004;75:669–77. doi: 10.1086/424492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, Baker A, Jonasdottir A, Ingason A, Gudnadottir VG, Desnica N, Hicks A, Gylfason A, Gudbjartsson DF, Jonsdottir GM, Sainz J, Agnarsson K, Birgisdottir B, Ghosh S, Olafsdottir A, Cazier JB, Kristjansson K, Frigge ML, Thorgeirsson TE, Gulcher JR, Kong A, Stefansson K. A common inversion under selection in Europeans. Nat Genet. 2005;37:129–37. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- Sundar PD, Yu CE, Sieh W, Steinbart E, Garruto RM, Oyanagi K, Craig UK, Bird TD, Wijsman EM, Galasko DR, Schellenberg GD. Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia. Hum Mol Genet. 2007;16:295–306. doi: 10.1093/hmg/ddl463. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–9. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, Liggett SB, Gelfand EW, Rosenwasser LJ, Richter B, Israel E, Wechsler M, Gabriel S, Altshuler D, Lander E, Drazen J, Weiss ST. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–59. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Lazarus R, Litonjua AA, Klanderman B, Weiss ST. Chromosome 17: association of a large inversion polymorphism with corticosteroid response in asthma. Pharmacogenet Genomics. 2008;18:733–7. doi: 10.1097/FPC.0b013e3282fe6ebf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001;9:267–79. [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Vermulst AA, Franke B, Buitelaar J, Verkes RJ, Scholte RH. Interaction between dopamine D2 receptor genotype and parental rule-setting in adolescent alcohol use: evidence for a gene-parenting interaction. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.4. e-pub ahead of print 24 Feb 2009. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, Kwon JM, Wu W, Dick DM, Rice J, Jones K, Nurnberger JI, Jr, Tischfield J, Porjesz B, Edenberg HJ, Hesselbrock V, Crowe R, Schuckit M, Begleiter H, Reich T, Goate AM, Bierut LJ. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–11. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A, Miller B, Bonasera S, Boxer A, Karydas A, Wilhelmsen KC. Role of the tau gene region chromosome inversion in progressive supranuclear palsy, corticobasal degeneration, and related disorders. Arch Neurol. 2008;65:1473–78. doi: 10.1001/archneur.65.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 2004. [20 Jan 2009]. Global Status Report on Alcohol 2004. Available at: http://www.who.int/substance_abuse/publications/alcohol/ Rep. [Google Scholar]


